Abstract
Objective
To investigate the relationship between the expression of androgen receptor (AR) and clinical characteristics in breast cancer.
Patients and methods
The clinical records of all 432 patients tested for AR in our institution between January 2020 and May 2023 were reviewed. Clinical characteristics, age, menopausal status, tumor node metastasis (TNM) stage, distant metastasis, pathological complete response (pCR), histopathological features histological grade, estrogen receptor (ER), progesterone receptor, Her-2, Ki-67, and molecular subtype were registered for all patients.
Results
About 377 (87.27%) of the 432 patients had AR expression.
No significant difference in AR expression was found with age, menopausal status, TNM stage of primary tumor, or pCR. AR was positively and significantly associated with the histological grade, and recurrence. The AR expression was significantly related with molecular subtypes, including ER, PR Her-2, Ki67 and molecular subtype. ER (OR = 10.489, 95%CI: 5.470–21.569), PR (OR = 7.690, 95%CI: 3.974–16.129, Her-2 (OR = 10.489, 95%CI: 2.779–23.490 and tumor recurrence (OR = 0.110, 95%CI: 0.031–0.377 were significant independent risk factors affecting AR expression.
Conclusions
AR expression can serve as a reliable basis for judging the clinical molecular types and poor prognosis for breast cancer. AR may be a novel biomarker and target in AR-positive breast cancer depending on significant difference in AR expression among different molecular types of breast cancer.
Similar content being viewed by others
Introduction
Breast cancer is the most common malignancy and the first leading cause of cancer-related death in women worldwide. It is highly heterogeneous at molecular and clinical levels [1]. The main breast cancer subtypes include luminal A, Luminal B, Her-2-enriched and basal-like type, and normal-like type, depending on molecular profiles and several biomarkers [2]. Estrogen receptor (ER) and progesterone receptor (PR) as sex steroid hormone receptors are important biological markers for prognosis prediction in breast cancer. Androgen receptor (AR), another sex steroid hormone receptor family, is expressed in 70%-90% of breast cancer patients. The AR expression varies among breast cancer subtypes, and accounts for a large proportion in ER-positive tumors [3]. AR is a ligand-activated transcription factor. Increasing research shows AR played a dominant role in the development of breast cancer. AR can reportedly accelerate cell proliferation of ER-negative breast cancer and triple-negative breast cancer (TNBC) [4,5,6]. The main reason is that AR competes with ER for binding to androgen-responsive elements, leading to tumor cell growth [2]. The synergy between Her-2 and AR is reinforced by a positive feedback loop mechanism, which promotes Her-2 transcriptional upregulation and then activates related downstream pathways, accelerating AR-positive tumor growth [3, 7]. Additionally, AR promotes tamoxifen resistance probably by regulating cyclin D1 expression and promoting cell cycle progression [8]. However, some studies show serum androgen is positively associated with breast cancer risk whether in premenopausal or postmenopausal women [9,10,11,12]. Moreover, high AR expression is correlated with better disease-free survival (DFS) and overall survival (OS) [13]. Therefore, the existing views concerning the prognostic value of AR in different breast cancer subtypes are controversial.
Although some research shows AR-target drugs are effective for AR-positive breast cancer, the patients who exactly benefit from AR-target therapies remain uncertain. The aim of this study was to investigate the clinical characteristics of AR in breast cancer, and to evaluate the prognostic value and provide a therapeutic tool for breast cancer.
Patients and methods
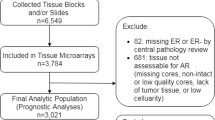
Totally 432 breast cancer patients receiving surgical resection at the First Affiliated Hospital of Nanjing Medical University between January 2020 and May 2023 were enrolled This study was approved by Ethics Committee of the Hospital (Ethics code 2022-SR-473).
