Abstract
Aims
To present a case series of 11 rare uterine tumors resembling ovarian sex cord tumors (UTROSCTs), and review the literature on this topic to offer up-to-date treatment management for UTROSCTs.
Method
Eight cases from Fujian Cancer Hospital between January 2017 and May 2023 and three patients from Fujian Union Hospital between October 2012 and October 2020 were retrospectively reviewed. All cases were pathologically confirmed as UTROSCTs by two senior and experienced pathologists. Clinical behaviors, medical data, histopathological features, therapy approaches, and survival outcomes were discussed.
Results
The median age at initial diagnosis was 53 years (29–70 years). 3 (27.3%) patients were under 40. Seven cases presented with abnormal vaginal bleeding, one with menstrual disorder, one with abnormal vaginal secretion, and two patients were accidentally found by physical examination without any symptoms. Three patients were initially misdiagnosed with endometrial cancer by MRI. Curettage was performed in all cases. Nine of them were well diagnosed by routine curettage, except for two samples, which were identified after surgery. Immunohistochemical biomarkers, such as CD99, Desmin, WT-1, CK, Vimentin, SMA, α-Inhibin, Ki67, CD56, ER, PR, and CR, tend to be positive in UTRO SCs patients. Six patients underwent hysterectomy with bilateral salpingo-oophorectomy. Two cases received a radical hysterectomy with bilateral salpingo-oophorectomy, retroperitoneal lymph node dissection, and omentum dissection. Three UTROSCTs were under observation after mass resection. The median PFS was 24 months (range 1–125 months).
Conclusion
UTROSCT is a rare mesenchymal tumor with low malignant potential. Treatment modalities should be carefully considered to balance the therapy outcomes and patient needs. Surgery conservative management might be suitable for young women with fertility desires.
Similar content being viewed by others
Introduction
Uterine tumor resembling ovarian sex cord tumor (UTROSCT) is a sporadic and controversial disease with unclear origins, named for resembling the morphology of ovarian sex cord tumors [1]. It mainly occurs in women in their 50 s and occasionally in young women with childbearing needs [2]. UTROSCT patients always present with irregular vaginal bleeding or chronic pelvic pain. However, some can be discovered by accidents [3]. Although UTROSCT is generally considered benign or has a low-malignant potential, it could metastasize sometimes. There are no established treatment protocols for UTROSCT so far. Surgical intervention is usually recommended with a favorable prognosis.
Although there has been modest literature on UTROSCT in recent years, focusing mainly on clinicopathological characteristics and gene variations, relatively few advances have been made in treatment strategies due to the low incidence. Here we reported 11 UTROSCT cases with different therapy approaches together with an updated literature review to expand our knowledge of UTROSCT management.
Materials and methods
Clinical data
A total of 11 UTROSCT patients from two institutions from October 2012 and October 2020 were included. Intrauterine tissue and surgical specimens were reviewed and confirmed by two senior and experienced pathologists. The clinical and follow-up information was obtained through medical files and telephone contact. We collected information such as menopausal status, parity, symptoms, tumor positions, tumor biomarkers, image data, immunohistochemical biomarkers, treatment approaches, and survival status. May 31, 2023, was the final censoring date for assessing the survival time.
Interventions
All patients underwent diagnostic curettage, three of which were hysteroscopically assisted. For further steps, six patients underwent hysterectomy with bilateral salpingo-oophorectomy, two cases received radical hysterectomy with bilateral salpingo-oophorectomy, retroperitoneal lymph node dissection, and omentum dissection, and three were under observation after tumorectomy due to their desires for children-bearing in the near future. All surgeries were performed by experienced gynecology surgeons. After treatment, patients were advised to have follow-up visits every three months in the first 2 years, every six months in the 3–5 years, and once a year after 5 years.
Results
Clinical features and treatment outcomes
The age ranged from 29 to 70 (median = 53) at the initial diagnosis. Seven cases presented with vaginal bleeding, one with menstrual disorder, and one with vaginal secretion. While two showed no symptoms. No elevation of serum tumor biomarkers such as CA125, CA199, and CEA were observed in 11 cases. The mass positions were in the endometrium (4 cases) and myometrium (7 cases). None of the patients received further postoperative treatment. The median follow-up time was 24 months (1–125 months), and no recurrence or metastasis was noted. Details are presented in Table 1.
