Abstract
Background
Luteinizing hormone (LH) is critical in follicle growth and oocyte maturation. However, the value of recombinant LH (r-LH) supplementation to recombinant follicle stimulating hormone (r-FSH) during controlled ovarian stimulation in the gonadotrophin releasing hormone (GnRH) antagonist regimen is controversial.
Methods
This multicenter retrospective cohort study recruited 899 GnRH antagonist cycles stimulated with r-LH and r-FSH in 3 reproductive centers and matched them to 2652 r-FSH stimulating cycles using propensity score matching (PSM) for potential confounders in a 1:3 ratio. The primary outcome was the cumulative live birth rate (CLBR) per complete cycle.
Results
The baseline characteristics were comparable in the r-FSH/r-LH and r-FSH groups after PSM. The r-FSH/r-LH group achieved a higher CLBR than the r-FSH group (66.95% vs. 61.16%, p = 0.006). R-LH supplementation also resulted in a higher 2-pronuclear embryo rate, usable embryo rate, and live birth rate in both fresh embryo transfer cycles and frozen-thawed embryo transfer (FET) cycles. No significant differences were found in the rate of moderate and severe ovarian hyperstimulation syndrome (OHSS), or cycle cancellation rate in the prevention of OHSS.
Conclusions
R-LH supplementation to r-FSH in the GnRH antagonist protocol was significantly associated with a higher CLBR and live birth rate in fresh and FET cycles, and improved embryo quality without increasing the OHSS rate and cycle cancellation rate.
Similar content being viewed by others
Background
Controlled ovarian stimulation (COS) is the first and critical procedure of in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). Gonadotropin releasing hormone (GnRH) antagonists are applied to conveniently downregulate the pituitary gland to reduce endogenous gonadotropins without the flare-up effect. Thus, follicle development is under the control of exogenous gonadotropins.
According to the “two cell-two gonadotropins” theory, follicle stimulating hormone (FSH) synergizes with luteinizing hormone (LH) to promote folliculogenesis and oocyte maturation. FSH stimulates granulosa cells to produce estradiol from androgen transformed from cholesterol by theca cells in response to LH stimulation. Recombinant FSH (r-FSH) alone can induce follicular development after pituitary downregulation, probably because residual endogenous LH secretion is not completely suppressed, thereby participating in the follicular development. However, follicular development was undermined [1], decidualization of the endometrium was affected, and implantation of embryos was disturbed [2] in the absence of exogenous LH. Therefore, the addition of exogenous recombinant LH (r-LH) in combination with FSH was inferred to promote the normal development of follicles. While conflicting opinions regarding this effect have been published.
A real-world study involving 9787 cycles suggested that LH supplementation for moderate and severe poor ovarian responders can improve the cumulative live birth rate (CLBR) [3]. When comparing the 2nd ICSI cycles stimulated by r-FSH and r-LH in the GnRH antagonist protocol with the 1st cycles stimulated with r-FSH for 228 cycles, the implantation rate was increased by 8% in the 2nd cycles (1st cycle: 18.57 ± 0.52 vs. 2nd cycle: 26.47 ± 0.62, p < 0.001) [4]. A study involving 320 cycles receiving the GnRH antagonist treatment [5] revealed that exogenous LH supplementation resulted in more oocytes retrieved (5.60 vs. 3.97, p = 0.04) and good-quality embryos (3.07 vs. 1.93, p = 0.01) for patients over 40 years old compared with the r-FSH alone group. Another study involving 1565 cycles [6] concluded that r-LH supplementation based on the r-FSH stimulation was associated with increased pregnancy rate (61% vs. 54%, p = 0.006), live birth rate (49% vs. 42%, p = 0.01), fertilization rate (74% vs. 72%, p = 0.04), and implantation rate (41% vs. 37%, p = 0.03). A meta-analysis [7] concluded that r-LH combined with r-FSH probably improved ongoing pregnancy rates (OR 1.20, 95% CI 1.01 to 1.42) compared to using r-FSH alone.
