Abstract
Background
To our knowledge, the treatment, outcome, clinical presentation, risk stratification of patients with venous thromboembolism and COVID-19 have not been well characterized.
Methods
We searched for systematic reviews, cohorts, case series, case reports, editor letters, and venous thromboembolism COVID-19 patients’ abstracts following PRISMA and PROSPERO statements. We analyzed therapeutic approaches and clinical outcomes of venous thromboembolism COVID-19 patients. Inclusion: COVID-19 patients with venous thromboembolism confirmed by an imaging method (venous doppler ultrasound, ventilation-perfusion lung scan, computed tomography pulmonary angiogram, pulmonary angiography). We assessed and reported the original Pulmonary Embolism Severity Index for each pulmonary embolism patient. In addition, we defined major bleedings according to the International Society of Thrombosis and Haemostasis criteria.
Results
We performed a systematic review from August 9 to August 30, 2020. We collected 1,535 papers from PubMed, Scopus, Web of Science, Wiley, and Opengrey. We extracted data from 89 studies that describe 143 patients. Unfractionated and low-molecular-weight heparin was used as parenteral anticoagulation in 85/143 (59%) cases. The Food and Drug Administration-approved alteplase regimen guided the advanced treatment in 39/143 (27%) patients. The mortality was high (21.6%, CI 95% 15.2-29.3). The incidence of major bleeding complications was 1 (0.9%) in the survival group and 1 (3.2%) in the death group. Pulmonary Embolism Severity Index was class I in 11.6% and II in 22.3% in survivors compared to 0% and 6.5% in non-survivors, respectively. Patients who experienced venous thromboembolism events at home were more likely to live than in-hospital events.
Conclusions
We determined a high mortality incidence of pulmonary embolism and a low rate of bleeding. Unfractionated and low-molecular-weight heparin drove parenteral anticoagulation and alteplase the advanced treatment in both groups. The original Pulmonary Embolism Severity Index could be helpful in the risk stratification.
Similar content being viewed by others
Background
The rapidly evolving coronavirus disease 2019 (COVID-19) global pandemic has been one of the most significant public health challenges since the Spanish flu pandemic over 100 years ago [1]. COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a multifaceted disease characterized by a wide range of clinical presentations and degrees of severity [2]. In the beginning, the target organ seemed to be only the respiratory system, inducing severe pneumonia and acute respiratory distress syndrome. However, an important lesson learned was that SARS-CoV-2 causes a high prothrombotic state, venous and arterial thrombosis [1]. Thus, the clinical presentation eventually resembles a thrombotic storm characterized by higher D-dimer measurements and high von Willebrand factor levels [3]. Additionally, thrombosis mechanisms linking inflammation pathways, coagulation system activity, immunothrombosis, cytokine storm, and renin-angiotensin-aldosterone system dysregulation [4,5,6,7,8] seem to be involved.
Therefore, in severe COVID-19, venous thromboembolism (VTE) emerges as a critical and frequent complication [9, 10], with a high incidence (15.3%, CI 95% 9.8-21.9) and mortality rate (45.1%, CI 95% 22.0-69.4), in pulmonary embolism (PE) patients [11]. Although there is a trend to better survival in patients treated with heparins (anticoagulation and anti-inflammatory effect) [12, 13], we do not have enough data on the best primary prevention doses, therapeutic approaches, and outcomes [9, 14, 15]. Also, there are no advanced treatment recommendations in massive and submassive PE [16, 17]. Therefore, we performed a systematic review using the Preferred Reporting Items for Systematic Reviews and Metanalyses (PRISMA) statement to determine the therapeutic trends and outcomes in VTE COVID-19 patients. Also, we assessed the original Pulmonary Embolism Severity Index (PESI) in PE patients.
Methods
Search strategy
We searched for systematic reviews, cohorts, case series, case reports, editor letters, and VTE COVID-19 patients’ abstracts through the PRISMA statement search [18]. We register the protocol in the International Prospective Register protocol of Systematic Reviews (PROSPERO); registration number: CRD42020203688). The patients must have received anticoagulation or thrombolysis. The objective was to assess the therapeutic trends and clinical outcomes of VTE COVID-19 patients.
