Abstract
Background
Miscarriage is a common complication in pregnancy and there is still a lack of biomarkers usable in asymptomatic patients before the event occurs. Periostin (PER), whose levels rise particularly during injury or inflammation, has been shown to play an important local role in implantation and early embryonic development. As PER has been described as a biomarker in various medical conditions we intended to evaluate if changes in PER serum levels may help to identify women at risk for spontaneous abortion in the first trimester.
Methods
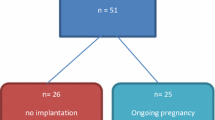
Women between 18 and 42 years without confounding comorbidities who conceived by IVF/ICSI and ovarian hyperstimulation were analysed in the study after informed consent. Maternal serum samples from 41 patients were assessed at the time of pregnancy testing (PT) and the following first ultrasound checkup (US). Patients were subsequently divided in two groups: (1) patients with subsequent miscarriage in the first trimester (n = 18) and (2) patients with ongoing pregnancy (n = 23), allowing for statistical analysis and investigating the change of PER levels per individual. PER levels were measured using enzyme-linked immunosorbent assay. Statistical analysis was performed using the Fisher exact and Student’s t test. p ≤ 0.05 was considered to be significant.
Results
There was no significant difference concerning possible confounders between the two groups. We did not find any significant difference in PER levels at the time point of PT or US. By investigating the interindividual changes of PER between the two time points however, we observed that patients with a following miscarriage showed increasing levels of PER at the time point of PT compared to US in contrast to patients with an ongoing pregnancy who demonstrated a decrease in PER levels. These alterations were significant in the absolute as well as in the relative comparison.
Conclusion
The relative expression of PER between PT and US is significantly altered in asymptomatic women with subsequent miscarriage compared to women with ongoing pregnancy. Therefore systemic PER levels might represent a potential promising biomarker for the assessment of pregnancy outcome.
Trial registration
Not applicable.
Similar content being viewed by others
Background
Despite the lack of consistent definitions, miscarriage (spontaneous abortion) is often defined as intrauterine pregnancy demise confirmed by ultrasound before the 24th week of gestation [1]. Miscarriage is a common complication in pregnancy and affects about 8 to 20% of clinically recognized pregnancies in the first 20 weeks of gestation [2]. Among others, the causes of early pregnancy loss are various, including cytogenetic abnormalities [3] as well as placental development, advanced maternal age [4], previous miscarriage [5], body mass index before pregnancy less than 18.5 or above 25 kg/m2 [6] as well as heavy maternal smoking [7].
As the fetus represents a semi-allograft, immune mechanisms are discussed as contributing factors.
Periostin (PER) is a 90 kDa extracellular protein which is expressed in multiple compartments of the body, especially in aorta, stomach, lower gastrointestinal tract, placenta and uterus [8]. Recent studies have been published evaluating its role in skin, bone, kidney, heart, lung, blood vessel, and allergic reactions, as well as cancer [8,9,10]. Levels rise particularly during injury or inflammation reactions. Furthermore, PER is a ligand for ανβ3- and ανβ 5-integrins, proteins relevant in implantation [11], and promotes cell motility [12, 13]. Its expression is triggered by transforming growth factor (TGF)-b, IL-4, and IL-13 [14, 15].
Of note many mechanisms of embryo implantation have first been described in cancer and metastasis. In oncology PER plays a crucial role especially in metastasis where it has been shown to increase Wnt signaling, a pathway that is also involved in embryo implantation [16] and in cancer stem cells [17, 18].
PER seems to be essential for metastatic cell maintenance and blocking PER results in metastasis prevention [19]. Another possible pathway of PER in facilitating metastasis is the formation of the immunosuppressive premetastatic niche formation [20].
In order to evaluate the regulation of PER during early pregnancy Anh et al. showed that periostin gene expression in the bovine endometrium is triggered by progesterone, while interferon tau seems to lower local PER levels [21]. On the other hand, Hiroi et al. describe that PER-levels are increased during the proliferative phase and decrease in the second half of menstrual cycle [22].
In addition, PER plays an important role in embryo development as it was shown to be present at amniotic membranes and to be higher expressed in neonatal umbilical cord than in adult serum [23,24,25]. Furthermore, an important pathway during early pregnancy, Wnt-pathway,is targeted by PER [26].
