Summary
Embryo transfer is a crucial step in IVF cycle, with increasing trend during the last decade of transferring a single embryo, preferably at the blastocyst stage. Despite increasing evidence supporting Day 5 blastocyst-stage transfer, the optimal day of embryo transfer remains controversial. The crucial questions are therefore, whether the mechanisms responsible to embryos arrest are embryo aneuploidy or others, and whether those embryos arrested in-vitro between the cleavage to the blastocyst stage would survive in-vivo if transferred on the cleavage-stage. We therefore aim to explore whether aneuploidy can directly contribute to embryo development to the blastocyst stage. Thirty Day-5 embryos, that their Day-3 blastomere biopsy revealed a single-gene defect, were donated by 10 couples undergoing preimplantation genetic testing treatment at our center. Affected high quality Day-3 embryos were cultured to Day-5, and were classified to those that developed to the blastocyst-stage and those that were arrested. Each embryo underwent whole genome amplification. Eighteen (60%) embryos were arrested, did not develop to the blastocyst stage and 12 (40%) have developed to the blastocyst stage. Nineteen embryos (63.3%) were found to be euploid. Of them, 12 (66.6%) were arrested embryos and 7 (58.3%) were those that developed to the blastocyst-stage. These figures were not statistically different (p = 0.644). Our observation demonstrated that the mechanism responsible to embryos arrest in vitro is not embryo aneuploidy, but rather other, such as culture conditions. If further studies will confirm that Day-5 blastocyst transfer might cause losses of embryos that would have been survived in vivo, cleavage-stage embryo transfer would be the preferred timing. This might reduce the cycle cancellations due to failure of embryo to develop to the blastocyst stage and will provide the best cumulative live birth-rate per started cycle.
Similar content being viewed by others
Introduction
Embryo transfer (ET) is a crucial step in IVF cycle, with increasing trend during the last decade of transferring a single embryo, preferably at the blastocyst stage. Despite increasing evidence supporting Day 5 blastocyst-stage transfer, the optimal day of embryo transfer remains controversial [1,2,3,4,5]. It has been suggested that one third to one half of human embryos produced by IVF do not develop to the blastocyst-stage in-vitro [6, 7].
In their Cochrane review on cleavage versus blastocyst stage embryo transfer in assisted reproductive technology (ART), Glujovsky et al. [3] have concluded that although there is an advantage favoring blastocyst transfer in fresh cycles, it remains uncertain whether the day of transfer impacts on cumulative live birth and pregnancy rates. Studies that favor Day 5 blastocyst-stage transfer usually report on outcomes per embryo transfer, rather than per cycle. While blastocyst transfer result in higher implantation rate, cleavage-stage transfer is associated with higher numbers of embryos available for freezing, and lower cancellation rate due to no embryos available for transfer [8]. Therefore, since developmental arrest during culture to the blastocyst stage might impair live birth rate, comparison should be analyzed with reference point cycle start, and not, with embryo transfer.
Culturing cleavage-stage embryo on Day 3 to the blastocyst-stage will select the developing, while discarding the non-viable embryos. Embryos usually arrest for various reasons, such as culture conditions, poor metabolism or DNA damage [9]. Whether aneuploidy can directly contribute to embryo development to the blastocyst stage remains unclear.
For better understanding of the mechanisms of embryo development, and whether aneuploidy can directly contribute to embryo development to the blastocyst stage, Qi et al. [10] examined the chromosome integrity of blastocysts and arrested embryos that did not develop to the blastocyst stage, from patients undergoing IVF and preimplantation genetic testing for aneuploidy (PGT-A). Most of arrested embryos were aneuploid (44/45), compared to 32/45 of the developing embryos (blastocysts) (32/45, P < 0.01). Both euploid (92.9%) and aneuploid (42.1%) embryos developed to blastocyst- stage.