Radiological imaging characteristics
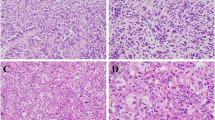
Radiological imaging sometimes could have been deceiving due to the small population size and limited knowledge about UTROSCT. The max SUV value in the myometrium is 4.5 under PET-CT examination after hysteroscopic curettage in case 3 (Fig. 1A) with conservative therapy. An increasing endometrial thickness was observed in cases 4 and 10 (Fig. 1B, C). A mass with myometrial invasion of case 11 which was misdiagnosed as endometrial cancer was finally proved to be UTROSCT (Fig. 1D). Case 6 was also suspected as endometrial cancer by MRI examination (Fig. 1E), so as case 10. No residual tumor was noted after curettage (Fig. 1F). Other patients didn't conduct a radiology examination. The radiological imaging findings are presented in Fig. 1.
UTROSCT (arrow) in different images of PET-CT and MRI. A A max SUV was 4.5 in the myometrium. B–D UTROSCT is mildly hypointense compared to endometrium signals on sagittal T2 sequence. E UTROSCT is similar to uterus signal and well-delineated on dynamic contrast-enhanced sequence. F No mass observed after curettage
Pathological findings
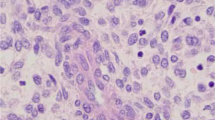
Glandular epithelium, basal-like, and myoepithelial cells are the three main kinds of cells that could be found in UTROSCT patients. Under the light microscope, we could see specific common structures: myxedema, sex cord-like structure, collagen degeneration, foam-like cells, organ-like structure, and muscle layer infiltration, which are often mixed and concurrence (Fig. 2).
Pathology is the golden standard for UTROSCT diagnosis. Patients with UTROSCT showed a diverse phenotype profile, according to immunohistochemistry performed on UTROSCT patients (Fig. 3, Table 2). Biomarkers such as inhibin, CD99, CD56, WT-1, and CK tend to be positive in UTROSCTs. Most tumors had exhibited at least one of the standard sex-cord markers, including CD99, WT-1, and α-Inhibin (Table 2). The positive expression rates of α-inhibin (Fig. 3A), CD99 (Fig. 3B), Vimentin (Fig. 3C) and WT-1 (Fig. 3D) were 3/10, 7/9, 5/6, 8/8, respectively. Almost all patients (9/11) expressed one or more smooth muscle markers, and the positive expression rates of desmin (Fig. 3E) and SMA (Fig. 3F) were 8/10 and 6/10, respectively. CK (Fig. 3G) as an epithelial biomarker was positive in most cases (10/11), and hormone receptors like ER (Fig. 3H) and PR (Fig. 3I) expressed variably among UTROSCTs. A low Ki67(Fig. 3J) index was observed among the patients (median 5%, range 2–30%). While CD56 (Fig. 3K) and Calretinin (Fig. 3L) are often used for differentiated diagnosis.
Discussion
In 1976, Clement and Scully first reported certain kinds of rare uterine tumors with histologic resemblances to ovarian sex-cord tumors [4]. Those types of tumors could be divided into two groups. The first group is endometrial stromal tumors with sex cord-like elements (ESTSCLE) with a particular propensity to recur or metastasize, and UTROSCT is the other group [5]. UTROSCT is rare and usually recognized as a benign tumor. Clinical observations and literature data indicated a better prognosis [6]. However, sporadic reports showed it could metastasize and recur sometimes. In 2017, Moore documented 34 UTROSCT cases, of which about 23.5% exhibited malignant behavior [7]. Due to the potential malignancy and limited experience, treatment recommendations have not been well established.
Surgery is recommended as the preferred treatment for UTROSCT patients [8]. Hysterectomy or mass resection alone are possible options for its management [9]. A systematic review of the literature on UTROSCTs’ treatment approaches is detailed in Table 3. With advancements in medicine and humanities, individualized-oriented tumor treatments have been deeply rooted in people’s hearts. We should pay more attention to patients with special needs or in different conditions, and then make personalized plans to balance the therapy outcomes and their needs.