Immunohistochemistry(IHC)
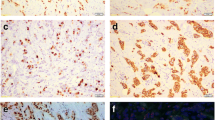
For IHC assay, the breast cancer tissue slides were incubated with the indicated primary antibodies (anti-AR, Clone number: EP120; ZSGB-BIO) overnight at 4℃. And then chromogenic detection was performed through a DBA detection kit (Kit-2031, Maixin, China). Data were obtained and analyzed by two experienced doctors of the pathology department of our hospital. and AR scores were presented as levels (0, none; 1 + , weak; 2 + , moderate; 3 + , strong) and the percentage of stained nuclei and divided into five groups (0: < 5%; 1:6%-25%; 2:26%-50%; 3:51%-75%; and 4–75%). Finally, the score was expressed as (staining intensity × percentage of positively stained cells).
Criteria for pathological results
The ER/PR status criterion is that ≥ 1% stained tumor nucleus is positive. The Her-2 status criteriona is that immunohistochemistry (IHC) confirms 3 + or 2 + (Fish confirm IHC) is positive. Ki67 is expressed in positive cells to calculate the percentage [14]. The 8th edition of Breast cancer TNM Staging Atlas from the American Joint Committee on Cancer (AJCC).
Statistical analysis
Statistical analysis was performed on SPSS 22.0. Associations between AR expression and clinicopathological features were assessed using Chi-square or Student's t-test and analysis of variance (ANOVA). Significant risk factors were used to predict AR expression using a multivariate logistic regression model. p < 0.05 was deemed as significant.
Results
AR expression in breast cancer tissue specimens
Of the 432 cases of ranged from 23 to 81 years (52.15 ± 11.10). Fifty five (12.7%) of the 432 patients were AR-negative and 377 (87.3%) patients were AR-positive (Table 1).
Relationship between AR expression and clinical features
Overall, 87 patients received neoadjuvant chemotherapy, among which 14 patients showed pathological complete response (pCR) (10 AR+ patients, 4 AR patients), and 73 patients were non-pCR (58 AR+ patients, 15 AR patients). In our series, AR expression was not significant in pCR (p = 0.5057, Table 1). Furthermore, no significant correlations were observed between AR expression and age, menopausal status, tumor size, lymph node, distant metastasis or tumor stage (Table 1).
In addition, 11 patients relapsed during follow-up. Our series showed a significant difference in AR expression and poor prognosis (p = 2.503e-05, Table 1). According to histological grade, there were significant differences in AR expression (p = 0.001363, Table 1).
Relationship between AR expression and molecular typing of breast cancer tissues
In all patients, 206 (47.7%) patients belonged to Luminal type, 83 (19.2%) patients were HR+/Her-2+, 54 (12.5%) patients were HR-/Her2+, and 82 (19.0%) patients were TNBC (Table 2). AR expression was related to the presence of ER (p=5.357e-15), PR (p=9.594e-11) and Her-2 (p= 8.408e-05). AR expression was also significantly associated with Ki-67 (p= 0.0458) and histological subtype (p<2.2e-16) (Table 2).
Expression difference of AR in TNBC and non-TNBC tissues
Among the patients, 82 (18.98%) patients were TNBC with 44 AR-positive cases (53.66%), and 350 (95.14%) patients were non-TNBC with 333 AR-positive cases (95.14%). AR expressions were significantly different between the TNBC group and the non-TNBC group (p < 2.2e-16) (Table 3).
Multivariate analysis with significant factors
Multivariate logistic regression confirmed ER, PR and Her-2 as independent predictors for AR expression (p < 0.05). In addition, AR expression was significantly related to tumor recurrence (p < 0.05) (Table 4).
Discussion
Breast cancer is hormone-dependent and routinely examined with ER/PR and AR, ER belong to the nuclear receptor superfamily [15,16,17]. Although AR has the same structure as ER/PR and is more extensively expressed AR is far less understood than ER and PR. We investigated the AR expression in breast cancer patients and correlated it with clinical-pathological characteristics.
We observed AR expression in about 87.3% (n = 377/432) of the cases in our cohort. This large proportion of AR expression is consistent with the literature. Research AR plays an important role in the pathogenesis of breast cancer, and is expressed in more than 70% breast cancer patients [18]. Other studies show the presence of AR in ER-positive breast cancer is correlated with tumor size, and histopathological grading [19, 20]. However, our investigation found AR expression was significantly associated with independent risk factors (e.g., ER, PR, Her-2 and Ki-67), but not with clinical-pathological characteristics (e.g., age, menopausal status, tumor size, lymph node, distant metastasis tumor stage) in breast cancer patients. This result may suggest AR is correlated with the histological subtype, and can be adopted as a potential biomarker.