Additionally, we analyzed the clinical presentation, risk stratification, and diagnostic approach. We included deep vein thrombosis (DVT) and PE confirmed by an imaging method (venous doppler US, ventilation-perfusion lung scan, computed tomography pulmonary angiogram, pulmonary angiography). We assessed the original PESI since it works better than the simplified PESI [19]. We established two groups, survivors and those who died. We performed a systematic review through PubMed, Scopus, Web of Science, Wiley, and OpenGrey and provided the complete search strategies in the e-Appendix. We used snowballing [20], a manual search to avoid lost reports, controlled vocabulary, and no language restriction. We do not contact authors to obtain additional information in cases with critical missing variables.
Study selection and data collection
We identified potentially eligible studies by examining titles and abstracts. We obtained full papers to assess eligibility criteria before the critical appraisal and extracted cases that met the eligibility criteria. All investigators analyzed data extraction of every case report to improve quality data extraction. The corresponding author is a cardiologist with expertise in the field (CJS). We conducted a group discussion daily to assess all the information extracted from the cases included in a database. Disagreements were solved posteriorly by consensus. We performed two meetings to ensure the data’s quality through a random review of 20% of the papers. The primary outcomes were therapeutic approaches, in-hospital death, intracranial hemorrhage (ICH), major, and minor.
Additionally, we analyzed the clinical presentation, the PE risk, COVID-19 severity, VTE primary prevention, and the thrombus’s location in the pulmonary circulation. According to the International Society of Thrombosis and Haemostasis criteria, we defined major bleedings [21]; we established the presence of right ventricular dysfunction according to the European Society of Cardiology guidelines of PE: right ventricular end-diastolic diameter/left ventricular end-diastolic diameter ratio ≥2:1, (b) regional or global right ventricular hypokinesis, (c) McConnell’s sign, (d) right ventricular diameter >35 mm, (e) systolic pulmonary arterial pressure ≥50 mm Hg; B-type brain natriuretic peptide (BNP) measurement (>90 pg/mL) or N-terminal proBNP (NT-proBNP) (>300 pg/mL); dynamic electrocardiographic changes (new complete or incomplete right bundle-branch block, anteroseptal ST elevation or depression, or anteroseptal T-wave inversion) [22]; other definitions, including the PESI score, massive PE and intensive care unit (ICU) VTE risk factors, are available in the e-Appendix.
Based on the high SARS-CoV-2 thrombogenicity and to understand its behavior in the venous system, we also analyzed acute cerebral venous sinus thrombosis (CVST), whether associated or not with VTE.
Statistical analysis
We used summary statistics for continuous and categorical variables according to their types and distributions. We report the frequency and percentage (n >20) for categorical variables, and for continuous variables, we report the mean and standard deviation. We used the IBM SPSS® software platform for descriptive statistical analysis.
Results
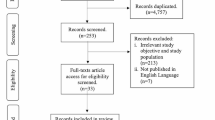
We carried out the systematic review from August 9 to August 30, 2020. Figure 1 shows the flowchart, including the four phases of PRISMA, and we obtained, eliminated, and excluded duplicated reports. In the identification phase, we collected 1,535 papers from PubMed, Scopus, Web of Science, Wiley, and Opengrey. Next, we carefully reviewed the full text for eligibility criteria and selected 107 reports for the quality assessment. Finally, we extracted the data for this review from 89 studies (references in supplementary material).
