Abstract
Background
Women with adenomyosis are characterized by having defective decidualization, impaired endometrial receptivity and/or embryo-maternal communication, and implantation failure. However, the molecular mechanisms underlying adenomyosis-related infertility remain unknown, mainly because of the restricted accessibility and the difficult preservation of endometrial tissue in vitro. We have recently shown that adenomyosis patient-derived endometrial organoids, maintain disease-specific features while differentiated into mid-secretory and gestational endometrial phase, overcoming these research barriers and providing a robust platform to study adenomyosis pathogenesis and the associated molecular dysregulation related to implantation and pregnancy disorders. For this reason, we aim to characterize the dysregulated mechanisms in the mid-secretory and gestational endometrium of patients with adenomyosis by RNA-sequencing.
Methods
Endometrial organoids were derived from endometrial biopsies collected in the proliferative phase of women with adenomyosis (ADENO) or healthy oocyte donors (CONTROL) (n = 15/group) and differentiated into mid-secretory (-SECorg) and gestational (-GESTorg) phases in vitro. Following RNA-sequencing, the significantly differentially expressed genes (DEGs) (FDR < 0.05) were identified and selected for subsequent functional enrichment analysis and QIAGEN Ingenuity Pathway Analysis (IPA). Statistical differences in gene expression were evaluated with the Student’s t-test or Wilcoxon test.
Results
We identified 1,430 DEGs in ADENO-SECorg and 1,999 DEGs in ADENO-GESTorg. In ADENO-SECorg, upregulated genes included OLFM1, FXYD5, and RUNX2, which are involved in impaired endometrial receptivity and implantation failure, while downregulated genes included RRM2, SOSTDC1, and CHAC2 implicated in recurrent implantation failure. In ADENO-GESTorg, upregulated CXCL14 and CYP24A1 and downregulated PGR were related to pregnancy loss. IPA predicted a significant inhibition of ID1 signaling, histamine degradation, and activation of HMGB1 and Senescence pathways, which are related to implantation failure. Alternatively, IPA predicted an inhibition of D-myo-inositol biosynthesis and VEGF signaling, and upregulation of Rho pathway, which are related to pregnancy loss and preeclampsia.
Conclusions
Identifying dysregulated molecular mechanisms in mid-secretory and gestational endometrium of adenomyosis women contributes to the understanding of adenomyosis-related implantation failure and/or pregnancy disorders revealing potential therapeutic targets. Following experimental validation of our transcriptomic and in silico findings, our differentiated adenomyosis patient-derived organoids have the potential to provide a reliable platform for drug discovery, development, and personalized drug screening for affected patients.
Similar content being viewed by others
Introduction
Adenomyosis is a benign uterine disease, defined as an infiltration of the endometrial glands and stroma into the myometrium [1]. It affects approximately 35% of reproductive-aged women [2], although the prevalence can vary depending on the study population, diagnostic methods, and geographic location [3]. Women with adenomyosis present abnormal uterine bleeding, chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility [4], driving them to seek assisted reproductive technologies [5]. However, in vitro fertilization efficacy for these patients remains highly controversial, with some studies reporting lower implantation rates but no effect on miscarriage rates [6, 7], and others describing frequent miscarriages without any adverse effects on implantation or pregnancy rates [8, 9]. Nevertheless, meta-analyses concluded that women with adenomyosis had higher miscarriage rates, lower implantation, pregnancy, and live birth rates compared to healthy patients [10,11,12,13], suggesting adenomyosis may impair embryo implantation and early pregnancy [13]. In this regard, understanding the underlying molecular mechanisms involved in adenomyosis pathogenesis is essential for managing adenomyosis-related infertility.
Defective decidualization [14], impaired endometrial receptivity [15], and/or embryo-maternal communication [16], and implantation failure [17] have been described in women with adenomyosis. However, the molecular mechanisms underlying these infertility-related alterations in adenomyosis women remain unknown, mainly due to the limited availability and difficult maintenance of the eutopic and ectopic endometrial tissues in vitro. As embryo implantation occurs in the endometrial mid-secretory phase [18], and events related to the embryo-maternal communication and early pregnancy stages happen in the endometrial gestational phase [19], deciphering the transcriptome of these endometrial phases in women with adenomyosis will represent a step forward in understanding the dysregulation that contributes to adenomyosis-associated infertility.
Organoids have emerged as a three dimensional (3D) in vitro platform capable of reproducing the phenotypes of native tissues remaining genetically stable in long-term culture [20]. Endometrial organoids have been developed from healthy and diseased endometrium, mimicking endometriosis [21], endometrial cancer [21], and adenomyosis [22], among other conditions. Notably, patient-derived adenomyosis endometrial organoids differentiated into mid-secretory and gestational phase phenotypes maintain disease-specific traits, overcoming the aforementioned research barriers and providing a reliable model to study adenomyosis pathogenesis and associated molecular dysregulation related to implantation and pregnancy disorders. In this regard, our adenomyosis organoids model allowed us to describe microRNAs contained in extracellular vesicles (EVs) secreted by these adenomyosis secretory and gestational organoids, involved in impaired embryo implantation and pregnancy disorders related with this disease [23]. However, there is not any study describing molecular mechanisms deregulated in eutopic endometrium in secretory and gestational phase from women with adenomyosis. Therefore, the aim of our study was to analyze the transcriptome of adenomyosis-derived endometrial organoids in the mid-secretory and gestational phases, to characterize the molecular mechanisms involved in adenomyosis-related infertility.
Materials and methods
Study design
Endometrial organoids were derived from the eutopic endometrium of women with (n = 15) or without adenomyosis (control; n = 15) and further differentiated into mid-secretory and gestational endometrial phases by supplementation with ovarian and pregnancy hormones [22], respectively. RNA was extracted from mid-secretory and gestational adenomyosis and control endometrial organoids for RNA-sequencing (RNA-seq) (Supplemental Fig. 1).
Patients and endometrial biopsies
Endometrial biopsies were obtained from patients (18 ≤ 45 years old; BMI ≤ 28 kg/m2) with and without adenomyosis, at the IVI Valencia Clinic (Table 1). Patients with any other suspected or diagnosed uterine pathologies were excluded. Control women were healthy egg donors with standard uterine volume, no evidence of adenomyotic lesions, and free of other gynecological pathologies and medication during previous three months.