The AR positive rate in ER-positive breast cancers is 66.9%, which is consistent with the reported rate of 60%-90% [17, 20, 21]. However, some studies indicate AR may act as a tumor suppressor in this sub-type [22, 23]. Meanwhile, AR expression is correlated with ER. One of the most likely mechanisms is that AR can competitively combine estrogen responsive elements to inhibit the transcriptionally active components of ER [22]. In addition, AR can directly bind to p300, a coactivator for competitive binding with ER, and then inhibits the function and downstream signaling pathways of ER [22], thus suppressing tumor growth in luminal breast cancer. Indeed, AR is also regarded as a good prognostic factor. For instance, the presence of AR in ER-positive breast cancer patients shows a better prognostic outcome in terms of DFS and OS [24, 25]. In HR + /Her2-T2N0 breast cancer, AR-positive patients have better DFS and lower risk of recurrence. In addition, AR negativity predicts a worse curative effect for adjuvant chemotherapy and endocrine therapy [13]. We did not discover significant relations between AR expression and time-to-event outcomes, which is because we did not follow up our patients for a long time and we will follow up full life.
AR expression accounts for 60% in Her-2-positive breast cancer patients, but the AR expression rate is low in this sub-type in our work, which may be related to the small sample size. Moreover, AR expression in Her-2-positive breast cancer patients predicts a worse prognosis, which may be involved in mediating Wnt/β catenin and Her-2 signaling pathways [26]. To the delight, we are conducting clinical trials on enzalutamide combined with trastuzumab for Her-2+/AR+ breast cancer patients, and hope to achieve good results.
As we know, TNBC have high heterogeneity and lacks target therapy, such as Her-2 and HR that predict poor prognosis and early metastasis. However, more than 50% of TNBC cases express AR, and more evidences suggest AR to be a solid target. Thike et Al. (2014) found AR-positive TNBC was related to better DFS [27]. Howeverother studies present opposite results. For instance, a clinical study with 559 TNBC cases shows AR negativity is correlated with a better prognostic outcome in terms of OS. For TNBC patients without lymph node metastasis, AR-positive patients also have a higher risk of death and recurrence [20, 24, 28]. Therefore, we are required to confirm the clinical role of AR. A. Di Leone disclosed that AR overexpression in TNBC showed a lower Ki67 rate and was related to a lower rate of pCR in neoadjuvant chemotherapy [29]. Currently, researchers pay more attention to TNBC to ensure long survival. In this aspect, AR-target therapy gives the choice for TNBC, which also need more clinical trials.
In summary, in-depth research on targeted therapy and combination therapy for breast cancer is demanded. Routine assessment of AR may help better personalize treatment for breast cancer, and can be used as a potential therapeutic target and novel biomarker in breast cancer. Furthermore, extensive research containing a large substantiation in patients, and more controlled prospective clinical trials are needed to validate the results.
Availability of data and materials
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Data availability
No datasets were generated or analysed during the current study.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/caac.21708.
Anestis A, Zoi I, Papavassiliou AG and Karamouzis MV (2020) Androgen Receptor in Breast Cancer-Clinical and Preclinical Research Insights. Molecules 25. https://doi.org/10.3390/molecules25020358
Vasiliou SK, Diamandis EP. Androgen receptor: A promising therapeutic target in breast cancer. Crit Rev Clin Lab Sci. 2019;56:200–23. https://doi.org/10.1080/10408363.2019.1575643.
Elebro K, Borgquist S, Simonsson M, Markkula A, Jirström K, Ingvar C, Rose C, Jernström H. Combined Androgen and Estrogen Receptor Status in Breast Cancer: Treatment Prediction and Prognosis in a Population-Based Prospective Cohort. Clin Cancer Res. 2015;21:3640–50. https://doi.org/10.1158/1078-0432.Ccr-14-2564.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. https://doi.org/10.1172/jci45014.