Baseline demographics and primary outcomes
Table 1 shows baseline demographics, clinical presentation, VTE and PE risk factors, DVT classification, PESI, and VTE onset. We identified 143 COVID-19 patients with VTE; most were relatively young overweight males with isolated PE with or without proximal DVT. The earliest clinical PE findings were severe oxygen desaturation, sudden dyspnea, and leg pain in DVT survived patients (Table 1). A remarkable characteristic was the lowest oxygen saturation in those who died. Among the usual comorbidities in COVID-19, hypertension had a higher incidence in patients who died. Cardiovascular risk factors (hypertension and diabetes) and those associated with in-hospital and ICU stay were more prevalent in those who died (Table 1). The proportion of low-risk and submassive PE was higher in patients who survived than those who died, where massive PE was predominant (Table 1). In this group, the detection of proximal or distal DVT was scarce. The original PESI classes II and III identified patients who lived (Table 1). Finally, patients with acute VTE events at home were more likely to live than in-hospital events. We identified reduced thromboprophylaxis use in both groups (Table 2). Initial treatment shows that unfractionated and low-molecular-weight heparin drove parenteral anticoagulation in both groups. Also, direct-acting oral anticoagulant use was rare. Alteplase 100 mg 2-hours infusion was the advanced treatment in both groups (Table 2). The mortality was high (21.6%, CI 95% 15.2-29.3), and there was a low incidence of bleeding complications, including ICH, in those who survived (Table 2).
Table 3 shows characteristics of VTE, including biomarkers, imaging studies, and severity, laboratories. Imagin and previous anticoagulation use related to COVID-19. Patients who died had a higher D dimer expression and right ventricular dysfunction (Table 3), and the use of biomarkers was low. [22] (Table 3). The computed tomography pulmonary angiography (CTPA) demonstrated a wide distribution of thrombus locations in surviving patients (Table 3). The variables mainly related to mortality were acute respiratory distress syndrome, mechanical ventilation, ICU stay, and higher C reactive protein measurements in patients with PE associated with severe COCID-19 patients.
Cerebral venous sinus thrombosis
We identified 15 young patients with a similar gender proportion practically without a history of contraceptives (Table 4). CVST clinical presentation included neurologic alterations at home, abnormal D dimer measurements, and only one case associated with a submassive PE. Most patients were asymptomatic or had COVID-19 pneumonia. Despite in-hospital primary prevention, five patients had CVST. We identified a remarkably high prevalence of ICH (10/15 patients) (66.7% CI95 38.4-88.2) and increased mortality (3/15 patients) (20% CI954.3-48.1) (Table 4).
Discussion
This systematic review highlights the therapeutic trends and outcomes of VTE survivors compared with those who died. The main observations were: First, unfractionated and low-molecular-weight heparin was the cornerstone in the VTE treatment. Also, 2-hours alteplase infusion was the most frequent advanced treatment in PE patients. Second, we identified high mortality in the ICU associated with severe COVID-19 with a low incidence of bleeding complications in massive PE. Third, the original PESI score II-III recognized patients who survived, suggesting its usefulness in the risk stratification in COVID-19 patients. Fourth, elevated C reactive protein and D dimer measurements and right ventricular dysfunction identified poor in-hospital outcomes. Finally, the exploratory analysis showed the same high ICH incidence in CVST mild COVID-19 patients than non-COVID-19 patients [23].
Recent systematic reviews and meta-analyses focused on the incidence, primary and secondary VTE prevention, bleeding complications [24,25,26,27], and the association of D-dimer with mortality [28, 29]. Therefore, therapeutic approaches, outcomes, clinical presentation, risk stratification, and patient characteristics are unclear.
Although still under debate, recent evidence from a small sample suggests that patients with severe COVID-19 disease are at high risk for thromboinflammation since they have SARS-CoV-2 infection, risk factors, cardiovascular, renal, or chronic pulmonary inflammatory comorbidities [2]. An increased frequency of arterial and venous thrombosis at the beginning of the pandemic was remarkable [30]. VTE is now recognized as among the predominant cardiovascular hazards [30], with the highest incidence in the intensive care unit setting (25%), increasing to 69% after surveillance venous ultrasonography [30]. Also, thromboprophylaxis, the foundation to prevent in-hospital VTE, fails in a subset of COVID-19 patients [30]. Additionally, quantifying the risk of thrombosis and cardiovascular complications is complicated in this heterogeneous population by reports of limited sample size, restriction of assessments to the ICU setting, outcome definitions, and differing thromboprophylaxis strategies [30].