Hysterectomy for UTROSCTs in the middle and old age
Hysterectomy resection with or without salpingo-oophorectomy should be a primary option for middle-aged or older women with UTROSCT, especially in patients whose follow-up cannot be guaranteed [9]. Baris Boyraz recorded that hysterectomy is the prior surgery approach in 71 out of 75 UTROSCT patients [40]. Besides, most patients had good survival outcomes simply by removing the uterine [21, 22, 40]. Moreover, some scholars claimed recurrence cases previously treated by hysterectomy [11, 12, 20] could still get prolonged survival by completely removing the recurrent mass. In our study, 6 out of 11 patients treated by hysterectomy did not experience recurrence and metastasis after a median follow-up time of 26.5 months (range 5–125 months). However, we still need a longer follow-up time and more cases to make survival outcomes more convincing.
Radical surgery for UTROSCTs with high risk of recurrence or metastasis
UTROSCT has uncertain malignant potential due to its low recurrence rate [7]. Distant metastasis and local recurrence have been occasionally reported [11, 22, 28, 29]. Our review found that the recurrence rate was low (19/117) during the median follow-up time between 20 days and 32 years. Currently, predictive features of aggressive UTROSCTs are poorly understood. Risk factors, such as myometrial invasion, serosal involvement, lymph-vascular space invasion (LVSI), GREB1-NCOA2 fusion, NCOA2 or NCOA3 [23, 41, 42], and high mitotic activity [40, 43]have been proven to be associated with tumor recurrence. Baris Boyraz concluded that high-risk UTROSCT showed more than three of the following five features: at least moderate cytologic atypia, tumor size > 5 cm, above 3 mitoses/10 at high power fields (HPFs), marginal infiltration, and necrosis [40].
High-risk UTROSCT patients could benefit from radical surgery. Miho believed hysterectomy alone was associated with a higher rate of recurrence. Extended radical surgery, including bilateral salpingo-oophorectomy, lymphadenectomy, and omentectomy, may reduce the recurrence rate of UTROSCT with sarcomatous features [34]. Based on the review in Table 3, no recurrence or distant metastasis was observed among 8 UTROSCT patients treated with radical surgery, indicating radical surgery seemed to be the optimal choice for high-risk patients. However, it should be attention that high-risk UTROSCT is often detected incidentally after a hysterectomy surgery, which makes the initial treatment insufficient. For this reason, the roles of adjuvant therapies should be carefully considered. Surprisingly, the literature showed adjuvant treatments such as chemotherapy, hormone therapy, or radiotherapy were still in debate for the UTROSCTs with high risks of recurrence or metastasis [35]. Oriana Marrucci reported a rare vaginal vault recurrence case 5 years after total hysterectomy with bilateral salpingo-oophorectomy. She had a favorable prognosis with only a second surgery [44]. Shigeaki Umeda also represented a case with epiploic appendix metastasis, who was treated with surgery and has been followed up for 8 years without evidence of recurrence [28]. However, Michelle presented an unusual case of an aggressive UTROSCT who underwent neoadjuvant chemotherapy followed by optimal cytoreductive surgery and adjuvant chemotherapy and still died 15 months after her initial diagnosis [31]. Therefore, the selection of auxiliary treatments needs to consider the specific situations of UTROSCT patients.
Still, the role of hormone therapy is also controversial. Sabrina M. Schraag recorded that surgery and endocrine treatment were applied for a young UTROSCT with recurrence. She was disease-free 34 months after the last surgery and was still on a monitor [38]. High-dose progesterone therapy was also applied after surgery in a UTROSCT with pelvic lymph node metastasis, and no recurrence was observed [28]. While Daisuke Endo pointed out that hormone therapy may be ineffective for recurrent tumors [45]. Some scholars even believed hormone therapy such as tamoxifen progressed this disease [33]. In our study, none patients received additional treatments, and no recurrences were observed. To be pointed out, two cases received radical surgery due to malignancy features shown in MRI images and pathology ambiguity. Based on the above, we believe the choice of surgical approaches should be carefully considered based on whether she is a high-risk UTROSCT.