However, a systematic review [8] has suggested that ovarian stimulation with r-LH and r-FSH did not differed from stimulation with r-FSH in terms of the number of oocytes retrieved, implantation rate and clinical pregnancy rate in women undergoing the GnRH antagonist cycles. A meta-analysis [9] showed that no significant difference presented in ongoing pregnancy rate, pregnancy rate, OHSS rate between LH supplementation and r-FSH alone in women.
The abovementioned studies did not reach a consensus on the value of LH supplementation. Furthermore, its effects on embryo development and its impact on the CLBR are less systematically evaluated. Thus, the present study aimed to investigate whether patients undergoing the GnRH antagonist protocol could benefit from r-LH supplementation in terms of embryo development, live birth rates in fresh cycles and frozen-thawed embryo transfer (FET) cycles, as well as the CLBR in complete cycles (when at least a live birth was achieved or all embryos from the same retrieval cycle were transferred) in 3 reproductive centers.
Methods
Study population and design
This multicenter retrospective cohort study recruited the autologous IVF/ICSI cycles using GnRH antagonist protocol conducted at the Reproductive Medicine Center of the Sixth Affiliated Hospital of Sun Yat-sen University, Northwest Women’s and Children’s Hospital, and Jiangsu Provincial Hospital from January 2014 to December 2018. The study group underwent ovarian stimulation with r-FSH and r-LH. R-LH was added on the day of GnRH-antagonist had been started. The control group received ovarian stimulation with r-FSH.
Only the first oocyte retrieval cycles of a patient and the subsequent FET cycles were included. Cycles were excluded if (1) the female partner underwent recurrent spontaneous miscarriages or suffered from hydrosalpinx, intrauterine adhesions, uterine malformations, submucosal myoma, adenomyosis or thyroid dysfunction; (2) either of the spouses presented chromosomal abnormalities; (3) human menopausal gonadotropin, letrozole or clomiphene citrate were used for ovarian stimulation; (4) cycles included in vitro matured oocytes, frozen-thawed embryos, biopsied embryos. Oocyte retrieval, fresh embryo transfer, FET and complete cycles (at least a live birth was achieved or all embryos from the same retrieval cycle were transferred) were included in the analyses. Cycles were followed up until December 2020. Subgroup analysis were conducted for the normal responders who were younger than 40 years old and the number of oocytes retrieved were between 4 and 15 [10].
This project was approved by Ethics Committees at the Sixth Affiliated Hospital of Sun Yat-sen University (2020ZSLYEC-295), Northwest Women’s and Children’s Hospital (2,019,013), and Jiangsu Provincial Hospital (2020-SR-046). The written informed consents were waived due to the retrospective nature of this study.
Controlled ovarian stimulation procedures and embryo evaluation
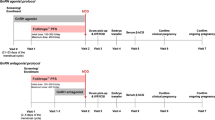
Fixed GnRH antagonist protocol (GnRH antagonist started on day 5 of r-FSH stimulation) and flexible GnRH antagonist protocol (GnRH antagonist started when the mean diameter of dominant follicles reached 12 mm) [11] were adopted for controlled ovarian stimulation following the routine of the three reproductive centers. R-FSH of 100 to 300 IU/day was administered according to the individual characteristics, such as anti-müllerian hormone, basal FSH, LH, estradiol, progesterone, and antral follicle counts (AFC) on days 2–3 of the menstrual cycle. Whether r-LH was supplemented was determined by the specialties.
Follicular growth was evaluated by serum concentration of estradiol, progesterone, FSH, and LH, and transvaginal ultrasonography. Once the diameters of three dominant follicles ≥ 17 mm or the diameters of two dominant follicles ≥ 18 mm, human chorionic gonadotrophin was injected, and oocytes were retrieved after 36-38 h. Most fresh embryos were transferred on day 3. Freezing all embryos was adopted when there was a risk of ovarian hyperstimulation syndrome (OHSS) [12], thin endometrium and elevated progesterone. Cleavaged embryos were evaluated by Scott’s criteria [13]: grades I and II with ≥ 4 cells were usable; with ≥ 6 cells were of good quality. Blastocysts (days 5 and 6) were evaluated by Gardner’s system [14]: blastocysts graded as 3–6, and inner cell mass and trophectoderm assessed as AA, AB, AC, BA, BB, BC, CA, or CB were usable embryos, grade 3–6 blastocysts graded with AA, AB, BA, and BB were good-quality embryos.