Diagnosis of adenomyosis
All patients were examined by transvaginal ultrasound. Adenomyosis was diagnosed in patients presenting a heterogeneous myometrium and a diffused endometrial border. Diffuse adenomyosis was diagnosed with a globally enlarged asymmetric uterus, hypoechoic striae, and areas with small cysts in the intramyometrial region, while focal adenomyosis was diagnosed by isolated intramyometrial clusters surrounded by areas of normal myometrium and altered vascularity [24, 25]. In all cases adenomyosis was confirmed by hysteroscopic evaluation of the endometrial cavity.
Establishment and differentiation of adenomyosis endometrial organoids
The adenomyosis and control endometrial organoids were derived from eutopic endometrium and differentiated into the mid-secretory and gestational phases modelling native endometrial tissue and disease-specific traits, which showed in vivo glandular epithelial phenotype (pan-cytokeratin, Mucin-1 [Muc-1], Periodic acid Schiff [PAS] staining, Laminin, and Ki67; assessed by immunostaining) and secretory and gestational features (α-tubulin, SRY-Box Transcription Factor 9 [SOX9], Secreted Phosphoprotein 1 [SPP1], Progestagen Associated Endometrial Protein [PAEP], LIF Interleukin 6 Family Cytokine [LIF], and Hydroxysteroid 17-Beta Dehydrogenase 2 [17βHSD2] expression and SPP1 secretion, assessed by immunostaining and quantitative real-time PCR (qRT-PCR)), as we previously described [22]. Immunohistochemistry of adenomyosis organoids showed higher expression of Transforming Growth Factor Beta 2 [TGFβ-2] and SMAD Family Member 3 [SMAD3] and increased gene expression of SPP1, PAEP, LIF, and 17βHSD2 by means of qRT-PCR [22]. Briefly, for mid-secretory phase differentiation, adenomyosis (ADENO-SECorg) and control organoids (CONTROL-SECorg) were treated with 10 nM estradiol (E2; Sigma-Aldrich, St. Louis, MO, USA, E4389), 1 µM progesterone (P4; Sigma-Aldrich, P7556) and 1 µM 8-bromoadenosine 3′,5′-cyclic monophosphate sodium salt (cAMP; Sigma-Aldrich, B7880). For gestational phase differentiation, adenomyosis (ADENO-GESTorg) and control organoids (CONTROL-GESTorg) were treated with 10 nM E2, 1 µM P4, 1 µM cAMP, with an additional 20 ng/mL prolactin (PRL; Peprotech, Cranbury, NJ, USA, 100-07) and 20 ng/mL human placental lactogen (hPL; R&D, Minneapolis, MN, USA, 5757-PL).
Library construction and RNA-sequencing
Total RNA was extracted from the ADENO-SECorg, CONTROL-SECorg, ADENO-GESTorg and CONTROL-GESTorg groups (n = 15/group) using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA, 74,104) according to the manufacturer’s protocol, and quantified with a Qubit 3 Fluorometer (Invitrogen, Waltham, MA, USA).
Next, cDNA libraries were generated employing the TruSeq Stranded mRNA Library Prep (Illumina, San Diego, CA, USA, 20,020,595) and TruSeq RNA CD Index Plate (Illumina, 20,019,792) according to manufacturer’s instructions. The quality and concentration of the libraries was assessed with the Agilent Technologies 2100 (Agilent Technologies, Santa Clara, CA, USA, G2939BA). Paired-end sequencing (2 × 75 bp) was performed on Illumina’s NextSeq 550 NGS platform.
Pre-processing, quality control and normalization
RNA-seq data libraries were processed within R computing environment (v 4.1.1). Library quality was analyzed with FastQC software [26]. Low-quality sequences (e.g., from one CONTROL-SECorg and two CONTROL-GESTorg samples) were removed with bbduk software [27]. Sequencing samples yielded an average of 14.1 million reads per sample. RNA-seq reads were aligned with the GRCh38 version of the human genome using subread software [28]. Read counts were normalized using the geometric median ratio method for each mRNA, using the DESeq2 R package. All raw sequencing data are available through the Gene Expression Omnibus (GEO) under accession number GSE244236.
Differentially expressed genes and functional enrichment analysis
Differential expression analysis (DEA) was carried out with the DESeq2 package to identify the differentially expressed genes (DEGs) between: (i) ADENO-SECorg versus CONTROL-SECorg; and (ii) ADENO-GESTorg versus CONTROL-GESTorg. Differentially expressed genes (DEGs) were considered significant when the P-value adjusted by false discovery rate (FDR) < 0.05. Gene ontology functional enrichment analysis and KEGG pathway analysis were performed by gene set enrichment analysis (GSEA) implemented in clusterProfiler [29]. Finally, the QIAGEN Ingenuity Pathway Analysis (IPA) was used to analyze the dysregulated pathways in both comparisons.
Validation
To corroborate RNA-seq data, we selected DEGs implicated in dysregulated pathways described in ADENO-SECorg and evaluated their gene expression by qRT-PCR using Power-Up SYBR Green (Thermo Fisher Scientific, USA) on a StepOnePlus Real-Time PCR System (Applied Biosystems, USA). The selected genes included Aldehyde Dehydrogenase 1 Family Member A1 (ALDH1A1), Aldehyde Dehydrogenase 9 Family Member A1 (ALDH9A1), Monoamine Oxidase B (MAOB), Lysine Acetyltransferase 2B (KAT2B), Poly (ADP-Ribose) Polymerase 1 (PARP1), Forkhead Box O3 (FOXO3), Superoxide Dismutase 2 (SOD2) and Sequestosome 1 (SQSTM1). Relative gene expression levels were determined by the ∆∆Ct method and normalized to β-actin (ACTB) housekeeping gene expression. Fold change was calculated using the CONTROL-SECorg as the reference group.
Statistical analysis
All statistical analyses of omics data were carried out in R (v 4.1.1). Graphics were generated using the R core package, gplots, ggplot2, or GraphPad Prism 8.0. Statistical differences in gene expression were evaluated with the Student’s t-test or Wilcoxon test in GraphPad Prism 8.0. In all cases, P < 0.05 was considered statistically significant.
Results
Global transcriptomic behaviour of adenomyosis patient-derived organoids
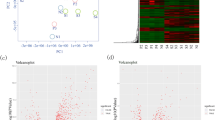
Principal Component Analysis (PCA) revealed distinct transcriptomic behaviour between the ADENO-SECorg and CONTRO-SECorg samples (Fig. 1A) and between the ADENO-GESTorg and CONTROL-GESTorg samples (Fig. 1B). In corroboration, the hierarchically-clustered heatmaps of the significant mRNAs (FDR < 0.05) showed different expression patterns between ADENO-SECorg and CONTROL-SECorg (Fig. 1C) and between ADENO-GESTorg and CONTROL-GESTorg (Fig. 1D).
