Abstract
Objective
Ovarian stimulation (OS) with high daily gonadotropin doses are commonly offered to patients attempting social/elective egg freezing. However, the optimal daily gonadotropin dose that would allow a higher oocyte yield in the successive IVF cycle attempt was not settled and should be determined.
Patients and methods
Data from all women admitted to our IVF unit for social/EEF, who underwent two consecutive IVF cycle attempts, with only those who used in the first attempt a starting daily gonadotropin dose of 300IU were analyzed. Patients characteristics and OS variables were used in an attempt to build a logistic model, helping in determining the daily gonadotropin dose that should be offered to patient during their second EEF attempt, aiming to further increase their oocyte yield.
Results
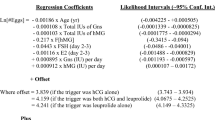
Three hundred and thirteen consecutive women undergoing two successive IVF cycle attempts were evaluated. Using logistic regression model, two equations were developed using individual patient-level data that determine the daily gonadotropin dose needed aiming to increase the oocyte yield in the successive cycle. (a): X=-0.514 + 2.87*A1 + 1.733*A2–0.194* (E2/1000) and (b): P = EXP(X) / [1 + EXP(X)].
Conclusions
Using the aforementioned equations succeeded in determining the daily gonadotropin dose that might result in increasing oocyte yield, with an AUC of 0.85. Any additional oocyte retrieved to these EEF patients might get them closer to fulfil their desire to parenthood.
Similar content being viewed by others
Introduction
Ovarian stimulation (OS) is considered a key factor in the success of in vitro fertilization-embryo transfer (IVF-ET), enabling the recruitment of multiple oocytes and, thereby, resulting in multiple embryos [1]. In 2019, the European Society for Human Reproduction and Embryology (ESHRE) special interest group (SIG) Reproductive Endocrinology [2] published has provided evidence-based information on the different options for OS for IVF/ICSI and concluded that a gonadotropin dose higher than 300 IU is not recommended for predicted poor responders.
What about normal, or high responder patients? Benadiva et al. [3] compared the hormonal profiles of ovulatory high and low responder patients who were treated with different daily doses (225 and 150 IU) of gonadotropins in successive IVF cycles. The magnitude of the ovarian response was found to be altered by changing the dose of gonadotropins in high responders, but not in normal responders. Moreover, while the increases in hormone levels accompanying a high response to gonadotropins could be dampened by lowering the dose, hormone concentrations were not influenced by changing the dose of gonadotropins in low responders.
Elective egg freezing (EEF) has spread globally following its endorsement by the American Society for Reproductive Medicine (ASRM) in 2018 [4]. In Israel, EEF for non-medical reasons is allowed for women age 30 to 41yrs. Moreover, while according to the Israel Health Law, almost every woman up to age 45 may legally seek government-subsidized infertility treatment with her own oocytes for as many cycles as necessary for the birth of two children, social/EEF for non-medical reason is not subsidized.
Ovarian stimulation (OS) with high daily gonadotropin doses, is therefore commonly offered to this group of patients, aiming to achieve the maximal oocytes cohort with minimum IVF cycle attempts. In a previous study of women undergoing EEF [5], the first OS stimulation attempt with high daily gonadotropin doses (300IU) yielded 8–9 oocytes, while the second attempt, using lower, same or increased daily gonadotropin dose, yielded an unpredictable increased, no change or decreased oocyte yield. In this study, it was therefore not possible to determine the optimal daily gonadotropin dose that would allow a higher oocyte yield in the successive IVF cycle attempt.
Prompted by the aforementioned observations, we aim to determine the daily gonadotropin dose in the second IVF cycle attempt, based on data retrieved from patients undergoing two successive attempts for EEF.
Patients and methods
We reviewed the computerized files of all consecutive women admitted to our IVF unit, between February 2018 and March 2023, and reached the ovum pick-up (OPU) stage. The elimination of bias in this selection was achieved by including only women undergoing OS, using a starting daily gonadotropin dose of 300IU in the first IVF cycle attempt. The study was approved by our institutional review board (SMC-9589-22).