Conservative therapy for UTROSCTs with fertility desire
With the development of minimally invasive surgery, tumorectomy by hysteroscopy or laparoscopy could be expected in young UTROSCTs who desire to preserve their organs and fertility. Many scholars reported successful pregnancies in young UTROSCT women treated with fertility-preserving surgery and had no sign of recurrence [38, 39], even in a myometrial invasion case [36]. Only one relapsed case after 20 months of conservative surgery [38]. In our review, all UTROSCTs with conservative therapy had favorable prognoses. Most researchers believed UTROSCT patients could maintain fertility without affecting the survival rate. In our study, three cases (Nos. 1, 2, and 3) were done with mass resection. After a comprehensive examination without signs of residue tumor, case 2 opted for surveillance. The menstruation returned to normal after conservative therapy. She has been followed up for two months with disease-free. In case 3, a PET/CT showed a max SUV value of 4.5 in the myometrium, which cannot be confirmed as postoperative changes or residue tumors. After exhaustive discussions with patients, she still chose to observe due to her eager solid of fertility preservation. Surprisingly, there are no signs of recurrence or metastasis after a follow-up of 54 months. Case 1 had a myomectomy and showed no sign of recurrence. She strongly desired to preserve her organs and decided to be closely observed. A conservative, uterus-preserving treatment appears justified in those whose close follow-up can be guaranteed, even in high-risk ones. Once recurrence, patients could still get a favorable prognosis after salvage surgery [7]. Further investigations are needed to prove the safety of organ-preserving strategy in UTROSCT.
UTROSCT is somehow inert and quickly got the attention of irregular uterine bleeding. All patients in our research were diagnosed in the early stages. A hysterectomy seems enough. However, a 40-year-old female still firmly chose to have extra salpingo-oophorectomy due to psychological fear of tumor recurrence, even after being fully educated on UTROSCT. Moreover, pre-surgery examinations, such as MRI images and curettage pathology, could sometimes be confusing, leading to overtreatment for oncological safety. Clinicians should focus more on aggressive UTROSCT features patients shared, such as tumor size, myometrial invasion, LVSI, and gene fusion, to determine the surgical approaches. Working with an experienced pathologist is necessary.
As far as we know, few articles have summarized and compared the different therapy approaches to UTROSCT. Our study gave a comprehensive knowledge of UTROSCT therapy methods. However, the main limitation of our study is that it was designed retrospectively. Specific information about related cases is lacking, the follow-up time for the young UTROSCT needs longer, and genetic alterations and molecular detection remain to be explored. However, we still contributed 11 UTROSCTs to the medical literature, which might aid clinical decision-making.
Conclusion
UTROSCT patients are often diagnosed in the early stage. Hysterectomy with or without salpingo-oophorectomy should be the primary treatment for UTROSCT patients. Radical surgery is a favored choice for patients with invasive features. Mass resection is safe for young fertility women in clinical surveillance. Further studies are needed to validate these findings.
Availability of data and materials
The data used or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Hillard JB, Malpica A, Ramirez PT. Conservative management of a uterine tumor resembling an ovarian sex cord-stromal tumor. Gynecol Oncol. 2004;92(1):347–52.
Ye S, Wu J, Yao L, He J. Clinicopathological characteristics and genetic variations of uterine tumours resembling ovarian sex cord tumours. J Clin Pathol. 2022;75(11):776–81.
Wang XY, Zhang MC, Chen J, Huang JH. Uterine tumor resembling ovarian sex cord tumor: A rare case report. Medicine (Baltimore). 2022;101(35):e30414.
Clement PB, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am J Clin Pathol. 1976;66(3):512–25.
McCluggage WG, Singh N, Gilks CB. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology. 2022;80(5):762–78.
Bakula-Zalewska E, Danska-Bidzinska A, Kowalewska M, Piascik A, Nasierowska-Guttmejer A, Bidzinski M. Uterine tumors resembling ovarian sex cord tumors, a clinicopathologic study of six cases. Ann Diagn Pathol. 2014;18(6):329–32.
Moore M, McCluggage WG. Uterine tumour resembling ovarian sex cord tumour: first report of a large series with follow-up. Histopathology. 2017;71(5):751–9.