Global transcriptomic behavior of secretory and gestational endometrial organoids from patients with adenomyosis compared to healthy oocyte donors. Principal component analyses (A-B), heatmaps representing the fold-enrichment score of genes after unsupervised clustering (C-D), and volcano plots of the significantly differentially expressed genes (adjusted p value < 0.05 and log2FC>|2|; E-F) between ADENO-SECorg (red; n = 15) and CONTROL-SECorg (blue; n = 14), or ADENO-GESTorg (red; n = 15) and CONTROL-GESTorg (blue; n = 13)
Differential gene expression of mid-secretory phase adenomyosis endometrial organoids
DEA identified 1,430 DEGs (500 up- and 930 downregulated; FDR < 0.05) between the ADENO-SECorg and CONTRO-SECorg in the mid-secretory phase (Fig. 1E). Among the top 20 downregulated DEGs selected for subsequent analysis (Fig. 2A), we highlight ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 2 [CHAC2], Metallothionein 1M [MT1M], Sclerostin Domain Containing 1 [SOSTDC1], and Ribonucleotide Reductase Regulatory Subunit M2 [RRM2] (log2 Fold change [FC] = -2.20, -2.13, -2.07, and − 1.95, respectively) based on their possible implication in recurrent implantation failure (RIF). Alternatively, among the top 20 upregulated DEGs (Fig. 2B) we point out RUNX Family Transcription Factor 2 [RUNX2], Olfactomedin 1 [OLFM1], FXYD Domain Containing Ion Transport Regulator 5 [FXYD5], and MT-RNR2 Like 1 [MTRNR2L1] (log2FC = 1.84, 1.70, 1.70, and 1.44, respectively) due to their involvement in impaired endometrial receptivity and embryo implantation.
Top 20 significantly differentially expressed genes between adenomyosis and control patient-derived endometrial organoids in mid-secretory and gestational phases. (A) Downregulated and (B) upregulated genes in ADENO-SECorg compared to CONTROL-SECorg. (C) Downregulated and (D) upregulated genes in ADENO-GESTorg compared to CONTROL-GESTorg. Statistical significance of the presented genes was established with adjusted p value < 0.05
Differential gene expression of gestational phase adenomyosis endometrial organoids
DEA identified 1,999 DEGs (153 up- and 1,846 downregulated; FDR < 0.05 and) between the ADENO-GESTorg and CONTROL-GESTorg in the gestational phase (Fig. 1F). Among the top 20 downregulated DEGs (Fig. 2C), we highlight ZW10 Interacting Kinetochore Protein (ZWINT), Establishment Of Sister Chromatid Cohesion N-Acetyltransferase 2 (ESCO2), Minichromosome Maintenance Complex Component 6 (MCM6), progesterone receptor (PGR) and Minichromosome Maintenance Complex Component 4 (MCM4) (log2FC = -2.57, -2.53, -2.48, -2.21, and − 2.07, respectively) based on their possible associations with recurrent pregnancy loss (RPL) and preeclampsia. From the top 20 upregulated DEGs (Fig. 2D), we note Cytochrome P450 Family 24 Subfamily A Member 1 (CYP24A1), C-X-C Motif Chemokine Ligand 14 (CXCL14), Cyclin Dependent Kinase Inhibitor 2 A (CDKN2A), Chloride Voltage-Gated Channel Ka (CLCNKA) and Platelet Activating Factor Receptor (PTAFR) (log2FC = 2.38, 1.55, 1.02, 1.01, and 0.80, respectively) due to their implication in spontaneous miscarriage, trophoblast outgrowth and invasion inhibition, and gestational diabetes mellitus.
Functional implications of adenomyosis in the mid-secretory phase endometrium
GO enrichment analysis identified 176 dysregulated biological processes in ADENO-SECorg (Supplemental Table 1). These processes were assigned to different functional groups, such as oocyte and embryo development, DNA damage repair, response to oxygen levels and hormones, immune response, cell-cell adhesion, cell cycle and apoptosis, aligning with the described functions of the mid-secretory phase DEGs we emphasized herein (Fig. 3A). On the other hand, KEGG pathway analysis revealed nine dysregulated pathways related to the cell cycle, mismatch repair, homologous recombination, cellular senescence, estrogen and progesterone signaling, inflammation cascades and different types of viral infection, among others (Supplemental Table 1).
Functional enrichment analysis and canonical pathways predicted to be affected by Ingenuity Pathway Analysis (IPA). Functional implications of relevant significantly downregulated and upregulated genes in (A) ADENO-SECorg with respect to CONTROL-SECorg or (B) ADENO-GESTorg with respect to CONTROL-GESTorg. Differential expression of the genes is showed in a box under the gene in blue (downregulated) and red (upregulated) by means of log2FC scale. Downregulated and upregulated canonical pathways predicted by IPA and deemed relevant for adenomyosis pathogenesis and associated-infertility in (C) ADENO-SECorg and (D) ADENO-GESTorg. DEGs, differentially expressed genes
Functional implications of adenomyosis in the gestational phase endometrium
GO analysis revealed 356 dysregulated biological processes in ADENO-GESTorg (Supplemental Table 2). Among the corresponding functional groups, embryo development, vital processes, developmental maturation, recombination, response to oxygen levels, radiation, insulin and stimulus, signal transduction and immune response, all stood out for their possible involvement in pregnancy disorders and corroborated the previously published associations of the gestational phase DEGs we featured (Fig. 3B). Further, KEGG pathway analysis revealed 39 dysregulated pathways, related to homologous recombination, mismatch repair, apoptosis and p53 signaling, different types of cancer, viral infection, diabetic complications and inflammation signaling cascades, among others (Supplemental Table 2).
Adenomyosis-related dysregulated pathways in the mid-secretory phase endometrium
QIAGEN IPA predicted 36 downregulated and 21 upregulated canonical pathways in the mid-secretory endometrium of women with adenomyosis, compared to controls (Supplemental Table 3). Among the relevant downregulated pathways in the mid-secretory endometrium, we distinguished the degradation of noradrenaline and adrenaline (z-score=-2.4), dopamine (z-score=-2.4), and histamine (z-score=-2.0), along with the inhibitor of DNA binding 1 (ID1) signaling pathway (z-score=-1.8), ribonucleotide reductase signaling pathway (z-score=-1.4), inhibition of angiogenesis by thrombospondin 1 (TSP1; z-score=-1.3), ATM signaling (z-score=-1.1), and sirtuin signaling pathway (z-score=-0.60), (Fig. 3C). Alternatively, among the upregulated pathways, we emphasize acute phase response signaling (z-score = 2.7), high mobility group box 1 (HMGB1) signaling (z-score = 0.8), sumoylation (z-score = 0.7) and senescence pathways (z-score = 0.5) (Fig. 3C).