Morelli et al. demonstrated in a case-control-study that PER mRNA and protein levels were lower in decidual and trophoblastic tissues from women with miscarriages in late first trimester compared to women with intact pregnancies, who underwent abortion induction. They concluded therefore that PER represents a marker of a viable pregnancy in the first trimester [18]. However, since this analysis included patients at 12 weeks of gestation when the miscarriage already had occurred and tissue was examined rather than female serum, these results do not allow prognostic conclusions for the outcome of the pregnancy during the first trimester.
As PER has been described as a biomarker in various medical conditions [14, 27, 28] we evaluated if changes in PER serum levels may help identify women at risk for spontaneous abortion in the first trimester.
Methods
After informed consent, 41 pregnant women in the study who had undergone ovarian hyperstimulation for in vitro fertilization with or without intracytoplasmic sperm injection were included. Inclusion criteria were: age between 18 and 42 years, pregnancy after ovarian hyperstimulation followed by embryo transfer. Women with possible confounding comorbidities (autoimmune diseases, essential hypertonia, diabetes mellitus or the intake of confounding medication) or a history of recurrent miscarriage or implantation failure were excluded.
The study was approved by the Ethical Committee of Heidelberg University (protocol S-243/2015). Maternal serum samples were obtained at the time of pregnancy testing (PT) and the following first ultrasound checkup (US) after approximately 10 days (9.9 ± 1.5 (group 1) days vs. 10.7 ± 2.1 (group 2) days) for the measurement of PER. Pregnancies were adequately developed with normal HCG rise at the time of the first US. Patients with a positive pregnancy test were subsequently divided in two groups: (1) patients with subsequent miscarriage in the first trimester (missed abortion, incomplete spontaneous abortion or complete spontaneous abortion, n = 18) later in pregnancy and (2) patients with ongoing pregnancy (n = 23).
ELISA assays
PER levels were analysed using the AdipoGen® Elisa kit for Human Periostin, catalog no. AG-45B-004-KI01, Lot K2321605, Adipogen International, Liestal, Switzerland. Serum samples were obtained and centrifuged for 20 min, 1200 x g, 10 °C. ELISA reagent (Wash Buffer, ELISA buffer, Detection Antibody, HRP labeled Streptavidin and Human Periostin standard) preparations were performed as described by the manufacturer.
Standard curve was prepared as described by the manufacturer, obtaining concentrations of 5000 pg/ml, 2500 pg/ml, 1250 pg/ml, 625 pg/ml, 312 pg/ml, 156 pg/ml, 78 pg/ml and 0 pg/ml). Samples were diluted 1/50 in ELISA buffer, as recommended.
100 μl of different standards and 100 μl of diluted serum were added to each well of the prepared microplate in duplicates. After incubation (2 h at 37 °C), wells were aspirated and washed. 100 μl of diluted Detection Antibody was added to each well, followed by incubation for 1 h at 37 °C. Again, supernatant was aspirated and the coated wells were washed. Afterwards 100 μl of diluted HRP labeled Streptavidin was added. After incubation for 30 min at room temperature, supernatant was aspirated and wells were washed, before 100 μl of TMB substrate solution were overlayed. Color reaction was allowed to develop in the dark for 15 min before 50 μl of stop solution were added.
Optical density of each well was measured using a microplate reader set to 450 nm with wave length correction of 570 nm.
Statistical analysis
Statistical analysis was performed using the Kolmogorov–Smirnov-test, the Fisher-exact-test and unpaired Student’s t test. A p-value ≤ 0.05 was considered to be significant.
Results
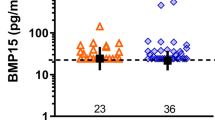
Descriptive data of patients are demonstrated in Table 1. There was no significant difference in age (34.2 ± 4.0 (group 1) years vs. 33.0 ± 6.3 (group 2) years) or body mass index in group 1 and 2 with 25.8 ± 5.1 kg/m2 in group 1 vs. 24.4 ± 4.4 kg/m2 in group 2. Embryo quality was similar in both groups. We grouped the embryos based on “The Istanbul consensus workshop on embryo assessment” [29] and had a transfer of poor embryos in 3 of 18 patients with miscarriage (16.7%) and in 8 of 23 patients with ongoing pregnancy (34.8%). Fishers exact-test revealed a p-value of 0.29 (Table 2). Additionally, no statistically significant difference in day of transfer with 3.8 ± 1.3 in group 1 vs. 4.5 ± 1.0 in group 2 (Table 2) or in time between pregnancy test and ultrasound control with 9.9 ± 1.5 days in group 1 vs. 10.7 ± 2.1 days in group 2 (Table 1) was observed. All values are given in mean ± STD.