The crucial questions are therefore, whether the mechanisms responsible to embryos arrest are culture conditions or embryo aneuploidy, and whether those embryos arrested in-vitro between the cleavage to the blastocyst stage would survive in-vivo if transferred on the cleavage-stage? Aiming to shed more light on these questions, we assessed the ploidy of top quality cleavage-stage embryos that were cultured to day-5, either arrested at the cleavage-stage or developed to the blastocyst-stage.
Patients and methods
Day-5 embryos, in which their Day-3 blastomere biopsy revealed a single-gene defect, were donated by couples undergoing PGT treatment at our center, from May 2021 to September 2021. All embryos were of high quality before the Day-3 biopsy, e.g. 7–8 equal blastomeres with no fragmentations. Affected embryos were cultured to Day-5 in the same condition, and were classified to those that developed to the blastocyst-stage and those that were arrested. Each embryo underwent whole genome amplification (WGA).
"WGA
Full-genomic amplification of the DNA was carried out by WGA-PCR PicoPlex SingleCell WGA Kit (Rubicon Genomics) [Takarabio. GenetiSure Pre-Screen Complete Protocol. Available from: https://www.takarabio.com/assets/documents/User%20Manual/ PicoPLEX%20WGA%20 Kit%20Protocol-At-A-Glance-070717.pdf.]. The quality and quantity of DNA received during amplification were controlled by electrophoresis using 1% agarose gel.
Array-CGH
WGA products were processed referring to the protocol of Agilent oligonucleotide array-based CGH for single cell G4410–90,003 Revision B0, October 2018. These products were fluorescently labelled with controls (Human Reference DNA Female/Male) according to the instructions of SureTag Complete DNA Labeling Kit (Agilent technologies, CA, USA), and then competitively hybridized to G9500A GenetiSure Pre-Screen Complete kit (8 × 60) Agilent technologies, CA, USA) [Agilent. Oligo aCGH/ChIP-Chip Hybridization Kit Protocol. Available from: https://www.agilent.com/en/product/cgh-cgh-snp-microarray-platform/cghcgh-snp-microarray-kits-reagents/oligo-acgh-chip-on-chip-hybridizationkit-228433]".
Interpretation of array-CGH results
Data analysis was accomplished according to the manufacturer recommended single cell analysis method. We only reported whole chromosome monosomies or trisomies. The operator of the molecular analysis (AJG) was blinded to the samples’ sources.
Informed consent was obtained from all patients before participation in the study, and the study was approved by our Institutional Clinical Research Committee (IRB SMC-19–6140). The study required no modification of patient’s routine follow-up or treatment.
Results
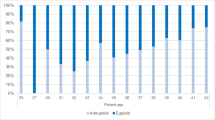
Ten women (age 31.7 ± 3.4 yrs, range 27–39 yrs) have donated 30 high-quality Day-3 embryos, which were found to be affected following Day-3 blastomere biopsy, and were cultured to the balastocyst-stage. Of which, 18 (60%) were arrested, did not develop to the blastocyst stage and 12 (40%) have developed to the blastocyst stage. Nineteen embryos (63.3%) were found to be euploid. Of them, 12 (66.6%) were arrested embryos and 7 (58.3%) were those that developed to the blastocyst-stage. These figures were not statistically different (p = 0.644). The CGH results of the embryos are presented in Table 1.
Discussion
In the present study of extended embryo culture, only 40% of good quality Day-3 embryos developed to the blastocyst stage. Figure that is in accordance with the reported figures in young patient [11]. Moreover, 11 embryos (63.3%) were found to be euploid. Again, figure that is in agreement with both the STAR study [12] of patients' age 33.7 ± 3.59yrs, and the study by Yan et al. [13] of patients' age 29.1 ± 3.6 yrs, with euploidy rate of good-quality blastocysts of 43.1%, and 69.8%, respectively.