Adenomyosis-related dysregulated pathways in the gestational phase endometrium
QIAGEN IPA analysis predicted 141 downregulated and 14 upregulated canonical pathways in the gestational phase endometrium of women with adenomyosis, compared to controls (Supplemental Table 4). Among the ones relevant for adenomyosis pathogenesis and infertility (Fig. 3D), D-myo-inositol-5-phosphate metabolism (z-score=-4.6), and signaling pathways for microRNA biogenesis (z-score=-4.5), the actin cytoskeleton (z-score=-4.2), extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) (z-score=-4.0) and ribonucleotide reductase (z-score=-2.7), together with vascular endothelial growth factor (VEGF) family ligand-receptor interactions (z-score=-1.9), were predicted as downregulated. Meanwhile, the predicted upregulated pathways included those for Rho GDP dissociation inhibitor (RHOGDI; z-score = 3.6), phosphatase and tensin homolog (PTEN; z-score = 3.4), p53 (z-score = 1.1) and peroxisome proliferator activated receptor alpha (PPARα)/retinoid X receptor alpha (RXRα) activation (z-score = 0.5).
Validation of differential gene expression in adenomyosis organoids
To validate RNA-sequencing results, eight DEGs were selected among the genes involved in the dysregulated pathways Histamine degradation, Dopamine degradation, Noradrenaline and Adrenaline degradation and Senescence in ADENO-SECorg. qRT-PCR results corroborated the differential gene expression pattern observed in ADENO-SECorg by RNA-seq compared to CONTROL-SECorg (Fig. 4). Specifically, ALDH1A1 (fold change = 0.235; p = 0.023), ALDH9A1 (fold change = 0.675; p = 0.035), MAOB (fold change = 0.136; p = 0.023), KAT2B (fold change = 0.624; p = 0.012), PARP1 (fold change = 0.746; p = 0.009), FOXO3 (fold change = 1.820; p = 0.011), SOD2 (fold change = 3.130; p = 0.003), SQSTM1 (fold change = 1.912; p = 0.034).
Discussion
Women with adenomyosis are characterized by impaired implantation and a higher number of miscarriages [10,11,12,13], thus, being able to study the dysregulated pathways and their putative causes in the endometrium, when these events occur, is crucial to improve fertility care for affected patients. Conventionally, the study of the endometrium in mid-secretory (implantation [30]) and gestational (early pregnancy [31]) phases was restricted by the difficulty of accessing and culturing the endometrium in these phases. However, the recent generation and differentiation of endometrial organoids overcomes these research barriers [20] and facilitates the study of specific endometrial disorders [21]. Going one step further, in this study, we performed next-generation sequencing of adenomyosis patient-derived organoids to identify the dysregulated genes and pathways in the eutopic secretory and gestational phase endometrium that may be responsible for the implantation failure and miscarriages experienced by affected women.
In ADENO-SECorg, we found CHAC2, MT1M, SOSTDC1 and RRM2 as significantly downregulated DEGs. CHAC2 has a pivotal role in the neutralization of reactive oxygen species, being necessary for the maintenance of human embryonic stem cell self-renewal [32]. MT1M is critical for regulating oxidative stress, inflammation and hormone signaling in term and preterm labor [33]. SOSTDC1, was found expressed in the uterine glandular epithelial cells of the receptive rat endometrium, and thus, may be involved in the onset of endometrial receptivity [34], while RRM2 expression was downregulated in the RIF endometrium, compared to fertile controls [35]. Based on this evidence, our findings suggest that the downregulation of these DEGs in the mid-secretory endometrium of women with adenomyosis advance the knowledge of adenomyosis and contributes to the endometrial dysfunction that impedes embryo implantation. Our findings indicate that adenomyosis-related infertility is also a product of the significant upregulation of certain DEGs, such as RUNX2, increased in the endometrium of infertile women with endometriosis [36]. OLFM1, related with a non-receptive endometrium and negatively regulates embryo attachment [37]; FXYD5, which drives the epithelial-to-mesenchymal transition [38] and promoted chronic inflammatory responses [39]; and MTRNR2L1, which was enhanced under hypoxic conditions in women with complicated pregnancies [40]. Based on the transcriptomic findings of the adenomyotic mid-secretory phase endometrium, IPA predicted the dysregulation of several pathways, that corresponded with those previously associated with poor reproductive outcomes. Particularly, downregulated histamine degradation was associated with pregnancy complications, such as diabetes, miscarriage, and trophoblastic disorders [41]; Sirtuin deficiency impaired embryo invasion and decidualization [42]; ATM-deficient dams had lower implantation rates [43]; and excessive noradrenaline inhibited decidualization, embryo, and fetal development in mice [44]. Further, impaired decidualization may be caused by aberrant stromal cell differentiation, mediated by downregulated ID1 expression, [45] stromal cell apoptosis, induced by the N-acyl dopamine family [46], or attenuation of ribonucleotide reductase signaling, which impeded decidualization and implantation in mice [35]. Finally, significant repression of TSP1 mRNA expression was linked to unexplained recurrent spontaneous abortion (URSA) [47]. On the other hand, IPA predicted mid-secretory phase adenomyosis etiologies may also include the upregulation of senescence pathway, as was observed in the peri-implantation endometrium and RPL [48]; hypersumoylation, since hyposumoylation was associated with a proper decidualization [49]; the premature activation of acute phase response signaling, which may interrupt early pregnancy [50]; and overactive HMGB1 signaling, which was related to the reduced adhesion ability of epithelial cells in patients with RIF [51] and at the maternal-fetal interface of URSA patients [52], as it was also previously described as deregulated in the endometrial tissue of adenomyosis women [53]. Findings from this study were corroborated by the validation in endometrial organoids of expression levels of DEG involved in these pathways, supporting that the dysregulated pathways in the mid-secretory endometrium of women with adenomyosis contribute to the disruption of endometrial receptivity and/or defective decidualization, resulting in these women failing to achieve implantation, and ultimately, pregnancy.