Our findings suggest that intravenous or subcutaneous anticoagulation remains the cornerstone of therapy in deep venous thrombosis and PE COVID-19 patients. Strategies for reperfusion therapy included the thrombolysis regimen recommended for international guidelines [22] or “safe dose” in PE patients [31,32,33]. The rationale for advanced treatment in PE is to avert or improve impending clinical instability secondary to right ventricular dysfunction to improve the outcome. The presence of several pulmonary hypertension mechanisms (PE, hypoxic vasoconstriction, pulmonary microthrombi, ACE2 dysregulation, and cytokine storm) inducing right ventricular dysfunction suggests the possibility to obtain a CTPA before clinical decision-making in this population [34]. In the presence of high clinical suspicion and clinical instability, systemic thrombolysis use has evidence level IC [22]. Despite systemic thrombolysis, bleeding complication incidence was lower (0.9% vs. 3.2%) than recent evidence (21.4%) using intermediate- or full-heparin dose without advanced treatment and bleeding definitions according to the individual studies [27]. This difference in the incidence of bleeding complications is unclear because relevant clinical or significant bleedings are usually reported. We showed high mortality (46% in massive PE in severe COVID-19 patients. It is higher than observed in massive PE non-COVID-19 patients (33%) [35]; the mortality rates observed are also related to severe COVID-19 and higher than previous other viral pandemics experienced in the past [36]. Additionally, mortality appears to be multifactorial and driven by adult respiratory distress syndrome (ARDS) and massive PE. In the absence of a validated risk score for patients with severe COVID-19 and PE, current risk stratification in PE [22] could lose accuracy and explain the high percentage of unclassified PE patients.
The original PESI score is a helpful tool for immediate and bedside risk stratification [22]; if this score helps to stratify bedside high clinical suspicion PE in COVID-19 patients is unanswered. The original PESI risk score had greater precision in identifying low and intermediate PE risks and identified a high proportion of high-risk patients with very high risk [19]. In addition, COVID-19 in the health systems usually conditions a delay recommended diagnostic approaches in high clinical suspicion PE patients [22]; thus, the original PESI score could be helpful in high clinical suspicion COVID-19 patients. However, clinicians should also consider that the simplified PESI score may fail [37], and a multimodal approach improves risk stratification accuracy. (PESI score definition is available in the e-Appendix).
Another remarkable finding shows VTE events despite thromboprophylaxis. Recent evidence indicates that thrombotic events occur primarily within the first ten days after admission [38]. In addition, Hardy et al. [39] observed an increase in thrombin generation associated with a decrease in overall fibrinolytic capacity during the first week of hospitalization, resulting in a strong procoagulant state. Thus, current evidence suggests administering heparin at standard doses in non-critically ill patients without risk factors for thrombosis or at a high dose for critically ill patients (intermediate or therapeutic dose) [40].
Additionally, high-dose thromboprophylaxis might be adjusted according to inflammation’s progression without increasing bleeding Risk in critically ill COVID-19 patients [38]. Randomized controlled trials comparing different thromboprophylaxis doses are needed to establish the best therapeutic approach [38]. The most consistent biomarker abnormalities related to mortality were higher C-reactive protein and D-dimer measurement levels, both associated with ICU admission and death [15]. Additionally, several plausible reasons for elevated D-dimer in patients with SARS-CoV-2: severe infection, VTE, pulmonary and coronary microthrombus, acute kidney, cardiac injury, and pro-inflammatory cytokines [29].
Overlapping severe COVID-19 pneumonia and PE is a challenge, and any pneumonia increases VTE risk [34, 41, 42]. A higher D-dimer measurement and severe oxygen desaturation are possible clinical markers to establish high clinical suspicion and PE severity. Recently, in a case series, the clinical presentation was similar: persistent or worsening respiratory symptoms increased oxygen requirements and DD levels several-fold higher [43]. We suggest that physicians in charge consider these clinical variables and never ignore abnormal or significantly elevated D-dimer because it is an expression of the coagulation system and secondary fibrinolysis activity, suggesting a high risk of acute thrombosis [34]. Sudden hypotension could be another clinical element for PE suspicion in the setting of pneumonia COVID-19 [34]. In the group with CVST, only two patients had a history of oral contraceptives and no history of hereditary prothrombotic factors. These findings suggest an essential role of SARS-CoV-2 in pathogenicity as a trigger of thrombosis. Although early ICH (present at the time of diagnosis) is a frequent complication (40%) [44, 45], current evidence demonstrates a low incidence of new ICH after initiating treatment with anticoagulation [23, 44,45,46]. Our findings identified a high ICH incidence, probably secondary to CVST. Although anticoagulation is the standard of care in CVST patients (avoid thrombus growth, prevent VTE), the high prevalence of ICH suggests that physicians in charge have to be warning for early detection of this feared complication [45].