One of the interesting observation obtained in the present study is the comparable (p = 0.644) euploidy rates between arrested embryos (66%) and those that developed to the blastocyst-stage (58%). These figures are reassuring and differ from those demonstrated by Qi et al. [10]. In the later, only 15.5% of all embryos were euploid, and 97.8% and 71.1% of the arrested embryos and the developing embryos (blastocysts) were aneuploid, respectively. These higher rates were explained and related to small sample size, and the delay between stopping to develop (Day 3) and the biopsy of the arrested embryos (Day 6), which might be the culprit of the DNA degeneration or damages. In the present study, euploidy rate in all the embryos and in the different subgroups (arrested and developing embryos) did not differ from those reported in the literature [12, 13] in young patients, ranging between 43.1% and 69.8%. We might therefore speculate that the cause of developmental arrest is not the embryo genetic integrity, but rather other reasons, such as the culture conditions. Moreover, since in the present study most embryos failing to progress to the blastocyst stage were chromosomally normal, we believe that in different conditions, they would progress to the blastocyst- stage and result in a live birth if transferred on Day-3.
Reinforcement to the aforementioned speculation might be found in the study by Xiao et al. [14], who uniquely examined the pregnancy rate per embryo in patients with only one surviving embryo. Day 3 embryo transfer resulted in significantly higher clinical pregnancy and live birth rates, when both adjusted and unadjusted for embryo grading. Notwithstanding that this advantage was observed despite the lower-quality of embryos transferred at the cleavage-stage, as compared to those cultured and planned to be transferred at the blastocyst-stage. It might be therefore concluded, that cleavage-stage embryos that arrest during in-vitro culturing to the blastocyst-stage, might still have the ability to implant if transferred on Day-3. It might be therefore possible that the developmental arrest of cleavage-stage embryos in vivo is lower than their attrition in vitro, and that blastocyst transfer leads to the loss of embryos that may have survived in vivo. It should be emphasized, that culturing cleavage-embryos to the blastocyst-stage is a selection measure, not treatment.
While the reported prevalence of mosaicism in human cleavage- and blastocyst-stage embryos, based on PGT-A, ranges from 50% to up to 90% [15], there is substantial evidence that an embryo’s chromosome complement might change throughout preimplantation development [16], with lower levels of mosaicism in blastocyst-stage, as compared to Day 3 embryos [17]. Moreover, "mosaic" embryos demonstrate increased cell proliferation and cell death in comparison to euploid embryos, observations suggestive of significant self-correction abilities of embryos [18, 19]. At the same time, Shahbazi et al. [20] have recently characterized the development of embryos with different specific aneuploidies up to day 9 and uncovered tissue-specific alterations. While some aneuploidies developed similarly to euploid embryos, others exhibited high rates of developmental arrest, endorsing the genetic plasticity that exists at preimplantation stages in human embryos and the possibility that, while chromosomal abnormalities do not appear to play a significant role in embryo arrest, some cases of embryos arrest due to chromosomal abnormalities cannot be ruled out.
The limitations of our study is the small sample size of affected embryos donated for the study, which are strongly influenced by the ethical concerns.
Conclusion
We might be therefore concluded, that in good prognosis patients (young patients with optimal ovarian response and high number and quality of embryos [11], transferring a fresh blastocyst might shorten the time to pregnancy, compared with cleavage-stage embryo transfer, probably without increasing the cumulative live-birth rate per started cycle [3]. Moreover, in unselected patients, or those with sub-optimal or poor ovarian response, cleavage-stage embryo transfer will reduce the incidence of cycle cancellation due to failure of embryo development to the blastocyst stage and will provide the best cumulative live birth-rate per started cycle. At our center, transferring embryos at the blastocyst-stage is offered to patients with > 8 Day-3 cleavage stage embryos. Further large well-designed studies are required to validate our observation.
Availability of data and materials
Not applicable.
References
Blake DA, Proctor M, Johnson NP. The merits of blastocyst versus cleavage stage embryo transfer: a Cochrane review. Hum Reprod. 2004;19:2174.