He L, Du Z, Xiong X, Ma H, Zhu Z, Gao H, Cao J, Li T, Li H, Yang K, Chen G, Richer JK, Gu H. Targeting Androgen Receptor in Treating HER2 Positive Breast Cancer. Sci Rep. 2017;7:14584. https://doi.org/10.1038/s41598-017-14607-2.
Chia KM, Liu J, Francis GD, Naderi A. A feedback loop between androgen receptor and ERK signaling in estrogen receptor-negative breast cancer. Neoplasia. 2011;13:154–66. https://doi.org/10.1593/neo.101324.
De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A, Lewis MT, Chamness GC, Hilsenbeck SG, Andò S, Fuqua SA. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010;121:1–11. https://doi.org/10.1007/s10549-009-0436-8.
Dorgan JF, Stanczyk FZ, Egleston BL, Kahle LL, Shaw CM, Spittle CS, Godwin AK, Brinton LA. Prospective case-control study of serum mullerian inhibiting substance and breast cancer risk. J Natl Cancer Inst. 2009;101:1501–9. https://doi.org/10.1093/jnci/djp331.
Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel-Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quirós JR, Roddam A, Thiebaut A, Tjønneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2005;97:755–65. https://doi.org/10.1093/jnci/dji132.
Fourkala EO, Zaikin A, Burnell M, Gentry-Maharaj A, Ford J, Gunu R, Soromani C, Hasenbrink G, Jacobs I, Dawnay A, Widschwendter M, Lichtenberg-Fraté H, Menon U. Association of serum sex steroid receptor bioactivity and sex steroid hormones with breast cancer risk in postmenopausal women. Endocr Relat Cancer. 2012;19:137–47. https://doi.org/10.1530/erc-11-0310.
Feng J, Li L, Zhang N, Liu J, Zhang L, Gao H, Wang G, Li Y, Zhang Y, Li X, Liu D, Lu J, Huang B. Androgen and AR contribute to breast cancer development and metastasis: an insight of mechanisms. Oncogene. 2017;36:2775–90. https://doi.org/10.1038/onc.2016.432.
Zhong W, Yi J, Wu H, Zou X, Feng J, Huang X, Li S, Wang X. Androgen receptor expression and its prognostic value in T1N0 luminal/HER2- breast cancer. Future Oncol. 2022;18:1745–56. https://doi.org/10.2217/fon-2021-1300.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. https://doi.org/10.1093/annonc/mdr304.
Wang D, Tang M, Zhang P, Yang K, Huang L, Wu M, Shen Q, Yue J, Wang W, Gong Y, Warner M, Dai L, He H, Yang Z, Gustafsson JA, Zhou S. Activation of ERβ hijacks the splicing machinery to trigger R-loop formation in triple-negative breast cancer. Proc Natl Acad Sci U S A. 2024;121:e2306814121. https://doi.org/10.1073/pnas.2306814121.
Sawada T, Kanemoto Y, Kurokawa T, Kato S. The epigenetic function of androgen receptor in prostate cancer progression. Front Cell Dev Biol. 2023;11:1083486. https://doi.org/10.3389/fcell.2023.1083486.
Narayanan R and Dalton JT (2016) Androgen Receptor: A Complex Therapeutic Target for Breast Cancer. Cancers (Basel) 8. https://doi.org/10.3390/cancers8120108
Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24:924–31. https://doi.org/10.1038/modpathol.2011.54.
Witzel I, Graeser M, Karn T, Schmidt M, Wirtz R, Schütze D, Rausch A, Jänicke F, Milde-Langosch K, Müller V. Androgen receptor expression is a predictive marker in chemotherapy-treated patients with endocrine receptor-positive primary breast cancers. J Cancer Res Clin Oncol. 2013;139:809–16. https://doi.org/10.1007/s00432-013-1382-8.
