Abstract
Background
Uterine cervical carcinoma is a severe health threat worldwide, especially in China. The International Federation of Gynecology and Obstetrics (FIGO) has revised the staging system, emphasizing the strength of magnetic resonance imaging (MRI). We aimed to investigate long-term prognostic factors for FIGO 2018 stage II–IIIC2r uterine cervical squamous cell carcinoma following definitive radiotherapy and establish a prognostic model using MRI-derived tumor volume.
Methods
Patients were restaged according to the FIGO 2018 staging system and randomly grouped into training and validation cohorts (7:3 ratio). Optimal cutoff values of squamous cell carcinoma antigen (SCC-Ag) and tumor volume derived from MRI were generated for the training cohort. A nomogram was constructed based on overall survival (OS) predictors, which were selected using univariate and multivariate analyses. The performance of the nomogram was validated and compared with the FIGO 2018 staging system. Risk stratification cutoff points were generated, and survival curves of low-risk and high-risk groups were compared.
Results
We enrolled 396 patients (training set, 277; validation set, 119). The SCC-Ag and MRI-derived tumor volume cutoff values were 11.5 ng/mL and 28.85 cm3, respectively. A nomogram was established based on significant prognostic factors, including SCC-Ag, poor differentiation, tumor volume, chemotherapy, and FIGO 2018 stage. Decision curve analysis indicated that the net benefits of our model were higher. The high-risk group had significantly shorter OS than the low-risk group in both the training (p < 0.0001) and validation sets (p = 0.00055).
Conclusions
Our nomogram predicted long-term outcomes of patients with FIGO 2018 stage II–IIIC2r uterine cervical squamous cell carcinoma. This tool can assist gynecologic oncologists and patients in treatment planning and prognosis.
Similar content being viewed by others
Introduction
Uterine cervical carcinoma ranks fourth in malignant tumors among women worldwide, after breast cancer, colorectal cancer, and lung cancer. In China, it is also the fourth leading cause of cancer death among women and is the most prevalent cancer in women [1]. Most uterine cervical carcinomas are squamous cell carcinomas, and the main therapies are surgery, radiotherapy, and chemotherapy. However, surgery is recommended only for early-stage patients, and most patients are at advanced stages when diagnosed; thus, radiotherapy and chemoradiotherapy play a crucial part in the treatment of these patients [2].
The International Federation of Gynecology and Obstetrics (FIGO), a global professional organization, developed a staging system for widely accepted independent prognostic risk factors used in clinical practice. The FIGO staging system is a clinical staging system based primarily on physical examination findings. In the FIGO 2009 staging system, lymph node metastasis was not included as a prognostic factor, despite being proven by several studies to be a strong prognostic factor for uterine cervical carcinoma [3, 4]. Therefore, the FIGO staging system was revised in 2018 and 2019 to account for lymph node status and emphasize the role of imaging in prognosis [5, 6]. While the FIGO 2018 staging system improved discriminatory power for stages I and IV [7], it did not improve discriminatory power for other stages, especially for stage IIIC [8]. This could be because tumor volume was not included in staging criteria, despite its significant correlation with prognosis even in the evaluation of same-stage tumors [9].
In the FIGO 2018 staging system, magnetic resonance imaging (MRI) plays a central role in uterine cervical cancer staging because it provides excellent contrast resolution, especially for soft tissue, and offers multiparametric imaging. Therefore, it has the advantages of delineating tumor extent and discriminating lymph node metastasis with high accuracy [10,11,12]. Numerous studies have shown that tumor diameter is an independent prognostic risk factor for uterine cervical carcinoma [13,14,15]. However, for a malignant tumor, estimation of the tumor volume based only on a single diameter produces inaccurate results because of its complicated and irregular shape. Kim et al. demonstrated that MRI-derived pretreatment tumor volume, but not pretreatment tumor diameter, was significantly correlated with the prognosis of patients with uterine cervical carcinoma who received concurrent chemotherapy and radiotherapy [16].
Squamous cell carcinoma antigen (SCC-Ag), which was discovered by Kato and Torigoe, is a characteristic biomarker for squamous cell carcinoma [17]. The expression level emerges synchronously with the squamous formation of the uterine cervix and increases during the neoplastic transformation of the cervical squamous epithelium [18]. SCC-Ag levels are elevated in 28–88% of patients with uterine cervical squamous cell carcinoma [19]. However, the ability of SCC-Ag to predict the prognosis of uterine cervical carcinoma remains controversial, with some researchers unable to demonstrate any predictive ability of the parameter at all [20]. In contrast, several researchers have demonstrated that SCC-Ag levels alone or in combination with other factors were significantly correlated with the prognosis and even had the capacity to predict the efficacy of treatment or risk of recurrence [21,22,23,24]. Thus, we sought to explore the role of SCC-Ag in the present study.
A nomogram can convert complicated results of multivariate analyses into a visual graph that is simple and easy to understand. Many studies have established a prognostic model using a nomogram for patients with uterine cervical carcinoma who received definitive radiotherapy [25, 13, 26]. However, these studies did not use the latest FIGO 2018 staging system.