Study limitations
The significant limitations of the study included a potential loss of case reports from search engines. There is a trend not to report patients with poor in-hospital outcomes or serious adverse events. In addition, it was not possible to obtain information on the timing of the D-dimer measurements and other biomarkers and bleeding complications outcome in the follow-up. We got the most information from case reports, and we did not contact any author. Additionally, the results should be analyzed with caution as most papers are case reports or case series. Despite a large number of published studies in Covid VTE, the number of studies that report outcomes based on treatments is unacceptably small to draw new conclusions, given the different stages of the pandemic, Covid-19 treatments, and international differences. The usable studies had in common and why the other studies were rejected; could this be the basis of reporting standards for the pandemic to help a unified assessment. The impact of VTE on critically ill patients seems no different from other diseases - so is it just that we cannot cure the underlying disease, or is there something unique about COVID-19 thrombosis.
Conclusions
This systematic review analyzes 143 survivors and non-survivors VTE COVI-19 patients. We determined a high mortality incidence of pulmonary embolism (21.6%) and a low rate of bleeding. Unfractionated and low-molecular-weight heparin drove parenteral anticoagulation and alteplase the advanced treatment in both groups. The original PESI could be helpful in risk stratification. However, the minuscule number of evaluated patients cannot possibly be representative, and therefore, the international community should urgently agree on reporting standards to answer the remaining questions in Covid-19. Prospective clinical trials are mandatory to elucidate the optimal primary or secondary prevention and advanced treatment in this population of patients.
Availability of data and materials
Not applicable.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- VTE:
-
Venous thromboembolism
- PE:
-
Pulmonary embolism
- PESI:
-
Original Pulmonary Embolism Severity Index
- PROSPERO:
-
The International Prospective Register protocol of Systematic Reviews
- PRISMA:
-
The Preferred Reporting Items for Systematic Reviews and Metanalyses
- DVT:
-
Deep vein thrombosis
- PESI:
-
Simplified Pulmonary Embolism Severity Index
- BNP:
-
B-type brain natriuretic peptide
- NT-proBNP:
-
N-terminal proBNP
- CVT:
-
Cerebral vein thrombosis
- ICU:
-
Intensive care unit
- ICH:
-
Intracranial hemorrhage
- CTPA:
-
Computed tomography pulmonary angiography
References
McFadyen JD, Stevens H, Peter K. The emerging threat of (Micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127(4):571–87.
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091.
Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67.
Vazquez-Garza E, Jerjes-Sanchez C, Navarrete A, Joya-Harrison J, Rodriguez D. Venous thromboembolism: thrombosis, inflammation, and immunothrombosis for clinicians. J Thromb Thrombolysis. 2017;44(3):377–85.
Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 pneumonia from Acute Respiratory Distress Syndrome (ARDS) and High Altitude Pulmonary Edema (HAPE): therapeutic implications. Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.047915.
Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. Pathology. 2020. [citado el 14 de abril de 2020]. Disponible en: https://doi.org/medrxiv.org/lookup/doi/10.1101/2020.04.06.20050575.
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–9.
Poor HD, Ventetuolo CE, Tolbert T, Chun G, Serrao G, Zeidman A, et al. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Respir Med. 2020. [citado el 2 de mayo de 2020]. Disponible en: https://doi.org/medrxiv.org/lookup/doi/10.1101/2020.04.17.20057125.
Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5(5):518–536.
Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020:CIRCULATIONAHA.120.047430.