Luteal phase support was performed with oral or vaginal progesterone until 8 weeks of gestation.
Outcome measures
The primary outcome was the CLBR which referred to the probability of achieving at least 1 live birth in a complete cycle.
Secondary outcomes assessed included number of oocytes retrieved, number of two-pronuclear (2PN) embryos and 2PN embryo rate after IVF, number of 2PN embryos and 2PN embryo rate after ICSI, usable embryo number and rate, good-quality embryo number and rate, mild/moderate OHSS [12] rate and cycle cancellation rate in prevention of OHSS. In terms of pregnancy outcomes, clinical pregnancy rate and live birth rate were evaluated. Clinical pregnancy was defined as the observation of gestational sac(s) through a transvaginal probe. Live birth was defined as a live-born infant(s) after 28 gestational weeks.
Statistical analysis
Propensity score-matching analysis (PSM) and data analysis were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA). Patients of the study group and the control group were randomly matched with the 1:3 nearest neighbor matching method. The covariates included maternal age, paternal age, maternal body mass index (BMI), infertility factors, infertility type, basal FSH, AFC, and fertilization type.
Continuous variables that followed normal distribution were expressed as mean values (± standard deviations, SDs), and compared by student’s t-test. Data with skewed distribution were described as medians (quartiles) and Mann–Whitney U test was adopted for comparisons. Categorical variables were described as counts (percentages) and compared by Pearson Chi-square test. P < 0.05 was regarded as statistically significant.
Results
The process of inclusion and exclusion is shown in Fig. 1. A total of 8,228 oocyte retrieval cycles met the inclusion criteria, of which 1,029 cycles received r-FSH/r-LH administration. After PSM, 884 oocyte retrieval cycles that received r-FSH/r-LH treatment were matched with 2,652 r-FSH stimulated cycles, 293 fresh embryo transfer cycles with r-FSH/r-LH treatment were matched with 879 r-FSH stimulated cycles, 753 FET cycles with r-FSH/r-LH treatment were matched with 2,259 r-FSH stimulated cycles, and 702 complete cycles with r-FSH/r-LH treatment were matched with 2,106 r-FSH stimulated cycles.
Baseline characteristics
The baseline characteristics of the oocyte retrieval cycles are presented in Table 1. Parental ages, female BMI, infertility factors, infertility type, AFC, basal LH and fertilization type were not statistically significant after PSM. The basal FSH of the study group was close to that of the control group (6.48 [5.48, 7.60] vs. 6.26 [5.32, 7.52]), although the difference was statistically significant (p = 0.012). Table 2 shows the baseline characteristics of the fresh embryo transfer cycles, Table 3 shows those of the FET cycles, and Table 4 displays those of the complete cycles. The distributions of these characteristics were comparable between the two groups after PSM except that the basal LH levels were significantly lower in the study group in Table 3 (p = 0.015) and Table 4 (p = 0.006). On the day of triggering, the estradiol, LH, progesterone level, and number of developed follicles were significantly higher in the study group from Tables 1, 2, 3, 4.
Embryo development
The comparisons of embryo development between the study and control groups are presented in Table 5. The study group had fewer retrieved oocytes than the control group (p < 0.001). The number of 2PN embryos was comparable between oocytes fertilized by IVF and those fertilized by ICSI. Thus, the study group presented a statistically higher 2PN embryo rate than the control group (IVF 2PN rate: p < 0.001; ICSI 2PN rate: p = 0.003) (Table 5). The number of usable embryos and good-quality embryos were comparable between the two groups. While the usable embryo rate in 2PN embryos was significantly higher in the study group (p < 0.001), no significant difference existed between the good-quality embryo rates of the two groups. Furthermore, the rate of mild or moderate OHSS was similar between the groups (p = 0.864). The cycle cancellation rate in the prevention of OHSS was not significantly different between the two groups.
Pregnancy outcomes
After fresh embryo transfer, 50.85% cycles of the study group, and 49.49% cycles of the control group achieved clinical pregnancy (p = 0.686). The live birth rate in the fresh cycles was significantly higher in the study group than in the control group (39.93% vs. 28.10%, p < 0.001).