This study aimed to investigate the long-term prognostic factors for patients with uterine cervical squamous cell carcinoma who received definitive radiotherapy and develop a model to predict prognosis in the context of the FIGO 2018 staging system and the widespread application of MRI.
Methods
Participants
We reviewed the medical records of patients with uterine cervical carcinoma who were diagnosed between 2013 and 2014 and received definitive radiotherapy in our institute. Patients with histologically confirmed squamous cell carcinoma of the uterine cervix that was restaged as II-IIIC2r according to the FIGO 2018 staging system received definitive radiotherapy and underwent an enhanced abdominopelvic MRI scan prior to treatment were enrolled (the flowchart is shown in Fig. 1). Patients with any of the following circumstances were excluded from the study: radiographic evaluation other than MRI, missing data, secondary cancer, or refusal to participate in this study. Overall survival (OS) was defined as the time between diagnosis and death.
SCC-Ag detection
Fasting venous blood was taken from patients in the morning before treatment, and serum was separated by centrifugation within 4 h. Abbott Diagnostics I2000 automatic chemiluminescence immunoassay analyzer and supporting reagents were used to detect SCC-Ag. The operation was carried out in strict accordance with the instrument’s operation specifications and the kit’s instructions. Since serum SCC-Ag level was not increased in all patients with cervical squamous cell carcinoma [19], was also elevated in other types of cancers [27, 28], and was affected by reduced kidney function [29], the sensitivity and specificity were both unsatisfactory. Thus, we aimed to explore the prognostic value of SCC-Ag combined with other clinical factors.
MRI analysis
A superconducting MRI scanner (Signa 1.5 T Excite iii HD, GE) was used to obtain the images before any treatment was started. The MRI scan sequences included fast spin echo (FSE), T1-weighted imaging (T1WI), FSE T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and coronal FSE T2WI. DWI was performed using a spin echo plane echo sequence. After plain scanning, gadopentetic acid was injected at 3 mL/s through the cubital vein at 0.1 mmol/kg, and then axial, sagittal, and coronal scans using a liver acquisition with volume acceleration sequences were carried out on gradient echo T1WI of 3D volumetric interpolation. On axial T2WI images showing the largest tumor, tumor length was defined as the largest diameter in the left–right direction and tumor width as the vertical diameter in the anterior–posterior direction. On sagittal T2WI images showing the largest tumor, tumor thickness was defined as the longest diameter in the foot–head direction. The tumor volume was calculated using the following equation: π × tumor length (cm) × tumor width (cm) × tumor thickness (cm)/6 = V. Enlarged lymph nodes with short diameters of > 1 cm were considered to be metastatic lymph nodes. All MRI data and medical records were retrospectively reviewed and confirmed by two radiologists with more than 10 years of experience in interpreting gynecologic oncology images.
Definitive radiotherapy
All patients received definitive radiotherapy. Radiotherapy volume, dose, and protocol were confirmed with a central review by gynecological oncologists Qin Chen and Min Wang. Radiotherapy in this study consisted of two parts: external beam radiotherapy (EBRT) and brachytherapy (BT). In EBRT, some patients received conventional radiotherapy, while others received intensity-modulated radiotherapy, which was administered through 6 MV photons once a day, 5 days a week. The prescription dose of EBRT was 45–56 Gy at 1.8–2.0 Gy per fraction. Patients with FIGO 2018 stage IIIc2r or IIIc1r with common iliac lymph node metastasis received extended-field irradiation, which included the whole pelvic and para-aortic lymph node area. Since BT was superior to inversely planned EBRT in both target doses and organs at risk (OAR) sparing, BT is irreplaceable in radical radiotherapy of locally advanced cervical carcinoma [30]. All these patients received high-dose-rate BT, which was started 2 weeks after the beginning of EBRT and was performed once a week, with the A point dose of 7 Gy per fraction. After the initial EBRT and BT was completed, evaluations were separately conducted by two gynecological oncologists. Patients were treated with an additional 1 or 2 times of BT, depending on the time of radiotherapy being more than 8 weeks, tumor regression, and tolerance doses of OAR. BT was administered 3–7 times. The overall treatment time of radical radiotherapy was controlled within 56 days, as much as possible. Subsequently, both the EBRT and BT doses were converted into 2-Gy equivalent doses (EQD2), respectively, using the following linear-quadratic model: prescription dose × (α/β + fractionated dose) / (α/β + 2) = EQD2, where α/β = 10. The EQD2 for EBRT and BT were then summed up to obtain the dose for analysis in this study.
Chemotherapy
Most patients in this study received chemotherapy, except for those who were older, had contraindications, and refused to receive it. These patients also received different chemotherapy regimens. Fourteen patients received platinum monotherapy with concurrent chemoradiation therapy. A total of 300 patients received platinum-based dual drug chemotherapy, of which 7 patients used docetaxel due to paclitaxel allergy, and the remaining 293 patients used paclitaxel. Two patients received intravenous gemcitabine and platinum chemotherapy due to taxane allergy. To stop massive vaginal bleeding, 8 patients with bulky tumors received interventional chemotherapy with paclitaxel, cisplatin, and bleomycin. Considering these scenarios, we included the presence of chemotherapy as a factor in the analysis.