In ADENO-GESTorg, we focused on the downregulation of ZWINT, ESCO2, MCM4/6, and PGR because of their roles in pregnancy-related processes. The knockdown of ZWINT1 was related to a high incidence of aneuploidy, leading to miscarriage, infertility, and newborn disorders [54]. Interestingly, elevated aneuploidy rates were also observed in ESCO2-mutant embryos [55]. Correct DNA replication requires the proper functioning of MCM family members, including MCM6 [56]. Indeed, MCM4 dysregulation causes genomic instability, and increases lethality of murine embryos [57]. Alternatively, dysregulated PGR expression was related to severe preeclampsia [58] and predisposition to RPL [59]. Among the upregulated genes, elevated CYP24A1 was observed in spontaneous miscarriage [60] and preeclamptic placentas [61]; CXCL14 is implicated in insulin [62] and inhibited trophoblast attachment and outgrowth, disrupting the establishment of pregnancy [63]; and CDKN2A and CLCNKA were respectively associated with gestational diabetes [64] and IGF-1 deficiency [65], while PTAFR induced preterm delivery in mice [66]. Taken together, the contributions of these dysregulated genes showcase the complexity of adenomyosis pathogenesis.
Based on the findings presented herein, we emphasize several putative causes for the pregnancy disorders in patients with adenomyosis. Particularly, the downregulated D-myo-inositol-5-phosphate metabolism may decrease oocyte and embryo quality [67]; the reduced VEGF family ligand-receptor interactions may restrict the trophoblasts’ hypoxia adaptation [68]; limited actin cytoskeleton signaling may impede the polymerization essential for trophoblast invasion and tube formation during placental development [69]; attenuated microRNA biogenesis (mediated by DICER and DROSHA ribonucleases) during the endometrial receptivity phase may lead to implantation failure [70]; and repressed ERK/MAPK signaling may directly lead to embryonic lethality, as observed with the placental malformations due to the loss of Map2k1 function in mice [71]. Given the reproductive impact of the biological processes involving these pathways, their downregulation is proposed as a potential contributor to the many miscarriages suffered by women with adenomyosis. Interestingly, several pathways predicted to be affected by gestational phase adenomyosis have been related to preeclampsia, including upregulated PPARα/RXRα activation, which negatively regulated trophoblast invasion and led to recurrent miscarriage [72]; excessive p53 signaling [73]; along with enhanced RHOGDI and PTEN, which also inhibited trophoblast invasion [74, 75].
To our knowledge, this is the first transcriptomic study of adenomyosis patient-derived endometrial organoids differentiated into mid-secretory and gestational phase. Although these in vitro models faithfully recapitulated the native microenvironment in which the events related to implantation and early pregnancy respectively occur, additional in vivo studies are required to validate the DEGs and predicted pathways we identified as altered in the eutopic endometrium of women with adenomyosis. Moreover, endometrial organoids only contain epithelial cells and the complexity of interactions present in the native tissues may not be fully reflected in this model. Therefore, further studies including stromal or immune system cells would be necessary to validate and to translate our findings to the clinical practice. Nevertheless, it is important to highlight the importance of endometrial epithelial cells in the implantation and pregnancy processes because they are the first maternal contact for an implanting embryo and thereby, our organoid model could define new biomarkers of adenomyosis pathogenesis and related infertility.
Conclusions
Dysregulated molecular mechanisms involved in defective decidualization, disrupted endometrial receptivity and impaired embryo implantation were identified in the mid-secretory phase endometrium of women with adenomyosis, whereas dysregulated molecular mechanisms associated with inhibition of trophoblast outgrowth and invasion, impaired embryo development, pregnancy loss, preeclampsia and placental defects were observed in gestational phase endometrium of women with adenomyosis. These findings represent potential therapeutic targets that can be exploited to develop pharmacological treatments, and ultimately, reduce the risk of adenomyosis-related infertility.
Our differentiated patient-derived adenomyosis organoids, together with the transcriptomic findings presented herein, can be used to develop and test targeted pre-conception therapies in vitro/ex vivo. Further, these pathological endometrial organoids can be used as personalized drug screening tools, to predict patient-specific drug efficacy in vitro prior to clinical administration.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. All raw sequencing data are available through the GEO under accession number GSE244236.
Abbreviations
- GESTorg:
-
Gestational phase derived organoids
- SECorg:
-
Mid-secretory phase derived organoids
- 17βHSD2 :
-
Hydroxysteroid 17-Beta Dehydrogenase 2
- ACTB:
-
β-actin
- ADENO:
-
Adenomyosis
- ALDH1A1:
-
Aldehyde Dehydrogenase 1 Family Member A1
- ALDH9A1:
-
Aldehyde Dehydrogenase 9 Family Member A1
- cAMP:
-
1 µM 8-bromoadenosine 3′,5′-cyclic monophosphate sodium salt
- CDKN2A:
-
Cyclin Dependent Kinase Inhibitor 2 A
- CHAC2 :
-
ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 2
- CLCNKA :
-
Chloride Voltage-Gated Channel Ka
- CONTROL:
-
Healthy oocyte donors
- CYP24A1 :
-
Cytochrome P450 Family 24 Subfamily A Member 1
- CXCL14 :
-
C-X-C Motif Chemokine Ligand 14
- DEA:
-
Differential expression analysis
- DEGs:
-
Differentially expressed genes
- E2:
-
Estradiol
- ERK:
-
Extracellular signal-regulated kinase
- ESCO2 :
-
Establishment Of Sister Chromatid Cohesion N-Acetyltransferase 2
- EVs:
-
Extracellular vesicles
- FC:
-
Fold change
- FDR:
-
False discovery rate
- FOXO3 :
-
Forkhead Box O3
- FXYD5 :
-