For FET cycles, the clinical pregnancy rate in the study group was 63.75% versus that of 61.97% in the control group, and no significant difference was observed. The study group obtained a significantly higher live birth rate than the control group (51.53% vs. 43.16%, p < 0.001).
The CLBR in complete cycles of the study group was also significantly higher than that of the control group (66.95% vs. 61.16%, p = 0.006).
Subgroup analysis of normal responders
Subgroup analysis of normal responders were conducted. Among the normal responders, 616 oocyte retrieval cycles of the study group were matched with 1,848 cycles of the control group. No significantly differences existed between the baseline characteristics of the study groups and the control groups of the oocyte retrieval cycles (Supplementary table 1), fresh embryo transfer cycles (Supplementary table 2), FET cycles (Supplementary table 3), and complete cycles (Supplementary table 4) except that the basal LH in the study group were significantly higher than that in the control group of the FET cycles (p = 0.041, Supplementary table 3). While the E2, LH, P and the number of developed follicles on the day of triggering were significantly higher in the study groups (Supplementary table 1, 2, 3, 4).
Consistent with the outcomes of the whole group analysis, the study groups of the normal responders achieved significantly better prognosis than the control groups. The number of oocytes retrieved were close in the two groups. The number of 2PN embryos fertilized by IVF (p = 0.003) and the rate of 2PN embryos by IVF (p < 0.001) were higher in the study group of normal responders (Supplementary Table 5). The number of oocytes retrieved, number and rate of 2PN embryos fertilized by ICSI, number and rate of usable embryos and good-quality embryos, and mild/moderate OHSS rate were comparable between the two groups. However, the cycle cancellation rate in the prevention of the study group was significantly higher in the study group (p < 0.001) (Supplementary table 5).
The clinical pregnancy rates in the fresh embryo transfer cycles were comparable (p = 0.413) between the study group (55.79%) and the control group (52.75%). However, the live birth rate in the fresh cycles was significantly higher in the study group than in the control group (42.98% vs.33.06%, p = 0.005).
No significantly difference was found in the clinical pregnancy rate in the FET cycles between the two groups (study group: 65.88% vs. control group: 63.27%, p = 0.287), But the study group in the FET cycles obtained a significantly higher live birth rate than the control group (53.73% vs. 45.56%, p = 0.001).
The study group also achieved significantly higher CLBR than that of the control group (69.79% vs. 61.56%, p = 0.001).
Discussion
Numerous publications [3, 7, 8, 15,16,17,18,19,20] have proven that r-FSH alone is capable of inducing satisfactory follicle development during controlled ovarian stimulation. Although LH is critical for follicle growth and oocyte maturation, the benefit of r-LH supplementation in the GnRH antagonist regimen remains disputable. In this multicenter retrospective cohort study, we investigated the effects of r-LH supplementation on the whole process of IVF/ICSI in the same cohort for the first time. R-LH supplementation was found to be associated with improved embryo development, live birth rates in both fresh and FET cycles, and the CLBR in complete cycles in patients receiving the GnRH antagonist regimen. The occurrence rates of OHSS and cycle cancellation in the prevention of OHSS were not increased.
The effects of r-LH supplementation on embryo development, OHSS rate, and cycle cancellation rate have not been clearly investigated. Although a randomized study reported a lower OHSS incidence and lower cycle cancellation rate in the r-LH supplementation group that was downregulated by GnRH agonists [21], no significant differences were observed in the present study. The different conclusions might be attributed to the GnRH antagonist protocol in our study, which reduced the occurrence of OHSS compared with the GnRH agonist protocol [22]. However, the subgroup analysis of normal responders revealed a higher cycle cancellation rate in the study group. It was probably because that the E2 level on the trigger day was significantly higher in the study group, thus the embryo transfer was more frequently cancelled in the study group to prevent OHSS.
A prospective randomized study, which focused on cycles in which the GnRH antagonist was administered, reported that the number of oocytes retrieved was similar whether r-LH was supplemented or not [19]. However, another prospective randomized study [23] showed that the number of oocytes recovered was relatively lower in the r-FSH/r-LH group (5.33 ± 4.8 vs. 7.00 ± 3, p > 0.05), whose trend was consistent with the results of our study. This phenomenon suggested that r-LH supplementation did not help to improve ovarian response.