Statistical analysis
Patient information was anonymized prior to analysis. Using computer-generated random numbers, we grouped the patients into training and validation cohorts at a ratio of 7:3. The function “surv_cutpoint” in the R package “survminer” was implemented to generate the optimal cutoff values of SCC-Ag and tumor volume for the training cohort [31]. Continuous parameters presented as means ± standard deviation or medians with interquartile ranges were compared between the training and validation sets using Student’s t-test or the Mann–Whitney U test, as appropriate. The chi-squared test or Fisher’s exact test was used to compare the frequency distribution of categorized parameters.
A nomogram was constructed based on significant predictors of overall survival (OS) selected by multivariate Cox proportional hazards regression using a stepwise selection method that included variables with a p-value of < 0.05 in the univariate analysis and of clinical importance. In the nomogram, points were assigned by drawing a line upward from the corresponding values to the “Points” line. The sum of the points, plotted on the “Total Points” line, corresponds to predictions of 3-year, 5-year, and 7-year OS rates in patients with uterine cervical squamous cell carcinoma. Based on the concordance index (C-index) and the area under the time-dependent receiver operating characteristic curve (AUC), the predictive accuracy of the constructed nomogram was evaluated. Calibrating this constructed nomogram, we used bootstraps of 1000 resamples with calibration curves. The performance of the nomogram was internally validated using the validation set and compared with that of the FIGO 2018 staging system. To measure the improvement in the predictive effect of our model, we used the net reclassification improvement (NRI) and integrated discrimination improvement (IDI). It is only possible to evaluate a diagnostic method by assessing its receiver operating characteristic curve from the specificity and sensitivity plot; however, although this is considered an accurate method, the patients are not guaranteed to benefit from it. A method of evaluation devised by Vickers and Elkin, called decision curve analysis (DCA), is capable of calculating the net benefits of the model [32]. In this study, the sum score of each patient was calculated from the nomogram. Subsequently, cutoff points for risk stratification (low and high) were generated using the “surv_cutpoint” function of “survminer.” The log-rank test was used to compare the Kaplan–Meier survival curves of the low-risk and high-risk groups using the training and validation sets. Our statistical analyses were performed using R software (version 4.0.3). We considered a p-value of 0.05 to be statistically significant.
Results
Patient OS and grouping
A total of 484 patients with uterine cervical squamous cell carcinoma were restaged according to the FIGO 2018 staging system. Four patients who had other malignancies, 72 patients who underwent non-MRI scanning prior to any treatment, and 12 patients who were lost to follow-up were excluded. Finally, 396 patients were enrolled in this study. The date of the last follow-up was December 12, 2021. The median follow-up time was 89.77 months. The 3-year, 5-year, and 7-year OS rates were 87.1%, 83.3%, and 81.8%, respectively.
Cutoff values, patient characteristics, and results of univariate and multivariate analyses of factors for OS
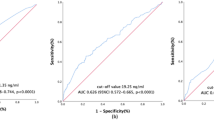
In total, 277 and 119 patients were randomized to the training and validation sets, respectively. The cutoff value of SCC-Ag derived from the training set was 11.5 ng/mL (Fig. 2). The cutoff value of tumor volume derived from MRI was 28.85 cm3 using the same method as that for SCC-Ag (Fig. 3). SCC-Ag and tumor volumes were converted into categorical variables depending on their cutoff values. Patient characteristics in both the training and validation sets are shown in Table 1. There were no significant differences between the training and validation sets in terms of age at diagnosis, SCC-Ag level, MRI-derived tumor volume, radiotherapy modality, presence of chemotherapy, time of radiotherapy, EQD2 of point A, histologically poor differentiation, hemoglobin level before treatment, parametrial invasion status, FIGO 2018 stage, and survival status. In the multivariate analysis, SCC-Ag level, histologically poor differentiation, MRI-derived tumor volume, presence of chemotherapy, and FIGO 2018 stage were found to be significant prognostic factors for uterine cervical squamous cell carcinoma (Table 2).
Establishment and evaluation of the prognostic model
The established nomogram for predicting OS is shown in Fig. 4. The corresponding score can be calculated through the top “Points” line of the nomogram for each prognostic factor, and then the 3-year, 5-year, and 7-year OS could be estimated by summing up the individual scores and checking the “Total Points” line at the bottom. The C-indices of our nomogram for the training and validation sets were 0.74 (95% confidence interval [CI]: 0.67–0.80) and 0.70 (95% CI: 0.57–0.82), while those of the FIGO 2018 staging system were 0.66 (95% CI: 0.59–0.73) and 0.63 (95% CI: 0.52–0.75), respectively (Table 3). For our model, the AUCs of the validation set for 3-year, 5-year, and 7-year OS were 0.67, 0.68, and 0.71, respectively. Meanwhile, the corresponding AUCs of the FIGO 2018 staging system were 0.65, 0.66, and 0.65. Notably, our model had higher C-indices and AUCs than the FIGO 2018 staging system, suggesting that our model has a better discrimination ability than the FIGO staging system (Fig. 5). Calibration curves with bootstraps of 1000 resamples also indicated a good agreement between the predicted OS and observed outcomes (Fig. 6). As shown in Table 4, the NRI values of our nomogram for 3-year, 5-year, and 7-year OS were 0.21, 0.38, and 0.38, respectively, in the validation set (all p < 0.01); the corresponding IDI values were 0.08, 0.14, and 0.10 (all p < 0.01). These values suggest that the predictive performance of our nomogram was substantially improved. DCA indicated that the net benefits of our model were higher than those of the FIGO staging system at 3, 5, and 7 years (Fig. 7).