Zhou FF, He YT, Li Y, Zhang M, Chen FH. Uterine tumor resembling an ovarian sex cord tumor: A case report and review of literature. World J Clin Cases. 2021;9(23):6907–15.
Blake EA, Sheridan TB, Wang KL, Takiuchi T, Kodama M, Sawada K, Matsuo K. Clinical characteristics and outcomes of uterine tumors resembling ovarian sex-cord tumors (UTROSCT): a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014;181:163–70.
Gutierrez-Pecharroman A, Tirado-Zambrana P, Pascual A, Rubio-Marin D, García-Cosío M, Moratalla-Bartolomé E, Palacios J. A uterine tumor resembling ovarian sex cord tumor associated with tamoxifen treatment: a case report and literature review. Int J Gynecol Pathol. 2014;33(2):151–5.
Kondo Y, Sakaguchi S, Mikubo M, Naito M, Shiomi K, Ohbu M, Satoh Y. Lung metastases of a uterine tumor resembling ovarian sex-cord tumor: report of a rare case. Diagn Cytopathol. 2018;46(1):88–91.
Croce S, Lesluyes T, Delespaul L, Bonhomme B, Pérot G, Velasco V, Mayeur L, Rebier F, Ben Rejeb H, Guyon F, et al. GREB1-CTNNB1 fusion transcript detected by RNA-sequencing in a uterine tumor resembling ovarian sex cord tumor (UTROSCT): A novel CTNNB1 rearrangement. Genes Chromosomes Cancer. 2019;58(3):155–63.
Dubruc E, Alvarez Flores MT, Bernier Y, Gherasimiuc L, Ponti A, Mathevet P, Bongiovanni M. Cytological features of uterine tumors resembling ovarian sex-cord tumors in liquid-based cervical cytology: a potential pitfall. Report of a unique and rare case. Diagn Cytopathol. 2019;47(6):603–7.
Zhang X, Zou S, Gao B, Qu W. Uterine tumor resembling ovarian sex cord tumor: a clinicopathological and immunohistochemical analysis of two cases and a literature review. J Int Med Res. 2019;47(3):1339–47.
Lee CH, Kao YC, Lee WR, Hsiao YW, Lu TP, Chu CY, Lin YJ, Huang HY, Hsieh TH, Liu YR, et al. Clinicopathologic Characterization of GREB1-rearranged Uterine Sarcomas With Variable Sex-Cord Differentiation. Am J Surg Pathol. 2019;43(7):928–42.
Vilos AG, Zhu C, Abu-Rafea B, Ettler HC, Weir MM, Vilos GA. Uterine tumors resembling ovarian sex cord tumors identified at resectoscopic endometrial ablation: report of 2 cases. J Minim Invasive Gynecol. 2019;26(1):105–9.
Dimitriadis GK, Wajman DS, Bidmead J, Diaz-Cano SJ, Arshad S, Bakhit M, Lewis D, Aylwin SJB. Ectopic hyperprolactinaemia due to a malignant uterine tumor resembling ovarian sex cord tumors (UTROCST). Pituitary. 2020;23(6):641–7.
Grither WR, Dickson BC, Fuh KC, Hagemann IS. Detection of a somatic GREB1-NCOA1 gene fusion in a uterine tumor resembling ovarian sex cord tumor (UTROSCT). Gynecol Oncol Rep. 2020;34:100636.
Chang B, Bai Q, Liang L, Ge H, Yao Q. Recurrent uterine tumors resembling ovarian sex-cord tumors with the growth regulation by estrogen in breast cancer 1-nuclear receptor coactivator 2 fusion gene: a case report and literature review. Diagn Pathol. 2020;15(1):110.
Bennett JA, Lastra RR, Barroeta JE, Parilla M, Galbo F, Wanjari P, Young RH, Krausz T, Oliva E. Uterine Tumor Resembling Ovarian Sex Cord Stromal Tumor (UTROSCT): A Series of 3 Cases With Extensive Rhabdoid Differentiation, Malignant Behavior, and ESR1-NCOA2 Fusions. Am J Surg Pathol. 2020;44(11):1563–72.