FXYD Domain Containing Ion Transport Regulator 5
- GEO:
-
Gene expression omnibus
- GSEA:
-
Gene set enrichment analysis
- HMGB1 :
-
High mobility group box 1
- hPL:
-
Human Placental Lactogen
- ID1:
-
Inhibitor of DNA binding 1
- IPA:
-
QIAGEN Ingenuity Pathway Analysis
- KAT2B :
-
Lysine Acetyltransferase 2B
- LIF :
-
LIF Interleukin 6 Family Cytokine
- MAOB :
-
Monoamine Oxidase B
- MAPK :
-
Mitogen-activated protein kinase
- MCM4 :
-
Minichromosome Maintenance Complex Component 4
- MCM6 :
-
Minichromosome Maintenance Complex Component 6
- MT1M :
-
Metallothionein 1 M
- MTRNR2L1 :
-
MT-RNR2 Like 1
- Muc-1:
-
Mucin-1
- OLFM1 :
-
Olfactomedin 1
- P4:
-
Progesterone
- PAEP :
-
Progestagen Associated Endometrial Protein
- PARP1 :
-
Poly (ADP-Ribose) Polymerase 1
- PAS:
-
Periodic acid Schiff
- PCA:
-
Principal Component Analysis
- PGR :
-
Progesterone receptor
- PPARα :
-
Peroxisome proliferator activated receptor alpha
- PRL:
-
Prolactin
- PTAFR :
-
Platelet Activating Factor Receptor
- PTEN :
-
Phosphatase and tensin homolog
- qRT-PCR:
-
Quantitative real-time PCR
- RHOGDI :
-
Rho GDP dissociation inhibitor
- RIF:
-
Recurrent implantation failure
- RNA-seq:
-
RNA-sequencing
- RPL:
-
Recurrent pregnancy loss
- RRM2 :
-
Ribonucleotide Reductase Regulatory Subunit M2
- RUNX2 :
-
RUNX Family Transcription Factor 2
- RXRα :
-
Retinoid X receptor alpha
- SMAD3:
-
SMAD Family Member 3
- SOD2 :
-
Superoxide Dismutase 2
- SOSTDC1 :
-
Sclerostin Domain Containing 1
- SOX9:
-
SRY-Box Transcription Factor 9
- SPP1 :
-
Secreted Phosphoprotein 1
- SQSTM1:
-
Sequestosome 1
- TGFβ-2:
-
Transforming Growth Factor Beta 2
- TSP1 :
-
Thrombospondin 1
- URSA:
-
Unexplained recurrent spontaneous abortion
- VEGF :
-
Vascular endothelial growth factor
- ZWINT :
-
ZW10 Interacting Kinetochore Protein
References
Vercellini P, Bonfanti I, Berlanda N. Adenomyosis and infertility: is there a causal link? Expert Rev Endocrinol Metab. 2019;14:365–7.
Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111:629–40.
Mishra I, Melo P, Easter C, Sephton V, Dhillon-Smith R, Coomarasamy A. Prevalence of adenomyosis in women with subfertility: systematic review and meta‐analysis. Ultrasound in Obstetrics & Gynecology. 2023;62:23–41.
Peric H, Fraser IS. The symptomatology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:547–55.
Puente JM, Fabris A, Patel J, Patel A, Cerrillo M, Requena A, et al. Adenomyosis in infertile women: prevalence and the role of 3D ultrasound as a marker of severity of the Disease. Reproductive Biology and Endocrinology. 2016;14:60.
Thalluri V, Tremellen KP. Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum Reprod. 2012;27:3487–92.
Mavrelos D, Holland TK, O’Donovan O, Khalil M, Ploumpidis G, Jurkovic D et al. The impact of adenomyosis on the outcome of IVF–embryo transfer. Reprod Biomed Online. 2017;35:549–54.
Salim R, Riris S, Saab W, Abramov B, Khadum I, Serhal P. Adenomyosis reduces pregnancy rates in infertile women undergoing IVF. Reprod Biomed Online. 2012;25:273–7.
Martínez-Conejero JA, Morgan M, Montesinos M, Fortuño S, Meseguer M, Simón C, et al. Adenomyosis does not affect implantation, but is associated with miscarriage in patients undergoing oocyte donation. Fertil Steril. 2011;96:943–950e1.
Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014;29:964–77.
Tamura H, Kishi H, Kitade M, Asai-Sato M, Tanaka A, Murakami T, et al. Clinical outcomes of infertility treatment for women with adenomyosis in Japan. Reprod Med Biol. 2017;16:276–82.
Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil Steril. 2017;108:483–490e3.
Cozzolino M, Tartaglia S, Pellegrini L, Troiano G, Rizzo G, Petraglia F. The effect of uterine adenomyosis on IVF outcomes: a systematic review and Meta-analysis. Reproductive Sci. 2022;29:3177–93.
Jiang Y, Jiang R, Cheng X, Zhang Q, Hu Y, Zhang H, et al. Decreased expression of NR4A nuclear receptors in adenomyosis impairs endometrial decidualization. Mol Hum Reprod. 2016;22:655–68.
Fischer CP, Kayisili U, Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil Steril. 2011;95:1133–6.
Campo S, Campo V, Benagiano G. Infertility and adenomyosis. Obstet Gynecol Int. 2012;2012:1–8.
Sudoma I. The evaluation of pinopode formation in patients with adenomyosis. Fertil Steril. 2002;77:27.
Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111:611–7.
Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154.
Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19:568–77.
Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, et al. Patient-derived organoids from endometrial Disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21:1041–51.
Juárez-Barber E, Francés-Herrero E, Corachán A, Vidal C, Giles J, Alamá P, et al. Establishment of Adenomyosis Organoids as a preclinical model to study infertility. J Pers Med. 2022;12:219.
Juárez-Barber E, Segura-Benítez M, Carbajo-García MC, Bas-Rivas A, Faus A, Vidal C, et al. Extracellular vesicles secreted by adenomyosis endometrial organoids contain miRNAs involved in embryo implantation and pregnancy. Reprod Biomed Online. 2023;46:470–81.
Lazzeri L, Morosetti G, Centini G, Monti G, Zupi E, Piccione E, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110:1154–1161e3.
Naftalin J, Hoo W, Nunes N, Holland T, Mavrelos D, Jurkovic D. Association between ultrasound features of adenomyosis and severity of menstrual pain. Ultrasound in Obstetrics & Gynecology. 2016;47:779–83.
Wingett SW, Andrews S. FastQ screen: a tool for multi-genome mapping and quality control. F1000Res. 2018;7:1338.
Bushnell B, Rood J, Singer E. BBMerge – Accurate paired shotgun read merging via overlap. Biggs PJ, editor. PLoS One. 2017;12:e0185056.
Liao Y, Smyth GK, Shi W. The subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108–8.
Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012/03/28. 2012;16:284–7.
Governini L, Luongo FP, Haxhiu A, Piomboni P, Luddi A. Main actors behind the endometrial receptivity and successful implantation. Tissue Cell. Churchill Livingstone; 2021. p. 101656.