Liao S-C, Shao S-C, Chen Y-T, Chen Y-C, Hung M-J. Incidence and mortality of pulmonary embolism in COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24(1):464.
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020. [citado el 14 de abril de 2020]. Disponible en: https://doi.org/doi.wiley.com/10.1111/jth.14817.
Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020. [citado el 14 de abril de 2020]. Disponible en: http://doi.wiley.com/https://doi.org/10.1111/jth.14821.
Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020:CIRCULATIONAHA.120.047549.
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020:S0735109720350087.
Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue Plasminogen Activator (tPA) treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): a case series. J Thromb Haemost. 2020. [citado el 14 de abril de 2020]. Disponible en: https://doi.org/doi.wiley.com/10.1111/jth.14828.
Oudkerk M, Büller HR, Kuijpers D, van Es N, Oudkerk SF, McLoud TC, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the national institute for public health of the Netherlands. Radiology. 2020:201629.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Vinson DR, Ballard DW, Mark DG, Huang J, Reed ME, Rauchwerger AS, et al. Risk stratifying emergency department patients with acute pulmonary embolism: does the simplified Pulmonary Embolism Severity Index perform as well as the original? Thromb Res. 2016;148:1–8.
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331(7524):1064–5.
Schulman S, Kearon C, the SUBCOMMITTEE ON CONTROL OF ANTICOAGULATION OF THE SCIENTIFIC AND STANDARDIZATION COMMITTEE OF THE INTERNATIONAL SOCIETY ON THROMBOSIS AND HAEMOSTASIS. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients: definitions of major bleeding in clinical studies. J Thromb Haemost. 2005;3(4):692–4.
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019:ehz405.
de Bruijn SFTM, Stam J, Randomized. Placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484–8.
Lu Y, Pan L, Zhang W-W, Cheng F, Hu S-S, Zhang X, et al. A meta-analysis of the incidence of venous thromboembolic events and impact of anticoagulation on mortality in patients with COVID-19. Int J Infect Dis. 2020;100:34–41.
Porfidia A, Valeriani E, Pola R, Porreca E, Rutjes AWS, Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. 2020;196:67–74.
Zhang C, Shen L, Le K-J, Pan M-M, Kong L-C, Gu Z-C, et al. Incidence of venous thromboembolism in hospitalized coronavirus disease 2019 patients: a systematic review and meta-analysis. Front Cardiovasc Med. 2020;7:151.
Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, et al. Incidence of venous thromboembolism and bleeding among hospitalized patients with COVID-19: a systematic review and meta-analysis. Chest. 2020:S0012369220351461.
Chi G, Lee JJ, Jamil A, Gunnam V, Najafi H, Memar Montazerin S, et al. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J Clin Med. 2020;9(8):2489.
Bansal A, Singh AD, Jain V, Aggarwal M, Gupta S, Padappayil RP, et al. The association of D-dimers with mortality, intensive care unit admission or acute respiratory distress syndrome in patients hospitalized with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Heart Lung. 2020:S0147956320303800.
Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–72.
Sharifi M, Larijani F, Wycliffe R, Loggins B, Schroeder B, Monteros DDL, et al. Low dose systemic thrombolysis and new oral anticoagulants in the treatment of large thrombi in the right heart. J Am Coll Cardiol. 2017;69(11):2079.
Sharifi M, Vajo Z, Freeman W, Bay C, Sharifi M, Schwartz F. Transforming and simplifying the treatment of pulmonary embolism: “safe dose” thrombolysis plus new oral anticoagulants. Lung. 2015;193(3):369–74.
Sharifi M, Bay C, Schwartz F, Skrocki L. Safe-dose thrombolysis plus rivaroxaban for moderate and severe pulmonary embolism: drip, drug, and discharge: thrombolysis plus rivaroxaban in PE. Clin Cardiol. 2014;37(2):78–82.