Glujovsky D, Farquhar C. Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril. 2016;106:244–50.
Glujovsky D, Farquhar C, QuinteiroRetamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;30(16):CD002118.
Levron J, Shulman A, Bider D, Seidman D, Levin T, Dor J. A prospective randomized study comparing day 3 with blastocyst-stage embryo transfer. Fertil Steril. 2002;77:1300–1.
Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C. Blastocyst versus cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol. 2017;49:583–91.
Ruangvutilert P, Delhanty JD, Serhal P, Simopoulou M, Rodeck CH. FISH analysis on day 5 post-insemination of human arrested and blastocyst stage embryos. Prenat Diagn. 2000;20:552–60.
Gardner DK, Vella P, Lane M, Wagley L, Schlenker T. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–8.
Glujovsky D, Farquhar C. Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril. 2016;106(2):244–50.
Jurisicova A, Acton BM. Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reprod. 2004;128:281–91.
Qi ST, Liang LF, Xian YX, Liu JQ, Wang W. Arrested human embryos are more likely to have abnormal chromosomes than developing embryos from women of advanced maternal age. J Ovarian Res. 2014;13(7):65.
Goldman RH, Racowsky C, Farland LV, Munné S, Ribustello L, Fox JH. Predicting the likelihood of live birth for elective oocyte cryopreservation: a counseling tool for physicians and patients. Hum Reprod. 2017;32(4):853–9.
Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M, Wells D, Gordon T, Stock-Myer S, Willman S, STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071–9.
Yan J, Qin Y, Zhao H, Sun Y, Gong F, et al. Live birt with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047–58.
Xiao JS, Healey M, Talmor A, Vollenhoven B. When only one embryo is available, is it better to transfer on day 3 or to grow on? Reprod Biomed Online. 2019;39(6):916–23.
Taylor TH, Gitlin SA, Patrick JL, et al. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20:571–81.
Barbash-Hazan S, Frumkin T, Malcov M, Yaron Y, Cohen T, Azem F, Amit A, Ben-Yosef D. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil Steril. 2009;92(3):890–6.
Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, Griffin DK, Wells D, PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703.
Victor AR, Griffin DK, Brake AJ, Tyndall JC, Murphy AE, Lepkowsky LT, Lal A, Zouves CG, Barnes FL, McCoy RC, Viotti M. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum Reprod. 2019;34(1):181–92.
Orvieto R, Shimon C, Rienstein S, Jonish-Grossman A, Shani H, Aizer A. Do human embryos have the ability of self-correction? Reprod Biol Endocrinol. 2020;18(1):982013 (100(6):1695-703).
Shahbazi MN, Wang T, Tao X, Weatherbee BAT, Sun L, Zhan Y, Keller L, Smith GD, Pellicer A, Scott RT, Seli E, Zernicka-Goetz M. Developmental potential of aneuploid human embryos cultured beyond implantation. Nat Commun. 2020;11(1):3987.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
R.O. Designed the study, wrote the first paper draft, edited it, proof read the paper and took part in discussions regarding the results. A.J.G. conducted the WGA experiments, proof read the paper and took part in discussions regarding the WGA interpretations. S.A.M., M.N.H. and O.D.S. Proof read the paper and took part in discussions regarding the results. A.A. Participated in designing the study, conducted the embryological work and retrieved the data, proof read the paper and took part in discussions regarding the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by our Institutional Clinical Research Committee (IRB SMC-19–6140).
Consent for publication
Informed consent was obtained from all patients before participation in the study.
Competing interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Orvieto, R., Jonish-Grossman, A., Maydan, S.A. et al. Cleavage-stage human embryo arrest, is it embryo genetic composition or others?. Reprod Biol Endocrinol 20, 52 (2022). https://doi.org/10.1186/s12958-022-00925-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-022-00925-2