Meresman GF, Olivares C, Vighi S, Alfie M, Irigoyen M, Etchepareborda JJ. Apoptosis is increased and cell proliferation is decreased in out-of-phase endometria from infertile and recurrent abortion patients. Reproductive Biology and Endocrinology. 2010;8:126.
Wang C-K, Yang S-C, Hsu S-C, Chang F-P, Lin Y-T, Chen S-F, et al. CHAC2 is essential for self-renewal and glutathione maintenance in human embryonic stem cells. Free Radic Biol Med. 2017;113:439–51.
Lappas M. Expression and regulation of metallothioneins in myometrium and fetal membranes. Am J Reprod Immunol. 2018;80:e13040.
Simmons DG, Kennedy TG. Uterine sensitization-associated gene-1: a novel gene induced within the rat endometrium at the time of uterine receptivity/sensitization for the decidual cell reaction. Biol Reprod. 2002;67:1638–45.
Lei W, Feng X-H, Deng W-B, Ni H, Zhang Z-R, Jia B et al. Progesterone and DNA damage encourage uterine cell proliferation and decidualization through up-regulating ribonucleotide reductase 2 expression during early pregnancy in mice. J Biol Chem. 2012/03/08. 2012;287:15174–92.
Cong S, Guo Q, Cheng Y, Gao J, Sun L, Wang J et al. Identification and analyzation of differentially expressed transcription factors in endometriosis. Front Mol Biosci. 2021;7.
Kottawatta KSA, So K-H, Kodithuwakku SP, Ng EHY, Yeung WSB, Lee K-F. MicroRNA-212 regulates the expression of Olfactomedin 1 and C-Terminal binding protein 1 in human endometrial epithelial cells to Enhance Spheroid attachment in Vitro1. Biol Reprod. 2015;93(109):1–10.
Bai Y, Li LD, Li J, Chen RF, Yu HL, Sun HF, et al. A FXYD5/TGF-β/SMAD positive feedback loop drives epithelial-to-mesenchymal transition and promotes Tumor growth and Metastasis in Ovarian cancer. Int J Oncol. 2020;56:301–14.
Song L, Li X, Sun Q, Zhao Y. Fxyd5 activates the NF-κB pathway and is involved in chondrocytes inflammation and extracellular matrix degradation. Mol Med Rep. 2022;25.
Nikolakopoulos P, Tzimagiorgis G, Goulis DG, Chatzopoulou F, Zepiridis L, Vavilis D. Serum humanin concentrations in women with pre-eclampsia compared to women with uncomplicated pregnancies. J Maternal-Fetal Neonatal Med. 2018;31:305–11.
Maintz L, Schwarzer V, Bieber T, van der Ven K, Novak N. Effects of histamine and diamine oxidase activities on pregnancy: a critical review. Hum Reprod Update. 2008. p. 485–95.
Cummings MJ, Yu H, Paudel S, Hu G, Li X, Hemberger M, et al. Uterine-specific SIRT1 deficiency confers premature uterine aging and impairs invasion and spacing of blastocyst, and stromal cell decidualization, in mice. Mol Hum Reprod. 2022;28:gaac016.
Bhuller Y, Jeng W, Wells PG. Variable in vivo Embryoprotective Role for Ataxia-Telangiectasia–mutated against constitutive and phenytoin-enhanced oxidative stress in atm knockout mice. Toxicol Sci. 2006;93:146–55.
Wang J, Tang Y, Wang S, Cui L, Li D, Du M. Norepinephrine exposure restrains endometrial decidualization during early pregnancy. J Endocrinol. 2021;248:277–88.
Deepak V, Ravikumar N, Badell ML, Sidell N, Rajakumar A. Transcription factor ID1 is involved in decidualization of stromal cells: implications in preeclampsia. Pregnancy Hypertens. 2020;21:7–13.
Gamisonia AM, Yushina MN, Fedorova-Gogolina IA, Akimov MG, Eldarov CM, Pavlovich S. N-Acyl dopamines induce apoptosis in endometrial stromal cells from patients with endometriosis. Int J Mol Sci. 2021;22:10648.
Jin Y, Wang X, Xiao Y, Lv C, Ding C, Lin Q. ORIGINAL ARTICLE: the role of TSP-1 on decidual macrophages involved in the susceptibility to unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2009;61:253–60.
Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22:104–15. 2015/09/22.
Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW-F et al. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci U S A. 2006/10/19. 2006;103:16272–7.
Gómez-Chávez F, Correa D, Navarrete-Meneses P, Cancino-Diaz JC, Cancino-Diaz ME, Rodríguez-Martínez S. NF-κB and its regulators during pregnancy. Front Immunol. 2021;12:679106.
Han M, Cao Y, Zhou W, Zhou M, Zhou X, Zhang D, et al. Increased expression of HMGB1 in the implantation phase endometrium is related to recurrent implantation failure. Mol Biol Rep. 2022;49:1701–10.
Zhu D, Zou H, Liu J, Wang J, Ma C, Yin J et al. Inhibition of HMGB1 ameliorates the maternal-fetal interface Destruction in unexplained recurrent spontaneous abortion by suppressing pyroptosis activation. Front Immunol. 2021;12.
Xiang Y, Sun Y, Yang B, Yang Y, Zhang Y, Yu T, et al. Transcriptome sequencing of adenomyosis eutopic endometrium: a new insight into its pathophysiology. J Cell Mol Med. 2019;23:8381–91.
Woo Seo D, Yeop You S, Chung W-J, Cho D-H, Kim J-S, Su Oh J. Zwint-1 is required for spindle assembly checkpoint function and kinetochore-microtubule attachment during oocyte meiosis. Sci Rep. 2015;5:15431.
Percival SM, Thomas HR, Amsterdam A, Carroll AJ, Lees JA, Yost HJ, et al. Variations in dysfunction of sister chromatid cohesion in esco2 mutant zebrafish reflect the phenotypic diversity of Roberts syndrome. Dis Model Mech. 2015;8:941–55.
Ohtani K, Iwanaga R, Nakamura M, Ikeda M, Yabuta N, Tsuruga H, et al. Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene. 1999;18:2299–309.
McNairn AJ, Chuang C-H, Bloom JC, Wallace MD, Schimenti JC. Female-biased embryonic death from inflammation induced by genomic instability. Nature. 2019/02/20. 2019;567:105–8.
Garrido-Gomez T, Castillo-Marco N, Clemente-Ciscar M, Cordero T, Muñoz-Blat I, Amadoz A, et al. Disrupted PGR-B and ESR1 signaling underlies defective decidualization linked to severe preeclampsia. Elife. 2021;10:e70753.