The area under the time-dependent receiver operating characteristic curves for 3-year, 5-year and 7-year OS from the validation set. A AUC for 3-year OS. B AUC for 5-year OS. C AUC for 7-year OS. OS overall survival, AUC area under the curve, FIGO International Federation of Gynecology and Obstetrics
Risk stratification by the nomogram
The cutoff point for risk stratification (low and high) was 171.21, based on the scores of patients in the training group. The OS of patients in the high-risk group was significantly shorter than that in the low-risk group in both the training (p < 0.0001) and validation sets (p = 0.00055) (Fig. 8).
Discussion
We established a prognostic model to predict the long-term OS of patients with FIGO 2018 stage II-IIIC2r uterine cervical squamous cell carcinoma who received definitive radiotherapy. In this study, we found that factors such as the SCC-Ag level, histologically poor differentiation, tumor volume derived from MRI prior to treatment, FIGO 2018 stage, and presence of chemotherapy were predictors of prognosis in these patients.
We found that uterine cervical squamous cell carcinoma with elevated pretreatment serum SCC-Ag concentrations of more than 11.5 ng/ml had a significantly worse prognosis than those with concentrations less than or equal to 11.5 ng/ml. The outcomes from some other studies were in line with ours. Cheng et al. found that pretreatment SCC Ag >10 ng/mL was a significant poor prognostic factor of progression-free survival (PFS), locoregional recurrence-free survival, and distant metastasis-free survival in patients with stage IB-IVA cervical cancer in patients who underwent concurrent chemoradiation therapy [33]. Chen et al. enrolled 203 patients with stage IIA–IVA cervical squamous cell carcinoma and found in their study that pretreatment SCC >11.4 ng/mL was an independent predictor of PFS [34]. Apparently, increased SCC-Ag level was associated with a worse patient outcome [35]. The underlying reason for this may be that SCC-Ag could promote radioresistance of tumor cells by suppressing radiation-induced cell death [36]. Also, Shou et al. found that high SCC-Ag (≥14.6 ng/mL) was associated with the FIGO 2018 stage [37], with a more advanced stage indicating a poorer prognosis.
Brambs et al. reexamined the histological slides of 467 patients with surgically treated FIGO stage IB1–IIB uterine cervical carcinoma and found that binary grading (grade 1/2 vs. grade 3) may be more suitable for evaluating prognostic survival than conventional tumor grading based on the degree of keratinization [38]. This is also the reason we only considered poor differentiation, and not tumor grade, in our analysis, and our results are consistent with those of other studies. Studies by Xie et al. and Luo et al. showed that patients with poor differentiation (grade 3) had a significantly worse OS than those with grade 1/2 uterine cervical carcinoma in the early stage (FIGO stages IA2–IIB) [39, 40]. Using data from 31,536 women with uterine cervical squamous cell carcinoma extracted from the Surveillance, Epidemiology, and End Results (SEER) Program between 1983 and 2013, Matsuo et al. found that grade 3 tumors (poor differentiation) were independently associated with decreased cause-specific survival, especially among patients with stage II–III disease [41]. These findings could be attributed to the keratin pattern being a component of aggregated cervical squamous cell carcinoma related to survival [42]. On the contrary, Kumar et al. analyzed patients who were diagnosed with uterine cervical squamous cell carcinoma between 1988 and 2004 using limited data from the SEER Program and figured out that nonkeratinized squamous cell carcinoma, rather than keratinized squamous cell carcinoma, might be more radiosensitive and associated with a better prognosis [43]. Notably, the racial composition of the Asian population was 11.4%, and the proportion of poorly differentiated cases in our study was approximately 16%—a reason why the results of the above-mentioned studies are different from ours and why our findings are only applicable to Chinese patients.
FIGO 2018 is a clinical staging system based on physical examination and imaging. Gynecologists rely heavily on physical examination when evaluating primary tumors. However, palpation as a component of physical examination is a subjective method that can only determine the axial diameter of the tumor but cannot estimate the contribution of normal cervical tissue. Thus, clinical estimation of tumor size through palpation cannot adequately represent the actual tumor volume [44]. Narayan et al. demonstrated in their study that tumor volume measured using MRI accurately reflected the extent of local disease and could be used as an objective measurement of the primary site of cervical cancer [45]. Other researchers also demonstrated that an increase in tumor volume is associated with lymph node metastasis and poor prognosis [46, 47]. Some investigators have even observed that MRI-derived tumor volume provides more accurate and useful prognostic information than that provided by the FIGO staging system [48]. However, not all these studies used the FIGO 2018 staging system. In our results, MRI-derived tumor volume was a critical prognostic factor for FIGO 2018 stage II–IIIC2r uterine cervical squamous cell carcinoma.