Kaur K, Rajeshwari M, Gurung N, Kumar H, Sharma MC, Yadav R, Kumar S, Manchanda S, Singhal S, Mathur SR. Uterine tumor resembling ovarian sex cord tumor: A series of six cases displaying varied histopathological patterns and clinical profiles. Indian J Pathol Microbiol. 2020;63(Supplement):S81-s86.
Goebel EA, Hernandez Bonilla S, Dong F, Dickson BC, Hoang LN, Hardisson D, Lacambra MD, Lu FI, Fletcher CDM, Crum CP, et al. Uterine Tumor Resembling Ovarian Sex Cord Tumor (UTROSCT): A Morphologic and Molecular Study of 26 Cases Confirms Recurrent NCOA1-3 Rearrangement. Am J Surg Pathol. 2020;44(1):30–42.
Devereaux KA, Kertowidjojo E, Natale K, Ewalt MD, Soslow RA, Hodgson A. GTF2A1-NCOA2-associated uterine tumor resembling ovarian sex cord tumor (UTROSCT) shows focal rhabdoid morphology and aggressive behavior. Am J Surg Pathol. 2021;45(12):1725–8.
Shibahara M, Kurita T, Murakami M, Harada H, Tsuda Y, Hisaoka M, Kagami S, Matsuura Y, Yoshino K. Uterine tumor resembling ovarian sex cord tumor: a case report. J Uoeh. 2022;44(2):161–6.
Pang L, Dai Y, Ren F, Peng X, Guo Z. Uterine tumor resembling ovarian sex cord tumors (UTROSCT): two case reports of the rare uterine neoplasm with literature review. Curr Med Imaging. 2022;18(10):1125–31.
Zhou Y, Chen YH, Wang YN, Sun L. Uterine tumor resembling ovarian sex cord tumor with an abnormal increase in CA125: a case report. Asian J Surg. 2023;46(6):2641–42.
Lu B, Xia Y, Chen J, Tang J, Shao Y, Yu W. NCOA1/2/3 rearrangements in uterine tumor resembling ovarian sex cord tumor: a clinicopathological and molecular study of 18 cases. Hum Pathol. 2023;135:65–75.
Umeda S, Tateno M, Miyagi E, Sakurai K, Tanaka R, Tateishi Y, Tokinaga A, Ohashi K, Furuya M. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) with metastasis: clinicopathological study of two cases. Int J Clin Exp Pathol. 2014;7(3):1051–9.
Mačák J, Dundr P, Dvořáčková J, Klát J. Uterine tumors resembling ovarian sex cord tumors (UTROSCT). Report of a case with lymph node metastasis. Cesk Patol. 2014;50(1):46–9.
Cetinkaya N, Bas S, Cuylan ZF, Erdem O, Erkaya S, Gungor T. Uterine tumors resembling ovarian sex cord tumors: a case report and literature review. Oncol Lett. 2016;11(2):1496–8.
Kuznicki ML, Robertson SE, Hakam A, Shahzad MM. Metastatic uterine tumor resembling ovarian sex cord tumor: a case report and review of the literature. Gynecol Oncol Rep. 2017;22:64–8.
Fan LL, Shen Y, Chanda K, Ren ML. Uterine tumors resembling ovarian sex-cord tumor: A case report and literature review. J Cancer Res Ther. 2018;14(Supplement):S1209-s1212.
Segala D, Gobbo S, Pesci A, Martignoni G, Santoro A, Angelico G, Arciuolo D, Spadola S, Valente M, Scambia G, et al. Tamoxifen related uterine tumor resembling ovarian sex cord tumor (UTROSCT): a case report and literature review of this possible association. Pathol Res Pract. 2019;215(5):1089–92.
Sato M, Yano M, Sato S, Aoyagi Y, Aso S, Matsumoto H, Yamamoto I, Nasu K. Uterine tumor resembling ovarian sex-cord tumor (UTROSCT) with sarcomatous features without recurrence after extended radical surgery: a case report. Medicine (Baltimore). 2020;99(11):e19166.
Watrowski R, Jäger C, Möckel J, Kurz P, Schmidt D, Freudenberg N. Hysteroscopic treatment of uterine tumor resembling ovarian sex cord-like tumor (UTROSCT). Gynecol Endocrinol. 2015;31(11):856–9.