Our data showed that r-LH supplementation was associated with an increased normal fertilization rate (2-pronuclear embryo rate of both IVF and ICSI), usable embryo rate, and live birth rate in FET cycles. These finding were consistent with the conclusions of Paterson’s [6] and Lisi’s study [24]. In the previous studies, LH was proven to promote folliculogenesis by (i) facilitating the synthesis of androgens for the production of estradiol and the induction of FSH receptor expression in granulosa cells [25]; (ii) recruiting local growth factors, such as EGF, GDF9, and TGF-β to promote oocyte maturation [23, 26]; (iii) decreasing the cumulus apoptosis rate [23]; (iv) resuming meiosis and ovulation [15, 27]. Thus, r-LH supplementation contributed to improve the quality of oocytes and promote embryogenesis.
Furthermore, the present study showed that the live birth rate in the fresh cycles was elevated in the study group, which was in accordance with previous studies showing that a higher live birth rate was achieved when r-LH was supplemented in the GnRH antagonist protocol [4,5,6,7]. Exposure to low endogenous LH by downregulation leads to stagnation of endometrial growth [28], decreasing endometrium receptivity [2], and decreasing the implantation rate [28]. The disturbance can be rescued by LH receptor stimulation through mid-cycle HCG supplementation [28]. LH supplementation probably achieved the same effects on endometrium as the HCG supplementation.
Many researches have discussed the “LH activity” supplementation on progesterone levels on the triggering day, but no consensus has been reached. In five previous studies [16, 19, 29,30,31], no significant difference in serum progesterone was observed between groups with or without r-LH supplementation during COS. One study [32] suggested the progesterone level was significantly lower in r-LH supplied group, and the number of follicles was significantly reduced. Three other studies [33,34,35] found that when LH activity (hCG) was provided during the late follicular phase, serum P-values were significantly increased. We also observed that the progesterone levels were significantly higher in the study group of the present study, which might result from the elevated number of developed follicles after r-LH supplementation. The inconsistency suggests that the effect of r-LH supplementation on progesterone may vary among patients with different characteristics [36], which requires further analysis in a larger sample size of patients.
The clinical pregnancy rates in fresh and FET cycles were comparable between the two groups, indicating a higher miscarriage rate in the r-FSH group, which might suggest the unsatisfactory developmental potential of embryos.
Traditionally, live birth rate per embryo transfer has been reported as the success rates of IVF/ICSI [37]. However, embryo freezing and thawing becomes increasingly universal that live birth rate of a single embryo transfer cycle is not adequate to evaluate the effect of the IVF/ICSI treatment. CLBR describes the probability that a person will deliver at least one baby after transferring all fresh and frozen embryos from the same oocyte retrieval cycle [38]. Not only was the quality of embryos assessed, but the number of usable embryos was also evaluated. Therefore, the CLBR has been regarded as the most valuable patient-centered outcome to assess the success of IVF/ICSI treatment [39]. However, the impact of r-LH supplementation on the CLBR has been less investigated perhaps because the calculation of the CLBR is more complicated than the live birth rate in an embryo transfer cycle. The former requires the selection of complete cycles (all embryos form an oocyte retrieval cycle were used up or at least a live birth was achieved) and also the integration of results of a series of fresh and frozen embryo transfer cycles. There is only one real-world study focusing on poor ovarian responders and reporting that the CLBR of moderate and severe poor ovarian responders is improved when r-LH is provided [3]. Our study is the first to report the effect of r-LH supplementation on CLBR in patients receiving the GnRH antagonist protocol. The conclusion suggests that the CLBR is elevated in r-FSH/r-LH stimulated patients with GnRH antagonist pituitary downregulation, perhaps through elevated oocyte quality, promoted embryo developmental potential, and optimized decidualization and receptivity.