The FIGO staging system is widely used in the clinical management of uterine cervical carcinoma and is a paramount factor affecting the treatment outcome. However, there are other prognostic factors that must be considered. Lymph node metastasis could strongly decrease the survival of patients with uterine cervical carcinoma, and in this regard, the FIGO 2018 staging system defined stage IIIC1 as pelvic lymph node metastasis and stage IIIC2 as para-aortic lymph node metastasis, both of which can be suffixed with the letter “r” or “p” to refer to a radiological or pathological finding, respectively [5]. Therefore, we contrasted a nomogram using the FIGO 2018 staging system and other clinical factors, with emphasis on MRI-derived tumor volume. NRI and IDI are indices indicating how a model’s predictive power improves after a new risk factor(s) is introduced. A value of > 0 indicates improvement. In this study, the NRI and IDI values for 3-year, 5-year, and 7-year OS were all > 0, suggesting that our model achieved a better predictive ability than the FIGO 2018 staging system. Thus, our nomogram could offer patients accurate individual predictions.
Moreover, synchronous radiotherapy and platinum-based chemotherapy is the standard treatment for locally advanced cervical cancer. Indeed, chemotherapy is thought to act as a radiosensitizer with the aim of eradicating occult metastases [49]. Notably, most patients enrolled in our study received platinum- and taxane-based dual drug chemotherapy. Our results confirmed that chemotherapy may improve the OS of patients, and chemotherapy was included in our model as a therapeutic factor. Garces et al. demonstrated that paclitaxel–carboplatin is an active and well-tolerated regimen for the treatment of advanced cervical cancer [50]. Moreover, a multicenter phase II trial conducted by Takekuma et al. demonstrated that intravenous paclitaxel and nedaplatin in patients with advanced/recurrent uterine cervical cancer exhibited favorable antitumor activity [51]. In addition, a systematic review and meta-analysis involving 17 published studies and 4106 patients identified that concurrent chemoradiotherapy with platinum-based dual drug therapy improved OS and PFS of locally advanced cervical carcinoma patients relative to concurrent chemoradiotherapy with platinum monotherapy [52]. All of above findings are consistent with our observations. This chemotherapy parameter could be very useful both to gynecologic oncologists when creating treatment plans and to patients during decision-making for accepting those plans. For instance, if a virtual 59-year-old patient with a histologically confirmed uterine cervical squamous cell carcinoma with FIGO stage IIIb, pretreatment SCC-Ag level of 15.0 ng/mL, and MRI-derived tumor volume of 35 cm3 decides to undergo chemotherapy, the total score for all parameters calculated from the nomogram will be 139, and the predictive 3-year, 5-year, and 7-year OS will be 86%, 80%, and 78%, respectively. However, if the patient refuses to receive chemotherapy, the total score will be 187, and the predictive 3-year, 5-year, and 7-year OS will be 73%, 63%, and 60%. In this example, radiotherapy without chemotherapy is associated with an apparent decrease in OS.
There are several nomograms established by other researchers for predicting uterine cervical carcinoma prognosis following radiotherapy [26, 53, 54]. Other researchers explored the predictive accuracy of the FIGO 2018 staging system and other significant prognostic factors; however, they have not investigated the value of MRI-derived tumor volume in predicting prognosis of these patients [55, 56]. To our knowledge, this nomogram is the first long-term model for predicting OS in patients with uterine cervical squamous cell carcinoma who received definitive radiotherapy using the FIGO 2018 staging system and pretreatment tumor volume derived from MRI.
This study has some limitations that should be acknowledged. First, it was a retrospective study and is therefore prone to selection bias. However, several measures were taken to minimize selection bias. From study design to implementation, we selected patients with cervical squamous cell carcinoma that were treated in our hospital from 2013 to 2014. Moreover, all case data were collected from electronic data. During this period, there were no significant changes in the treatment methods for cervical cancer, and long-term follow-up was conducted on the patients. Finally, during the analysis process, the patients were randomly divided into the training and a validation sets, with balanced baseline data. Second, the chemotherapy regimens in this study were heterogeneous.
Specific chemotherapy schemes included platinum single drug chemotherapy, platinum and taxane dual drug chemotherapy, such as paclitaxel and platinum, docetaxel and platinum, gemcitabine and platinum dual drug chemotherapy, as well as paclitaxel, cisplatin, and bleomycin interventional chemotherapy scheme. As a result, we only analyzed the presence of chemotherapy as a factor in our research and found that chemoradiotherapy could improve the OS of patients compared to radiotherapy alone. Nevertheless, further stratified analysis of the specific chemotherapy regimens is necessary. Third, radiotherapy plays a significant role in the treatment of cervical cancer. Compared with traditional 2D radiation therapy, intensity modulated radiotherapy (IMRT) has dosimetric advantages in organ preservation and has made possible safer dose escalation especially to the para-aortic region. This provides better clinical outcomes while reducing toxicity [57,58,59]. However, we did not observe a significant impact of IMRT on survival in our study. The underlying reason for this may be that patients with lymph node metastasis were more likely to receive IMRT, while patients without lymph node metastasis were more likely to receive conventional radiation therapy. FIGO 2018 stage of patients with lymph node metastasis were more advanced, this may have led to the effectiveness of IMRT being underestimated. Fourth, although our prediction model was developed using data from a single institution, the presented results are based on a follow-up period of 89.77 months. In addition, all the predictive factors included in this study are easy to obtain. Therefore, external validation for our findings at multiple centers is clinically feasible. However, patients with cervical squamous cell carcinoma have a relatively good prognosis, and hence our research focused on the long-term survival; this requires several years of follow-up. Therefore, we plan to perform this analysis in a future study.