Khan N, Zargar MH, Ahmed R, Godha M, Ahmad A, Afroze D, et al. Effect of steroid hormone receptor gene variants PROGINS (Alu insertion) and PGR C/T (rs1042839) as a risk factor for recurrent pregnancy loss in Kashmiri population (North India). J Obstet Gynecol Res. 2021;47:4329–39.
Hou H, Zhang JY, Chen D, Deng F, Morse AN, Qiu X, et al. Altered decidual and placental catabolism of vitamin D may contribute to the aetiology of spontaneous miscarriage. Placenta. 2020;92:1–8.
Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y, et al. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2012;303:928–35.
Cheong CY, Chng K, Lim MK ee, Amrithraj AI, Joseph R, Sukarieh R et al. Alterations to DNA methylation and expression of CXCL14 are associated with suboptimal birth outcomes. J Hum Genet. 2014;59:504–11.
Kuang H, Chen Q, Fan X, Zhang Y, Zhang L, Peng H, et al. CXCL14 inhibits trophoblast outgrowth via a paracrine/autocrine manner during early pregnancy in mice. J Cell Physiol. 2009;221:448–57.
Tarnowski M, Malinowski D, Safranow K, Dziedziejko V, Pawlik A. CDC123/CAMK1D gene rs12779790 polymorphism and rs10811661 polymorphism upstream of the CDKN2A/2B gene in women with gestational Diabetes. J Perinatol. 2017;37:345–8.
Li Y, Wu C, Gu J, Li D, Yang Y. A novel mutation associated with type III Bartter syndrome: a report of five cases. Mol Med Rep. 2019;20:65–72.
Agrawal V, Jaiswal MK, Ilievski V, Beaman KD, Jilling T, Hirsch E. Platelet-activating factor: a role in preterm delivery and an essential interaction with Toll-like receptor signaling in mice. Biol Reprod. 2014/09/24. 2014;91:119.
Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2016;32:69–73.
Wu S, Cui Y, Zhao H, Xiao X, Gong L, Xu H, et al. Trophoblast exosomal UCA1 induces endothelial Injury through the PFN1-RhoA/ROCK Pathway in Preeclampsia: a human-specific adaptive pathogenic mechanism. Oxid Med Cell Longev. 2022;2022:2198923.
Wang H, Wang P, Liang X, Li W, Yang M, Ma J, et al. Down-regulation of endothelial protein C receptor promotes preeclampsia by affecting actin polymerization. J Cell Mol Med. 2020;24:3370–83. 2020/01/30.
Loke H, Rainczuk K, Dimitriadis E. MicroRNA Biogenesis Machinery Is Dysregulated in the Endometrium of Infertile Women Suggesting a Role in Receptivity and Infertility. J Histochem Cytochem. 2019/05/30. 2019;67:589–99.
Nadeau V, Charron J. Essential role of the ERK/MAPK pathway in blood-placental barrier formation. Development. 2014;141:2825–37.
Singh S, Dhar R, Karmakar S. Fenofibrate mediated activation of PPARα negatively regulates trophoblast invasion. Placenta. 2022;126:140–9.
Sharp AN, Heazell AEP, Baczyk D, Dunk CE, Lacey HA, Jones CJP, et al. Preeclampsia is associated with alterations in the p53-pathway in villous trophoblast. PLoS ONE. 2014;9:e87621–1.
Song G, Jin F. RhoGDI1 interacts with PHLDA2, suppresses the proliferation, migration, and invasion of trophoblast cells, and participates in the pathogenesis of preeclampsia. Hum Cell. 2022;35:1440–52.
Xue P, Fan W, Diao Z, Li Y, Kong C, Dai X, et al. Up-regulation of PTEN via LPS/AP-1/NF-κB pathway inhibits trophoblast invasion contributing to preeclampsia. Mol Immunol. 2020;118:182–90.
Acknowledgements
The authors would like to express their sincere gratitude to the participants who made this study possible, all the clinical and laboratory staff of the IIS La Fe, IVI Valencia and Genomics and Epigenetics Department of UCIM-Universitat de València.
Funding
This research was funded by Health Institute Carlos III (FI19/00110 [E.J.-B.]). Instituto de Salud Carlos III and cofounded by the European Social Fund (ESF) “Investing in your future” through the Miguel Servet Program (CP20/00120 [H.F.]; CP19/00149 [I.C.]) Ministerio de Ciencia e Innovación. Generalitat Valenciana VALi + d Programme (APOSTD/2020/123 [A.C.] and ACIF/2019/139 [M.C.C.-G.]).
Author information
Authors and Affiliations
Contributions
Conceptualization, E.J.-B., H.F.; methodology, E.J.-B., A.C., M.C.C.-G., A.F., C.V., J.G.; software, E.J.-B., M.C.C.-G.; validation, E.J.-B., A.C., A.F.; formal analysis, E.J.-B., H.F.; investigation, E.J.-B., A.C., M.C.C.-G., A.F., I.C., H.F.; resources, H.F., A.P.; data curation, E.J.-B., A.C.; writing—original draft preparation, E.J.-B., A.C., H.F.; writing—review and editing, E.J.-B., A.C., I.C., H.F.; supervision, A.P., I.C., H.F.; project administration, A.P., H.F.; funding acquisition, E.J.-B., A.C., M.C.C.-G., I.C., H.F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Clinical Ethics Committee at Hospital La Fe (#2004-FIVI-039-HF; Valencia, Spain). All participants provided written informed consent prior to sample collection. All the experiments in our study were conducted in accordance to the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplementary Figure 1.
Experimental design. Created with BioRender.com
Supplementary Material 2: Supplementary Table 1.
Functional enrichment and KEGG pathway analysis of ADENO-SECorg
Supplementary Material 3: Supplementary Table 2.
Functional enrichment and KEGG pathway analysis of ADENO-GESTorg
Supplementary Material 4: Supplementary Table 3.
Canonical pathways predicted to be dysregulated in ADENO-SECorg
Supplementary Material 5: Supplementary Table 4.
Canonical pathways predicted to be dysregulated in ADENO-GESTorg
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Juárez-Barber, E., Corachán, A., Carbajo-García, M.C. et al. Transcriptome analysis of adenomyosis eutopic endometrium reveals molecular mechanisms involved in adenomyosis-related implantation failure and pregnancy disorders. Reprod Biol Endocrinol 22, 10 (2024). https://doi.org/10.1186/s12958-023-01182-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-023-01182-7