Betancourt-del Campo H, Jerjes-Sanchez C, Castillo-Perez M, López-de la Garza H, Paredes-Vázquez JG, Flores-Sayavedra YZ, et al. Systemic thrombolysis and anticoagulation improved biomarker measurements in massive-like pulmonary embolism and severe COVID-19 pneumonia: a case report. Brown RA, Bouzas-Mosquera A, Cankovic MZ, Rampat R, Sayers M, Thomson R, editores. Eur Heart J - Case Rep. 2020:ytaa448.
Piazza G. Advanced management of intermediate- and high-risk pulmonary embolism. J Am Coll Cardiol. 2020;76(18):2117–27.
Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29–30:100639.
Trevino AR, Perez L, Jerjes-Sanchez C, Rodriguez D, Panneflek J, Ortiz-Ledesma C, et al. Factor Xa inhibition and sPESI failure in intermediate-high-risk pulmonary embolism. Am J Emerg Med. 2018;36(10):1925.e3-1925.e4.
Tacquard C, Mansour A, Godon A, Godet J, Poissy J, Garrigue D, et al. Impact of high dose prophylactic anticoagulation in critically ill patients with COVID-19 pneumonia. Chest. 2021:S0012369221000477.
Hardy M, Michaux I, Lessire S, Douxfils J, Dogné J-M, Bareille M, et al. Prothrombotic disturbances of hemostasis of patients with severe COVID-19: a prospective longitudinal observational study. Thromb Res. 2021;197:20–3.
Susen S, Tacquard CA, Godon A, Mansour A, Garrigue D, Nguyen P, et al. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Crit Care. 2020;24(1):364.
The RIETE, Investigators, Frasson S, Gussoni G, Di Micco P, Barba R, Bertoletti L, et al. Infection as cause of immobility and occurrence of venous thromboembolism: analysis of 1635 medical cases from the RIETE registry. J Thromb Thrombolysis. 2016;41(3):404–12.
Zhang Y, Zhou Q, Zou Y, Song X, Xie S, Tan M, et al. Risk factors for pulmonary embolism in patients preliminarily diagnosed with community-acquired pneumonia: a prospective cohort study. J Thromb Thrombolysis. 2016;41(4):619–27.
Faggiano P, Bonelli A, Paris S, Milesi G, Bisegna S, Bernardi N, et al. Acute pulmonary embolism in COVID-19 disease: preliminary report on seven patients. Int J Cardiol. 2020;313:129–31.
Girot M, Ferro JM, Canhão P, Stam J, Bousser M-G, Barinagarrementeria F, et al. Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage. Stroke. 2007;38(2):337–42.
Saposnik G, Barinagarrementeria F, Brown RD, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–92.
Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhão P, et al. Safety and efficacy of dabigatran etexilate vs dose-adjusted warfarin in patients with cerebral venous thrombosis: a randomized clinical trial. JAMA Neurol. 2019;76(12):1457.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
MCP: Substantial contributions to the conception, design of the work; research idea development; database; the acquisition, analysis, interpretation of data; have drafted the work and substantively revised it. CJS: Leaded the research team, research idea development, revising and approving the project design, and moderating group discussions. Also, he elaborated the project’s protocol and final tables and manuscripts. ACV: The data acquisition, have drafted the work and substantively revised it. He contributed to revising the database and the elaboration and revision of the tables and final manuscript. JGPV: The data acquisition database; have drafted the work and substantively revised it. EVG: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. RERC: Managed the systematic search alongside MCP, created the database, collected and interpreted data. JASC: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. AMMR: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. AAMI: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. MAF: The design of the work, data acquisition, and revision of the manuscript. YZFS: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. JAGL: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. HLG: data acquisition, analysis, and interpretation; Have drafted the work and substantively revised it. HBC: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. DMM: contributions to the design, data interpretation, interpretation of initial data. Have drafted the work and substantively revised it. JP: Acquisition and analysis of the database and interpretation of initial data. Have drafted the work and substantively revised it. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Castillo-Perez, M., Jerjes-Sanchez, C., Castro-Varela, A. et al. Differences between surviving and non-surviving venous thromboembolism COVID-19 patients: a systematic review. Thrombosis J 19, 101 (2021). https://doi.org/10.1186/s12959-021-00346-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-021-00346-y