Conclusions
We established a nomogram using MRI-derived tumor volume to predict the long-term outcomes of patients with FIGO 2018 stage II–IIIC2r uterine cervical squamous cell carcinoma. The tool may be useful to gynecologic oncologists when creating treatment plans and predicting individual prognoses and to patients when making treatment decisions.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J. Estimates of incidence and mortality of cervical cancer in, et al. a worldwide analysis. Lancet Glob Health. 2018;8(2020):e191–203.
NCCN. The NCCN cervical cancer clinical practice guidelines in oncology (Version 1.2022). 2021. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed Oct 2021.
Bae HS, Kim YJ, Lim MC, Seo SS, Park SY, Kang S, et al. Predictors of radiation field failure after definitive chemoradiation in patients with locally advanced cervical cancer. Int J Gynecol Cancer. 2016;26:737–42.
Nanthamongkolkul K, Hanprasertpong J. Predictive factors of pelvic lymph node metastasis in early-stage cervical cancer. Oncol Res Treat. 2018;41:194–8.
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36.
Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019; 145:129–135.
Mohamud A, Høgdall C, Schnack T. Prognostic value of the. FIGO staging system for cervical cancer. Gynecol oncol. 2018;165(2022):506–13.
Kim J, Cho Y, Kim N, Chung SY, Kim JW, Lee IJ, et al. Magnetic resonance imaging-based validation of the 2018 FIGO staging system in patients treated with definitive radiotherapy for locally advanced cervix cancer. Gynecol oncol. 2021; 160:735–741.
Raut A, Chopra S, Mittal P, Patil G, Mahantshetty U, Gurram L, Classification FIGO, et al. validation study in patients with locally advanced cervix cancer treated with chemoradiation. Int j radiat oncol. 2018;108(2020):1248–56.
Stenstedt K, Hellström AC, Fridsten S, Blomqvist L. Impact of MRI in the management and staging of cancer of the uterine cervix. Acta Oncol. 2011; 50:420–6.
Liu Y, Liu H, Bai X, Ye Z, Sun H, Bai R, et al. Differentiation of metastatic from non-metastatic lymph nodes in patients with uterine cervical cancer using diffusion-weighted imaging. Gynecol Oncol. 2011;122:19–24.
Balleyguier C, Sala E, Da Cunha T, Bergman A, Brkljacic B, Danza F, et al. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2011;21:1102–10.
Yang J, Tian G, Pan Z, Zhao F, Feng X, Liu Q, et al. Nomograms for predicting the survival rate for cervical cancer patients who undergo radiation therapy: a SEER analysis. Future Oncol. 2019;15:3033–45.
Wang J, Wang T, Yang YY, Chai YL, Shi F, Liu ZI. Patient age, tumor appearance and tumor size are risk factors for early recurrence of cervical cancer. Mol Clin Oncol. 2015;3:363–6.
Xie G, Wang R, Shang L, Qi C, Yang L, Huang L, et al. Calculating the overall survival probability in patients with cervical cancer: a nomogram and decision curve analysis-based study. BMC Cancer. 2020;20:833.
Kim HJ, Kim WC. Pretreatment tumor diameter/volume and pelvic lymph node status assessed by magnetic resonance imaging for uterine cervical carcinoma treated with concurrent chemotherapy and radiotherapy. J Obstet Gynaecol Res. 2008;34:529–37.
Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–8.
Maruo T, Yoshida S, Samoto T, Tateiwa Y, Peng X, Takeuchi S, et al. Factors regulating SCC antigen expression in squamous cell carcinoma of the uterine cervix. Tumour Biol. 1998;19:494–504.
Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66:10–20.
Gaarenstroom KN, Kenter GG, Bonfrer JM, Korse CM, Van de Vijver MJ, Fleuren GJ, et al. Can initial serum cyfra 21–1, SCC antigen, and TPA levels in squamous cell cervical cancer predict lymph node metastases or prognosis? Gynecol Oncol. 2000;77:164–70.
Benito V, Lubrano A, Pérez-Regadera JF, Torné A, Gil-Moreno A, Tejerizo-Garcia Á, et al. Postreatment squamous cell carcinoma antigen as a survival prognostic factor in patients with locally advanced cervical cancer. A Spanish multicenter study. The SEGO Spain-GOG group. Gynecol Oncol. 2021; 162:407–412.
Yoo JG, Kim SI, Yeo SG, Park DC. Usefulness of short-term imaging and squamous cell carcinoma antigen to early predict response to con current chemoradiotherapy in patients with cervical cancer. Cancer Control. 2022;29:10732748221074530.