Based on the consensus on LH supplementation among the Asia Pacific Fertility Advisory Group in 2011 [40], LH supplementation has been recommended to patients with central ovarian failure, poor ovarian response histories with < 4 oocytes with FSH levels ≥ 300 IU/day, and unsatisfactory responses to the current COS cycle. Furthermore, patients aged > 35 years should consider r-LH supplementation due to the potential of poor or suboptimal responses, and the decreased bioactivity of endogenous LH [40]. Our study, on the other hand, provides new evidence on the value of r-LH supplementation for common patients receiving COS through the GnRH antagonist protocol.
Considered to the affection of the intrinsic nature of retrospective research, especially selection bias of LH supplementation, we adopted PSM to balance the differences between the two groups, including maternal age, paternal age, maternal BMI, infertility factors, infertility type, basal FSH, AFC, and fertilization type. After PSM, the bias was reduced as much as possible, so that the data between the two groups were comparable and the research results were reliable to a certain extent.
The first strength of this study is that the CLBR was set as the primary outcome, thereby providing a global overview of the effects of r-LH supplementation in such individuals. Secondly, the present study provides a robust analysis on the suitable populations for r-LH supplementation based on data from reproductive centers in three tertiary hospitals. The r-LH supplementation has been conventionally recommended to patients with central ovarian failure, poor ovarian response histories, and unsatisfactory responses to the current COS, or patients aged > 35 years [33]. But our study extended the suitable populations to the patients undergoing the GnRH antagonist protocol. No matter the individuals were normal responders or not, it is helpful to improve the prognosis of these people.
Conclusions
In conclusion, r-LH supplementation to r-FSH in the GnRH antagonist protocol resulted in a statistically significantly higher CLBR, live birth rate in fresh and FET cycles, and better embryos without increasing the OHSS rate and cycle cancellation rate. The effects might have been achieved through higher quality embryos, the promotion of embryo developmental potential, and the optimization of decidualization and receptivity.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- r-LH:
-
Recombinant Luteinization hormone
- GnRH:
-
Gonadotrophin releasing hormone
- r-FSH:
-
Recombinant-follicle stimulation hormone
- PSM:
-
Propensity score matching
- CLBR:
-
Cumulative live birth rate
- FET:
-
Frozen-thawed embryo transfer
- OHSS:
-
Ovarian hyperstimulation syndrome
- COS:
-
Controlled ovarian stimulation
- IVF:
-
In vitro fertilization
- ICSI:
-
Intracytoplasmic sperm injection
- AFC:
-
Antral follicle counts
- 2PN:
-
2 Pronuclear
- BMI:
-
Body mass index
- SD:
-
Standard deviation
References
Loumaye E, Engrand P, Shoham Z, Hillier SG, Baird DT. Clinical evidence for an LH “ceiling” effect induced by administration of recombinant human LH during the late follicular phase of stimulated cycles in World Health Organization type I and type II anovulation. Hum Reprod. 2003;18(2):314–22.
Freis A, Germeyer A, Jauckus J, Capp E, Strowitzki T, Zorn M, et al. Endometrial expression of receptivity markers subject to ovulation induction agents. Arch Gynecol Obstet. 2019;300(6):1741–50.
Arvis P, Massin N, Lehert P. Effect of recombinant LH supplementation on cumulative live birth rate compared with FSH alone in poor ovarian responders: a large, real-world study. Reprod Biomed Online. 2021;42(3):546–54.
Setti AS, Braga D, Iaconelli AJ, Borges EJ. Improving Implantation Rate in 2nd ICSI Cycle through Ovarian Stimulation with FSH and LH in GNRH Antagonist Regimen. Rev Bras Ginecol Obstet. 2021;43(10):749–58.
He W, Lin H, Lv J, Wen Y, Cai L. The impact of luteinizing hormone supplementation in gonadotropin-releasing hormone antagonist cycles: a retrospective cohort study. Gynecol Endocrinol. 2018;34(6):513–7.
Paterson ND, Foong SC, Greene CA. Improved pregnancy rates with luteinizing hormone supplementation in patients undergoing ovarian stimulation for IVF. J Assist Reprod Genet. 2012;29(7):579–83.
Mochtar MH, Danhof NA, Ayeleke RO, Van der Veen F, van Wely M. Recombinant luteinizing hormone (rLH) and recombinant follicle stimulating hormone (rFSH) for ovarian stimulation in IVF/ICSI cycles. Cochrane Database Syst Rev. 2017;5(5):Cd005070.
Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Bühler K, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril. 2018;109(4):644–64.
Xiong Y, Bu Z, Dai W, Zhang M, Bao X, Sun Y. Recombinant luteinizing hormone supplementation in women undergoing in vitro fertilization/ intracytoplasmic sperm injection with gonadotropin releasing hormone antagonist protocol: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2014;12:109.
Oudshoorn SC, van Tilborg TC, Hamdine O, Torrance HL, Eijkemans MJC, Lentjes E, et al. Ovarian response to controlled ovarian hyperstimulation: what does serum FSH say? Hum Reprod. 2017;32(8):1701–9.
Kolibianakis EM, Venetis CA, Kalogeropoulou L, Papanikolaou E, Tarlatzis BC. Fixed versus flexible gonadotropin-releasing hormone antagonist administration in in vitro fertilization: a randomized controlled trial. Fertil Steril. 2011;95(2):558–62.
Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–47.
Scott LA, Smith S. The successful use of pronuclear embryo transfers the day following oocyte retrieval. Hum Reprod. 1998;13(4):1003–13.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Canosa S, Carosso AR, Mercaldo N, Ruffa A, Evangelista F, Bongioanni F, et al. Effect of rLH Supplementation during Controlled Ovarian Stimulation for IVF: Evidence from a Retrospective Analysis of 1470 Poor/Suboptimal/Normal Responders Receiving Either rFSH plus rLH or rFSH Alone. J Clin Med. 2022;11(6):1575–85.
Griesinger G, Schultze-Mosgau A, Dafopoulos K, Schroeder A, Schroer A, von Otte S, et al. Recombinant luteinizing hormone supplementation to recombinant follicle-stimulating hormone induced ovarian hyperstimulation in the GnRH-antagonist multiple-dose protocol. Hum Reprod. 2005;20(5):1200–6.
Acevedo B, Sanchez M, Gomez JL, Cuadros J, Ricciarelli E, Hernández ER. Luteinizing hormone supplementation increases pregnancy rates in gonadotropin-releasing hormone antagonist donor cycles. Fertil Steril. 2004;82(2):343–7.
Baruffi RL, Mauri AL, Petersen CG, Felipe V, Martins AM, Cornicelli J, et al. Recombinant LH supplementation to recombinant FSH during induced ovarian stimulation in the GnRH-antagonist protocol: a meta-analysis. Reprod Biomed Online. 2007;14(1):14–25.
Cédrin-Durnerin I, Grange-Dujardin D, Laffy A, Parneix I, Massin N, Galey J, et al. Recombinant human LH supplementation during GnRH antagonist administration in IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2004;19(9):1979–84.
König TE, van der Houwen LE, Overbeek A, Hendriks ML, Beutler-Beemsterboer SN, Kuchenbecker WK, et al. Recombinant LH supplementation to a standard GnRH antagonist protocol in women of 35 years or older undergoing IVF/ICSI: a randomized controlled multicentre study. Hum Reprod. 2013;28(10):2804–12.
Caserta D, Lisi F, Marci R, Ciardo F, Fazi A, Lisi R, et al. Does supplementation with recombinant luteinizing hormone prevent ovarian hyperstimulation syndrome in down regulated patients undergoing recombinant follicle stimulating hormone multiple follicular stimulation for IVF/ET and reduces cancellation rate for high risk of hyperstimulation? Gynecol Endocrinol. 2011;27(11):862–6.
Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4(4):Cd001750.
Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril. 2007;87(3):542–6.
Lisi F, Rinaldi L, Fishel S, Caserta D, Lisi R, Campbell A. Evaluation of two doses of recombinant luteinizing hormone supplementation in an unselected group of women undergoing follicular stimulation for in vitro fertilization. Fertil Steril. 2005;83(2):309–15.
Kishi H, Kitahara Y, Imai F, Nakao K, Suwa H. Expression of the gonadotropin receptors during follicular development. Reproductive medicine and biology. 2018;17(1):11–9.