We obtained samples from both time points in 10 of 18 patients in group 1 (55.6%) and in 17 of 23 patients in group 2 (73.9%), allowing to investigate if there were any temporal or relative changes between pregnancy test and the time of the ultrasound control between the two groups.
Absolute and relative concentrations of PER by Kolmogorov–Smirnov-test revealed a normal distribution pattern.
We did not observe any significant difference in PER levels at the time point of pregnancy testing or the time point of ultrasound control (Table 3).
However, by investigating the temporal changes of PER levels within the two groups, we observed that patients with a miscarriage showed increasing levels of PER at the time point of PT compared to US (+3622.5 ± 1768.47 pg/ml) in contrast to patients with an ongoing pregnancy who showed a decrease in PER levels (− 1750.29 ± 1461.51 pg/ml, p < 0.05) (Table 4).
Based on this observation, we built the ratio of PER expression at the time point of first ultrasound relative to pregnancy testing. The relative expression of PER was significantly higher in patients with miscarriage compared to control patients with ongoing pregnancy (1.24 ± 0.12 vs. 0.97 ± 0.06, p < 0.05).
Ratio of hCG-levels between the 6. week of gestation and the time of pregnancy testing did not differ significantly between the two groups (53.46 ± 54.01 in patients with miscarriage vs. 67.79 ± 54.37 in women with ongoing pregnancy, p = 0.52).
Discussion
This study is the first study, to the best of our knowledge, evaluating systemic levels of PER in the first weeks of pregnancy in order to investigate the differences between women who are going to abort compared to ongoing pregnancy.
As ultrasound cannot determine pregnancy progress, different hormone assessments have been published in order to help predict pregnancy outcome, such as human chorionic gonadotropin (hCG) [30, 31], progesterone [30, 31], kisspeptin [32], activin A [33], activin B [34], follistatin [35], CA-125 [31, 36], pregnancy associated plasma protein A (PAPP-A) [31, 37] or macrophage inhibitory cytokine-1 [38]. However, none of these factors have been established as a biomarker in early pregnancy for miscarriage.
The most appropriate time point to evaluate the risk of miscarriage would be already at the time of pregnancy testing. However, we could not identify any significant changes in PER expression at the time of pregnancy testing. The next possible time point in routine practical use to evaluate a possible screening biomarker for miscarriage is the time point of ultrasound control in the 6th week of gestation. Again, we could not identify significant changes between the two groups. This observation may be due to high interindividual variations and the small sample size, however the intraindividual variation seems to be low, as the sequential values show significant changes over time. Therefore we aimed to investigate if there were any intraindividual alterations in the temporal expression to differentiate between the two groups. The relative expression of PER over time was significantly higher in patients with miscarriage compared to control patients with ongoing pregnancy. The fact that PER is overexpressed in different diseases characterized by inflammation [8] can represent a possible explanation for our observations. The important role of PER in embryo invasion has been described as well as local PER expression at 12 weeks of gestation when miscarriage has already occurred [18, 23]. However, none of these studies evaluated systemically serum levels. Additionally, our study assesses PER levels in an earlier period of gestation.
Intraindividual relative expression, which eliminates the risk of confounding comorbidities, of PER over time represents a new possibly independent tool in risk assessment. However one of the limitations of our study is its small sample size and the fact that not all participations presented themselves in the 6. week of gestation, further minimizing our sample size, therefore our results need further validation in larger study cohorts in order to define a precise threshold at this very early stage in pregnancy to develop a predictive test.
Conclusion
In conclusion, we have shown for the first time that the relative expression of PER between 4 weeks of gestation and control ultrasound in the 6th week of gestation is significantly altered in asymptomatic women with subsequent miscarriage compared to women with ongoing pregnancy, therefore suggesting systemic PER levels as a potential promising biomarker for pregnancy outcome assessment. The development of an early-screening test to identify patients who are at risk of miscarriage in the actual pregnancy would be useful for several reasons: a miscarriage means an enormous distress for the patient and a predictive test with a negative result could be used to reassure anxious patients [31, 32, 36, 37, 39, 40]. On the other hand, a predictive test with a positive result can warn the patients in a very early stage of pregnancy [39], and will prohibit unnecessary prolongation of the current pregnancy by supplementation of high doses of progesterone, as often done in women after ART.