Choi KH, Lee SW, Yu M, Jeong S, Lee JW, Lee JH. Significance of elevated SCC-Ag level on tumor recurrence and patient survival in patients with squamous-cell carcinoma of uterine cervix following definitive chemoradiotherapy: a multi-institutional analysis. J Gynecol Oncol. 2019;30: e1.
Wen YF, Cheng TT, Chen XL, Huang WJ, Peng HH, Zhou TC, et al. Elevated circulating tumor cells and squamous cell carcinoma antigen levels predict poor survival for patients with locally advanced cervical cancer treated with radiotherapy. PLoS ONE. 2018;13: e0204334.
Rose PG, Java J, Whitney CW, Stehman FB, Lanciano R, Thomas GM, et al. Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG oncology/gynecologic oncology group randomized trials of chemoradiotherapy. J Clin Oncol. 2015;33:2136–42.
Sturdza AE, Pötter R, Kossmeier M, Kirchheiner K, Mahantshetty U, Haie-Meder C, et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with radiochemotherapy including image-guided brachytherapy: a retro-EMBRACE study. Int J Radiat Oncol Biol Phys. 2021; 111:168–177.
Jiang C, Zhao M, Hou S, Hu X, Huang J, Wang H, et al. The indicative value of serum tumor markers for metastasis and stage of non-small cell lung cancer. Cancers (Basel). 2022; 14:5064.
Kanie Y, Okamura A, Maruyama S, Sakamoto K, Fujiwara D, Kanamori J, et al. Clinical significance of serum squamous cell carcinoma antigen for patients with recurrent esophageal squamous cell carcinoma. Ann surg oncol. 2021;28:7990–6.
Obata K, Yutori H, Yoshida K, Sakamoto Y, Ono K, Ibaragi S. Relationships between squamous cell carcinoma antigen and cytokeratin 19 fragment values and renal function in oral cancer patients. Int J Oral Max Surg. 2022.
Georg D, Kirisits C, Hillbrand M, Dimopoulos J, Potter R. Image-guided radiotherapy for cervix cancer: high-tech external beam therapy versus high-tech brachytherapy. Int J Radiat Oncol Biol Phys. 2008;71:1272–8.
Sun L, Ke X, Wang D, Yin H, Jin B, Xu H, et al. Prognostic value of the albumin-to-γ-glutamyltransferase ratio for gallbladder cancer patients and establishing a nomogram for overall survival. J Cancer. 2021;12:4172–82.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–74.
Cheng YK, Kuo SH, Yen HH, Wu JH, Chen YC, Huang MY. The prognostic significance of pretreatment squamous cell carcinoma antigen levels in cervical cancer patients treated by concurrent chemoradiation therapy and a comparison of dosimetric outcomes and clinical toxicities between tomotherapy and volumetric modulated arc therapy. Radiat Oncol. 2022;17:91.
Chen W, Xiu S, Xie X, Guo H, Xu Y, Bai P, et al. Prognostic value of tumor measurement parameters and SCC-Ag changes in patients with locally-advanced cervical cancer. Radiat Oncol. 2022;17:6.
Charakorn C, Thadanipon K, Chaijindaratana S, Rattanasiri S, Numthavaj P, Thakkinstian A. The association between serum squamous cell carcinoma antigen and recurrence and survival of patients with cervical squamous cell carcinoma: a systematic review and meta-analysis. Gynecol oncol. 2018;150:190–200.
Murakami A, Suminami Y, Hirakawa H, Nawata S, Numa F, Kato H. Squamous cell carcinoma antigen suppresses radiation-induced cell death. Br J Cancer. 2001;84:851–8.
Shou H, Yasuo Y, Yuan S, Lou H, Ni J. Association of pretreatment SUVmax of cervix and SCC-antigen with FIGO2018 stage in stage IIB-IVB squamous cervical cancer and relationship to prognosis. Int j gynecol obstet. 2021;152:112–7.
Brambs CE, Höhn AK, Hentschel B, Fischer U, Bilek K, Horn LC. The prognostic impact of grading in FIGO IB and IIB squamous cell cervical carcinomas. Geburtshilfe Frauenheilkd. 2019; 79:198–204.
Xie XZ, Song K, Cui B, Jiang J, Zhang YZ, Wang B, et al. Clinical and pathological factors related to the prognosis of Chinese patients with stage Ib to IIb cervical cancer. Asian Pac J Cancer Prev. 2012;13:5505–10.
Luo H, Yao H, Xu X, Li Z, Zhao H, Zhu H. Prognostic significance of poorly differentiated histology and impact of adjuvant chemotherapy in early squamous cell carcinoma of cervix uteri. Cancer Med. 2021;10:2611–7.
Matsuo K, Mandelbaum RS, Machida H, Purushotham S, Grubbs BH, Roman LD, et al. Association of tumor differentiation grade and survival of women with squamous cell carcinoma of the uterine cervix. J Gynecol Oncol. 2018;29: e91.
N. Cancer Genome Atlas Research, M. Albert Einstein College of, S. Analytical Biological, H. Barretos Cancer, M. Baylor College of, H. Beckman Research Institute of City of, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017; 543:378–384.