Jeong KH, Lee HN, Kim MK, Kim ML, Seong SJ, Shin E. Successful delivery after conservative resectoscopic surgery in a patient with a uterine tumor resembling ovarian sex cord tumor with myometrial invasion. Obstet Gynecol Sci. 2015;58(5):418–22.
De Franciscis P, Grauso F, Ambrosio D, Torella M, Messalli EM, Colacurci N. Conservative resectoscopic surgery, successful delivery, and 60 months of follow-up in a patient with endometrial stromal tumor with sex-cord-like differentiation. Case Rep Obstet Gynecol. 2016;2016:5736865.
Schraag SM, Caduff R, Dedes KJ, Fink D, Schmidt AM. Uterine tumors resembling ovarian sex cord tumors - treatment, recurrence, pregnancy and brief review. Gynecol Oncol Rep. 2017;19:53–6.
Carbone MV, Cavaliere AF, Fedele C, Vidiri A, Aciuolo D, Zannoni G, Scambia G. Uterine tumor resembling ovarian sex-cord tumor: conservative surgery with successful delivery and case series. Eur J Obstet Gynecol Reprod Biol. 2021;256:326–32.
Boyraz B, Watkins JC, Young RH, Oliva E. Uterine tumors resembling ovarian sex cord tumors: a clinicopathologic study of 75 cases emphasizing features predicting adverse outcome and differential diagnosis. Am J Surg Pathol. 2023;47(2):234–47.
Dickson BC, Childs TJ, Colgan TJ, Sung YS, Swanson D, Zhang L, Antonescu CR. Uterine tumor resembling ovarian sex cord tumor: a distinct entity characterized by recurrent NCOA2/3 gene fusions. Am J Surg Pathol. 2019;43(2):178–86.
Chen Z, Lan J, Chen Q, Lin D, Hong Y. A novel case of uterine tumor resembling ovarian sex-cord tumor (UTROSCT) recurrent with GREB1-NCOA2 fusion. Int J Gynaecol Obstet. 2021;152(2):266–8.
Yin X, Wang M, He H, Ru G, Zhao M. Uterine tumor resembling ovarian sex cord tumor with aggressive histologic features harboring a GREB1-NCOA2 fusion: case report with a brief review. Int J Gynecol Pathol. 2023;42(1):54–62.
Marrucci O, Nicoletti P, Mauriello A, Facchetti S, Patrizi L, Ticconi C, Sesti F, Piccione E. Uterine tumor resembling ovarian sex cord tumors type II with vaginal vault recurrence. Case Rep Obstet Gynecol. 2019;2019:5231219.
Endo D, Todo Y, Okamoto K, Suzuki H. A case of recurrent group II uterine tumor resembling ovarian sex-cord tumors, against which two hormonal agents were ineffective. Taiwan J Obstet Gynecol. 2016;55(5):751–3.
Acknowledgements
The author Jie Lin thanks her husband Deliang Li for the unconditional support.
Funding
This work was sponsored by the Startup Fund for scientific research, Fujian Medical University, China (Grant number: 2021QH1141), Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number 2021Y9209), the Fujian Provincial Health Technology Project (Grant number 2021QNA043) and the Natural Science Foundation of Fujian Province (Grant number 2023J011257).
Author information
Authors and Affiliations
Contributions
Yang Sun designed the present study. Jie Lin and Linying Liu wrote the manuscript. Jie Lin checked and revised the manuscript. Linghua Wang helped to reviewed and collected all data, Ning Ma reviewed, collected and checked MRI data, Kailin Zhang reviewed, collected and checked pathology data, Ning Xie, Haijuan Yu and Sufang Deng contributed to collecting and reviewing materials.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of Fujian Medical Cancer Hospital and Fujian Union Hospital and adhered to the Helsinki Declaration. Oral consent was obtained from both patients for the publication of their information and images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, J., Liu, L., Wang, L. et al. The management of uterine tumor resembling an ovarian sex cord tumor (UTROSCT): case series and literature review. World J Surg Onc 22, 42 (2024). https://doi.org/10.1186/s12957-024-03319-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03319-3