All women underwent the multiple dose GnRH-antagonist protocol with GnRH-agonist for triggering final follicular maturation, as previously described [5]. Data on patients’ characteristics and OS variables were collected from the files.
Results were presented as means + standard deviations. Differences in variables between the two cycles were statistically analyzed by student’s paired t-test and differences between the two doses, in the second cycle, were analyzed by the independent t-test. Multivariate analysis to estimate dose selection was performed by logistic regression models. The analyses included independent variables/covariates that were statistically significant in the univariate analyses. Level of significance was set at 0.05, and was two-tailed. The final logistic model equation was used to the estimate dose prediction.
Results
Three hundred and thirteen consecutive women undergoing two successive IVF cycle attempts were evaluated. Women age and body mass index were 35.3 ± 3.5yrs and 23.3 ± 4.3 kg/m2, respectively. The IVF cycle characteristics are shown in Table 1. While there was no between- cycle difference in the duration of OS, peak E2 and progesterone levels and the number of follicles > 13 mm in diameter on the day of trigger, were significantly higher during the second IVF cycle attempt compared to the first attempt (Table 1). Moreover, during the second IVF cycle attempt women used a significantly higher daily gonadotropin dose (366 ± 76 vs. 300 ± 0; p < 0.001) and yielded more follicles ≥ 13 mm in diameter on day of trigger (9.7 ± 5.0 vs. 8.6 ± 4.1; p < 0.001), and more oocytes (12.37 ± 6.5 vs. 10.5 ± 5.9; p < 0.001) and mature oocytes (9.3 ± 5.3 vs. 7.8 ± 5.3; p < 0.001).
An equation was developed using the logistic regression model for an individual patient-level data. This equation calculated the daily gonadotropin dose based on previous results including the oocyte yield in the first cycle.
Step 1: Calculate X using the first equation (a)
X=-0.514 + 2.87*A1 + 1.733*A2–0.194* (E2/1000)
If the number of oocyte retrieved in the first cycle is ≤ 7: A1 = 1 and A2 = 0.
If the number of oocyte retrieved in the first cycle is between 8 and 12: A1 = 0 and A2 = 1.
And If the number of oocyte retrieved in the first cycle is ≥ 13, A1 = 0 and A2 = 0.
E2 is the estradiol level (pmol/L).
Step 2: After calculating the X value, it should be placed in the following logistic model (b)
P = EXP(X) / [1 + EXP(X)].
If P > 0.5 then the suggested daily gonadotropin dose in the successive cycle should be 450IU, while if P < 0.5 it should be 300IU.
The correlation between the predicted and actual values was found to be good (AUC = 0.85).
For example, patient yielding 10 oocytes in the first cycle, using a daily gonadotropin dose of 300IU, with peak E2 of 8000 pmol/L.
Using equation (a):
X=-0.514 + 2.87*0 + 1.733*1–0.194* (8000/1000)= -0.33
Now, placing the X value in (b):
P = EXP(-0.333) / [1 + EXP(-0.333)] = 0.4175
Since P is < 0.5, the suggested daily gonadotropin dose should be 450IU.
Calculator: To simplify the calculation of the suggested daily FSH dose in the second IVF cycle attempt, a calculator is attached, where we have to place the # of oocytes retrieved and the peak E2 level in the first IVF cycle attempt, in the red and blue square, respectively.
Discussion
In the present study, by analyzing the data of women undergoing two successive IVF cycle attempts for EEF, we could build a logistic model, helping in determining the daily gonadotropin dose that should be offered to patient during their second EEF attempt, aiming to further increase their oocyte yield. Since any improvement in patients’ response to OS, such as from low (1–3 oocytes) to suboptimal response (4–9 oocytes) was demonstrated to improve cumulative LBR [6], any additional oocyte retrieved to patients undergoing EEF might get them closer to parenthood.