Kumar S, Shah JP, Bryant CS, Imudia AN, Ali-Fehmi R, Malone JM, et al. Prognostic significance of keratinization in squamous cell cancer of uterine cervix: a population based study. Arch Gynecol Obstet. 2009;280:25–32.
Burghardt E, Baltzer J, Tulusan AH, Haas J. Results of surgical treatment of 1028 cervical cancers studied with volumetry. Cancer. 1992;70:648–55.
Narayan K, McKenzie A, Fisher R, Susil B, Jobling T, Bernshaw D. Estimation of tumor volume in cervical cancer by magnetic resonance imaging. Am J Clin Oncol. 2003;26:e163–8.
Trattner M, Graf AH, Lax S, Forstner R, Dandachi N, Haas J, et al. Prognostic factors in surgically treated stage ib-iib cervical carcinomas with special emphasis on the importance of tumor volume. Gynecol Oncol. 2001;82:11–6.
Kim H, Kim W, Lee M, Song E, Loh JJ. Tumor volume and uterine body invasion assessed by MRI for prediction of outcome in cervical carcinoma treated with concurrent chemotherapy and radiotherapy. Jpn J Clin Oncol. 2007;37:858–66.
Narayan K, Fisher R, Bernshaw D. Significance of tumor volume and corpus uteri invasion in cervical cancer patients treated by radiotherapy. Int J Gynecol Cancer. 2006;16:623–30.
Liontos M, Kyriazoglou A, Dimitriadis I, Dimopoulos MA, Bamias A. Systemic therapy in cervical cancer: 30 years in review. Crit Rev Oncol Hematol. 2019;137:9–17.
Garces ÁH, Mora PA, Alves FV, do Carmo CC,Grazziotin R, Fernandes AC et al. First-line paclitaxel and carboplatin in persistent/recurrent or advanced cervical cancer: a retrospective analysis of patients treated at Brazilian National Cancer Institute. Int J Gynecol Cancer. 2013; 23:743–8.
Takekuma M, Hirashima Y, Ito K, Tsubamoto H, Tabata T, Arakawa A, et al. Phase II trial of paclitaxel and nedaplatin in patients with advanced/recurrent uterine cervical cancer: a Kansai Clinical Oncology Group study. Gynecol Oncol. 2012;126:341–5.
Deng T, Gu S, Wu J, Yu Y. Comparison of platinum monotherapy with concurrent chemoradiation therapy versus platinum-based dual drug therapy with concurrent chemoradiation therapy for locally advanced cervical cancer: a systematic review and meta-analysis. Infect Agent Cancer. 2022; 17:18.
Seo Y, Yoo SY, Kim MS, Yang KM, Yoo HJ, Kim JH, et al. Nomogram prediction of overall survival after curative irradiation for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2011;79:782–7.
Polterauer S, Grimm C, Hofstetter G, Concin N, Natter C, Sturdza A, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer. 2012;107:918–24.
Yang X, An J, Zhang Y, Yang Y, Chen S, Huang M, et al. Prognostic nomograms predicting survival in patients with locally advanced cervical squamous cell carcinoma: the first nomogram compared with revised FIGO 2018 staging system. Front Oncol. 2020;10: 591700.
Feng Y, Wang Y, Xie Y, Wu S, Li Y, Li M. Nomograms predicting the overall survival and cancer-specific survival of patients with stage IIIC1 cervical cancer. BMC Cancer. 2021;21:450.
Fernandez-Ots A, Crook J. The role of intensity modulated radiotherapy in gynecological radiotherapy: present and future. REP PRACT ONCOL RADI. 2013;18:363–70.
Wang Y, Lo TT, Wang L, Hsu ST, Hwang SF, Lu CH, et al. Long-term efficacy and toxicity of intensity-modulated radiotherapy in bulky cervical cancer. Int J Environ Res Public Health. 2023; 20:1161.
Du XL, Sheng XG, Jiang T, Yu H, Yan YF, Gao R, et al. Intensity-modulated radiation therapy versus para-aortic field radiotherapy to treat para-aortic lymph node metastasis in cervical cancer: prospective study. Croat Med J. 2010;51:229–36.
Acknowledgements
We thank Congmin He for her help with the preparation of data in this paper.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Lele Zang: data collection, writing—original draft, writing—review and editing. Qin Chen: writing—original draft, writing—review and editing. An Lin: conceptualization, writing—review and editing. Jian Chen: investigation, writing—review and editing. Xiaozhen Zhang: investigation, writing—review and editing. Yi Fang: investigation, writing—review and editing. Min Wang: conceptualization, project administration, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Fujian Cancer Hospital (approval no. k2021-087–1). Medical records of patients and images of locally advanced uterine cervical squamous cell carcinomas were retrospectively reviewed, and therefore, the requirement for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zang, L., Chen, Q., Lin, A. et al. A prognostic model using FIGO 2018 staging and MRI-derived tumor volume to predict long-term outcomes in patients with uterine cervical squamous cell carcinoma who received definitive radiotherapy. World J Surg Onc 21, 210 (2023). https://doi.org/10.1186/s12957-023-03116-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03116-4