You CP, Leung MH, Tsang WC, Khoo US and Tsoi H (2022) Androgen Receptor as an Emerging Feasible Biomarker for Breast Cancer. Biomolecules 12. https://doi.org/10.3390/biom12010072
Kono M, Fujii T, Lim B, Karuturi MS, Tripathy D, Ueno NT. Androgen Receptor Function and Androgen Receptor-Targeted Therapies in Breast Cancer: A Review. JAMA Oncol. 2017;3:1266–73. https://doi.org/10.1001/jamaoncol.2016.4975.
Hickey TE, Selth LA, Chia KM, Laven-Law G, Milioli HH, Roden D, Jindal S, Hui M, Finlay-Schultz J, Ebrahimie E, Birrell SN, Stelloo S, Iggo R, Alexandrou S, Caldon CE, Abdel-Fatah TM, Ellis IO, Zwart W, Palmieri C, Sartorius CA, Swarbrick A, Lim E, Carroll JS, Tilley WD. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat Med. 2021;27:310–20. https://doi.org/10.1038/s41591-020-01168-7.
You CP, Tsoi H, Man EPS, Leung MH and Khoo US (2022) Modulating the Activity of Androgen Receptor for Treating Breast Cancer. Int J Mol Sci 23. https://doi.org/10.3390/ijms232315342
Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA, Tamimi RM. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17:1867–74. https://doi.org/10.1158/1078-0432.Ccr-10-2021.
Kensler KH, Poole EM, Heng YJ, Collins LC, Glass B, Beck AH, Hazra A, Rosner BA, Eliassen AH, Hankinson SE, Winer EP, Brown M, Tamimi RM. Androgen Receptor Expression and Breast Cancer Survival: Results From the Nurses’ Health Studies. J Natl Cancer Inst. 2019;111:700–8. https://doi.org/10.1093/jnci/djy173.
Kolyvas EA, Caldas C, Kelly K, Ahmad SS. Androgen receptor function and targeted therapeutics across breast cancer subtypes. Breast Cancer Res. 2022;24:79. https://doi.org/10.1186/s13058-022-01574-4.
Thike AA, Yong-Zheng Chong L, Cheok PY, Li HH, Wai-Cheong Yip G, Huat Bay B, Tse GM, Iqbal J, Tan PH. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol. 2014;27:352–60. https://doi.org/10.1038/modpathol.2013.145.
Choi JE, Kang SH, Lee SJ, Bae YK. Androgen receptor expression predicts decreased survival in early stage triple-negative breast cancer. Ann Surg Oncol. 2015;22:82–9. https://doi.org/10.1245/s10434-014-3984-z.
Di Leone A, Fragomeni SM, Scardina L, Ionta L, Mulè A, Magno S, Terribile D, Masetti R, Franceschini G. Androgen receptor expression and outcome of neoadjuvant chemotherapy in triple-negative breast cancer. Eur Rev Med Pharmacol Sci. 2021;25:1910–5. https://doi.org/10.26355/eurrev_202102_25087.
Acknowledgements
None.
Funding
This research was supported by the National Natural Science Foundation of China (No. 82102780, 82303449), High-level Innovative and Entrepreneurial Talent Introduction Plan of Jiangsu Province (303073540ER21), Scientific Research Project of Jiangsu Provincial Health Commission (H2023088), and National Natural Science Foundation Youth Fund Cultivation Program of the First Affiliated Hospital of Nanjing Medical University (PY2022030), and Jiangsu Province Hospital (the First Affiliated Hospital with Nanjing Medical University) Clinical Capacity Enhancement Project (JSPH-MC-2022–26).
Author information
Authors and Affiliations
Contributions
HDZ and SLZ designed the study and wrote the manuscript. DDW and LHJ primarily wrote the manuscript and prepared the tables. JZ and XC made substantial contributions to the acquisition and analysis of data. JZ and HLZ made contributions to the analysis and interpretation of data and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
In this research were approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Ethics code 2022-SR-473), which deemed that written informed consent was not necessary due to the retrospectiveness of the research and the concealment of patient information.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
He-da Zhang is the first corresponding author.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Dd., Jiang, Lh., Zhang, J. et al. Androgen receptor expression and clinical characteristics in breast cancer. World J Surg Onc 22, 243 (2024). https://doi.org/10.1186/s12957-024-03525-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03525-z