As was previously demonstrated by Drakopoulos et al. [7], a dose increment of FSH remained the only significant predictor of the number of oocytes retrieved in the subsequent IVF cycle, with an increase of 50 IU of the initial rFSH dose leading to one more oocyte. Moreover, increasing the daily gonadotropin dose above 300IU was also shown to result in higher mature oocytes yield [5] in EEF patients. However, when analyzing the data according to the number of oocytes retrieved in the second IVF cycle attempt as compared to the first attempt [5], an increase, no change or reduced oocyte yield in the second cycle attempt was observed regardless the daily gonadotropin dose in the second cycle attempt (whether it was increased, no change and decreased compared to the first attempt).
In the present study, we managed to create equations, based on the OS variable in the first EEF cycle attempt, which helped determining the required daily gonadotropin dose that should be offered to EEF patients in their second IVF cycle attempt, in an attempt to increased their oocyte yield, with AUC of 0.85.
A major strength of our study is that we used and compared the IVF outcome in same cohort of “healthy” patients, undergoing two successive IVF cycle attempts for elective egg freezing (EEF). The fact that all women that participated in our study had two consecutive treatment cycles helps to eliminate multiple bias factors and to attribute the study results daily gonadotropin dose. On the other hand, biases might still exist either because of differences in time periods between the two cycles, statistical return to the mean, or other potential biases inherent in a retrospective analysis of the IVF cycles.
To conclude, patients attempting social/elective egg freezing has spread globally. While OS with high daily gonadotropin doses (300IU) should be offered to this group of patients in their first IVF cycle attempt, the daily gonadotropin dose in their second attempt aiming to achieve the maximal oocytes cohort with minimum IVF cycle attempts need to be determine. In the present study we offer a logistic model aiming to resolve this query. Further well designed prospective studies are needed to improve and validate our observation.
Data availability
No datasets were generated or analysed during the current study.
References
Penzias AS. Improving results with assisted reproductive technologies: individualized patient-tailored strategies for ovulation induction. Reprod Biomed Online. 2004;9:43–6.
Ovarian Stimulation TEGGO, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, Kunicki M, La Marca A, Lainas G, Le Clef N, Massin N, Mastenbroek S, Polyzos N, Sunkara SK, Timeva T, Töyli M, Urbancsek J, Vermeulen N. Broekmans F.ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open. 2020;2020(2):hoaa009.
Benadiva CA, Ben-Rafael Z, Strauss JF, Mastroianni L, Flickinger GL. Ovarian response of individuals to different doses of human menopausal gonadotropin. Fertil Steril. 1988;49:997–1001.
Ethics Committee of the American Society for Reproductive Medicine. Planned oocyte cryopreservation for women seeking to preserve future reproductive potential: an Ethics Committee opinion. Fertil Steril. 2018;110:1022–8.
Orvieto R, Aizer A, Saar–Ryss B, Marom–Haham L, Noach–Hirsh M, Haas J, Nahum R. Elective egg freezing patients may benefit from increasing the maximal daily gonadotropin dose above 300IU. Reprod Biol Endocrinol. 2022;20:171.
Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, Polyzos NP. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;31(2):370–6.
Drakopoulos P, Santos-Ribeiro S, Bosch E, Garcia-Velasco J, Blockeel C, Romito A, Tournaye H, Polyzos NP. The effect of dose adjustments in a subsequent cycle of women with suboptimal response following conventional ovarian stimulation. Front Endocrinol. 2018;9:361.
Acknowledgements
N/A.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
R.O. Designed the study, analyzed the data, wrote the first paper draft, edited it, proof read the paper and took part in discussions regarding the results. A.S.K. Contributed to the data collection, analyzed the data, proof read the paper and took part in discussions regarding the results. N.M. Analyzed the data, proof read the paper and took part in discussions regarding the results. A.S.Z. Proof read the paper and took part in discussions regarding the results. R.N. Contributed to the data collection, proof read the paper and took part in discussions regarding the results.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by our institutional (Sheba Medical Center) review board (SMC-9589-22), in accordance with the Declaration of Helsinki. Due to the retrospective design of the study, there was no need for participate declaration consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Orvieto, R., Kadmon, A.S., Morag, N. et al. Determining the optimal daily gonadotropin dose to maximize the oocyte yield in elective egg freezing cycles. Reprod Biol Endocrinol 22, 64 (2024). https://doi.org/10.1186/s12958-024-01236-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-024-01236-4