Abbreviations
- ART:
-
Assisted Reproductive Technology
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- PER:
-
Periostin
- PT:
-
Pregnancy testing
- US:
-
Ultrasound checkup
References
Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11.
Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):839-54.
van den Berg MMJ, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. BBA. Elsevier B.V. 2012;1822:1951–9.
Andersen AN, Wolfarth J, Christens P, Olsen J, Medbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000:1708–12.
Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ. 1989;299(6698):541–5. https://www.ncbi.nlm.nih.gov/pubmed/2507063.
Balsells M, García-Patterson A, Corcoy R. Systematic review and meta-analysis on the association of prepregnancy underweight and miscarriage. Eur J Obstet Gynecol. Elsevier Ireland Ltd. 2016;207:73–9.
Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 2000;340:333–9.
Idolazzi L, Ridolo E, Fassio A, Gatti D, Montagni M, Caminati M, et al. Periostin: the bone and beyond. Eur J Intern Med. European Federation of Internal Medicine. 2017;38:12–6.
Izuhara K, Arima K, Ohta S, Suzuki S, Yamamoto K-I, Inamitsu M. Periostin in allergic inflammation. Allergol Int. Elsevier Masson SAS. 2014;63:143–51.
Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–75.
Germeyer A, Savaris RF, Jauckus J, Lessey B. Endometrial beta3 integrin profile reflects endometrial receptivity defects in women with unexplained recurrent pregnancy loss. Reprod Biol Endocrinol. 2014;12:53. doi:10.1186/1477-7827-12–53.
Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707–18.
Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62(18):5358–64. https://www.ncbi.nlm.nih.gov/pubmed/?term=Periostin+secreted+by+epithelial+ovarian+carcinoma+is+a+Ligand+for.
Izuhara K, Matsumoto H, Ohta S, Ono J, Arima K, Ogawa M. Recent developments regarding periostin in bronchial asthma. Allergol Int. Elsevier B.V. 2015;64:S3–S10.
Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CTJ, Matthews JG, et al. Roles of Periostin in respiratory disorders. Am J Respir Crit Care Med. 2016;193:949–56.
Tepekoy F, Akkoyunlu G, Demir R. The role of Wnt signaling members in the uterus and embryo during pre-implantation and implantation. J Assist Reprod Genet. 2014;32:337–46.
Ng YH, Zhu H, Leung PCK. Twist modulates human Trophoblastic cell invasion via regulation of N-Cadherin. Endocrinology. 2012;153:925–36.
Morelli M, Misaggi R, Di Cello A, Zuccalà V, Costanzo F, Zullo F, et al. Tissue expression and serum levels of periostin during pregnancy: a new biomarker of embryo–endometrial cross talk at implantation. Eur J Obstet Gynecol. Elsevier Ireland Ltd; 2014;175:140–144.
Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr H-A, Delaloye J-F, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. Nature Publishing Group. 2011;481:85–9.
Wang Z, Xiong S, Mao Y, Chen M, Ma X, Zhou X, et al. Periostin promotes immunosuppressive premetastatic niche formation to facilitate breast tumour metastasis. J Pathol. 2016;239:484–95.
Ahn HW, Farmer JL, Bazer FW, Spencer TE. Progesterone and interferon tau-regulated genes in the ovine uterine endometrium: identification of periostin as a potential mediator of conceptus elongation. Reproduction. 2009;138:813–25.
Hiroi H, Momoeda M, Nakazawa F, Koizumi M, Tsutsumi R, Hosokawa Y, et al. Expression and regulation of Periostin/OSF-2 gene in rat uterus and human Endometrium. Endocr J. 2008;55:183–9.
Zhu S, Barbe MF, Amin N, Rani S, Popoff SN, Safadi FF, et al. Immunolocalization of Periostin-like factor and Periostin during embryogenesis. J Histochem Cytochem. 2008;56:329–45.
Dobreva MP, Lhoest L, Pereira PNG, Umans L, Chuva de Sousa Lopes SM, Zwijsen A. Periostin as a biomarker of the amniotic membrane. Stem Cells Int. 2012;2012:1–10.
Song H-J, Zhang P, Guo X-J, Liao L-M, Zhou Z-M, Sha J-H, et al. The proteomic analysis of human neonatal umbilical cord serum by mass spectrometry. Acta Pharmacol Sin. Nature Publishing Group. 2009:1550–8.
Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol. 2010;338:127–35.
Nagasaki T, Matsumoto H, Izuhara K. Utility of serum periostin in combination with exhaled nitric oxide in the management of asthma. KiHAC Respiratory Medicine Group. Allergol Int. 2017;66(3):404–410. doi:10.1016/j.alit.2017.02.003, https://www.ncbi.nlm.nih.gov/pubmed/28256388. Epub 2017 Feb 28.
Quaresima B, Crugliano T, Gaspari M, Faniello MC, Cosimo P, Valanzano R, et al. A proteomics approach to identify changes in protein profiles in serum of familial Adenomatous polyposis patients. Cancer Lett. Elsevier Ireland Ltd. 2008;272:40–52.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83.
Duan L, Yan D, Zeng W, Yang X, Wei Q. Predictive power progesterone combined with beta human chorionic gonadotropin measurements in the outcome of threatened miscarriage. Arch Gynecol Obstet. 2010;283:431–5.
Pillai RN1, Konje JC2, Tincello DG1, Potdar N3. Role of serum biomarkers in the prediction of outcome in women with threatened miscarriage: a systematic review and diagnostic accuracy meta-analysis. Hum Reprod Update. 2016;22(2):228–39.
Jayasena CN, Abbara A, Izzi-Engbeaya C, Comninos AN, Harvey RA, Gonzalez Maffe J, et al. Reduced levels of plasma Kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J Clin Endocrinol Metabol. 2014;99:E2652–60.
Warrick J, Gronowski A, Moffett C, Zhao Q, Bishop E, Woodworth A. Serum activin a does not predict ectopic pregnancy as a single measurement test, alone or as part of a multi-marker panel including progesterone and hCG. Clin Chim Acta. Elsevier B.V. 2012;413:707–11.
Dhiman P, Senthilkumar GP, Rajendiran S, Sivaraman K, Soundararaghavan S, Kulandhasamy M. Serum activin B concentration as predictive biomarker for ectopic pregnancy. Clin Biochem. Elsevier B.V. 2016;49:609–12.
Daponte A, Deligeoroglou E, Garas A, Pournaras S, Hadjichristodoulou C, Messinis IE. Activin a and Follistatin as biomarkers for ectopic pregnancy and missed abortion. Dis Markers. 2013;35:497–503.
Al Mohamady M, Fattah GA, Elkattan E, Bayoumy R, Hamed D. Correlation of serum CA-125 and progesterone levels with ultrasound markers in the prediction of pregnancy outcome in threatened miscarriage. Int J Fertil Steril. 2016;9:506–11.
Hanita O, Roslina O, Nor Azlin M. ORIGINAL ARTICLEMaternal level of pregnancy-associated plasma protein a as a predictor of pregnancy failure in threatened abortion. Malaysian J Pathol. 2012;34:145–51.
Tong S, Ngian G-L, Onwude JL, Permezel M, Saglam B, Hay S, et al. Diagnostic accuracy of maternal serum macrophage inhibitory Cytokine-1 and pregnancy-associated plasma protein-a at 6–10 weeks of gestation to predict miscarriage. Obstet Gynecol. 2012;119:1000–8.
Hannan NJ, Bambang K, Kaitu’u-Lino TJ, Konje JC, Tong S. A Bioplex analysis of cytokines and chemokines in first trimester maternal plasma to screen for predictors of miscarriage. Proost P, editor. PLoS One. 2014;9:e93320–7.
Barnhart KT, Guo W, Cary MS, Morse CB, Chung K, Takacs P, et al. Differences in serum human chorionic Gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504–11.
Acknowledgements
We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing.
Funding
This project was supported by the FRONTIER innovation funding, University of Heidelberg.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
AF, TS and AG designed the study. AF, JS, RJK and JJ conducted the sample collection and compiled the data. AF, JJ and AD analyzed and interpreted Periostin levels using ELISA. AF and AG performed statistical analysis and AF, RJK, TS and AG generated the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of the Heidelberg University (protocol S-243/2015). All patients included gave their written agreement after informed consent.
Consent for publication
Written informed consent for publication was obtained.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Freis, A., Schlegel, J., Kuon, R.J. et al. Serum periostin levels in early in pregnancy are significantly altered in women with miscarriage. Reprod Biol Endocrinol 15, 87 (2017). https://doi.org/10.1186/s12958-017-0307-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-017-0307-9