Barberi M, Ermini B, Morelli MB, Ermini M, Cecconi S, Canipari R. Follicular fluid hormonal profile and cumulus cell gene expression in controlled ovarian hyperstimulation with recombinant FSH: effects of recombinant LH administration. J Assist Reprod Genet. 2012;29(12):1381–91.
Pan B, Li J. The art of oocyte meiotic arrest regulation. Reprod Biol Endocrinol. 2019;17(1):8.
Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online. 2003;7(1):59–64.
Kovacs P, Kovats T, Kaali SG. Results with early follicular phase recombinant luteinizing hormone supplementation during stimulation for in vitro fertilization. Fertil Steril. 2010;93(2):475–9.
Ferraretti AP, Gianaroli L, Magli MC, D’Angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril. 2004;82(6):1521–6.
Sauer MV, Thornton MH 2nd, Schoolcraft W, Frishman GN. Comparative efficacy and safety of cetrorelix with or without mid-cycle recombinant LH and leuprolide acetate for inhibition of premature LH surges in assisted reproduction. Reprod Biomed Online. 2004;9(5):487–93.
Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25(8):2092–100.
Gomes MK, Vieira CS, Moura MD, Manetta LA, Leite SP, Reis RM, et al. Controlled ovarian stimulation with exclusive FSH followed by stimulation with hCG alone, FSH alone or hMG. Eur J Obstet Gynecol Reprod Biol. 2007;130(1):99–106.
Filicori M, Cognigni GE, Pocognoli P, Tabarelli C, Spettoli D, Taraborrelli S, et al. Modulation of folliculogenesis and steroidogenesis in women by graded menotrophin administration. Hum Reprod. 2002;17(8):2009–15.
Filicori M, Cognigni GE, Tabarelli C, Pocognoli P, Taraborrelli S, Spettoli D, et al. Stimulation and growth of antral ovarian follicles by selective LH activity administration in women. J Clin Endocrinol Metab. 2002;87(3):1156–61.
Hugues JN. Impact of “LH activity” supplementation on serum progesterone levels during controlled ovarian stimulation: a systematic review. Hum Reprod. 2012;27(1):232–43.
Duffy JMN, Bhattacharya S, Bhattacharya S, Bofill M, Collura B, Curtis C, et al. Standardizing definitions and reporting guidelines for the infertility core outcome set: an international consensus development study. Fertil Steril. 2021;115(1):201–12.
Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod. 2015;30(12):2703–7.
Wilkinson J, Roberts SA, Vail A. Developments in IVF warrant the adoption of new performance indicators for ART clinics, but do not justify the abandonment of patient-centred measures. Hum Reprod. 2017;32(6):1155–9.
Wong PC, Qiao J, Ho C, Ramaraju GA, Wiweko B, Takehara Y, et al. Current opinion on use of luteinizing hormone supplementation in assisted reproduction therapy: an Asian perspective. Reprod Biomed Online. 2011;23(1):81–90.
Acknowledgements
Not applicable
Funding
The study is supported by the Fertility Research Program of Young and Middle-aged Physicians in 2019.
Author information
Authors and Affiliations
Contributions
MW analyzed and interpreted the patient data, and was a major contributor to the manuscript. RH, XL, YM, WS collected the patients’ clinical data and contributed to the essay writing. QL designed the study and took part in the result interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study obtained approvals from the Ethics Committees at the Sixth Affiliated Hospital of Sun Yat-sen University (2020ZSLYEC-295), Northwest Women’s and Children’s Hospital (2019013), and Jiangsu Provincial Hospital (2020-SR-046). The written informed consents were waived by the ethics committees because it is a retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary table 1.
Characteristics of oocyte retrieval cycles of normal responders. Supplementary table 2. Characteristics of the fresh embryo transfer cycles of normal responders. Supplementary table 3. Characteristics of the FET cycles of normal responders. Supplementary table 4. Characteristics of the complete cycles of normal responders. Supplementary table 5. Number of oocytes retrieved and embryo assessment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, M., Huang, R., Liang, X. et al. Recombinant LH supplementation improves cumulative live birth rates in the GnRH antagonist protocol: a multicenter retrospective study using a propensity score-matching analysis. Reprod Biol Endocrinol 20, 114 (2022). https://doi.org/10.1186/s12958-022-00985-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-022-00985-4