Abstract
Background
The role of prophylactic drainage (PD) in gastrectomy for gastric cancer (GC) is not well-established. The purpose of this study is to compare the perioperative outcomes between the PD and non-drainage (ND) in GC patients undergoing gastrectomy.
Methods
A systematic review of electronic databases including PubMed, Embase, Web of Science, the Cochrane Library, and China National Knowledge Infrastructure was performed up to December 2022. All eligible randomized controlled trials (RCTs) and observational studies were included and meta-analyzed separately. The registration number of this protocol is PROSPERO CRD42022371102.
Results
Overall, 7 RCTs (783 patients) and 14 observational studies (4359 patients) were ultimately included. Data from RCTs indicated that patients in the ND group had a lower total complications rate (OR = 0.68; 95%CI:0.47–0.98; P = 0.04; I2 = 0%), earlier time to soft diet (MD = − 0.27; 95%CI: − 0.55 to 0.00; P = 0.05; I2 = 0%) and shorter length of hospital stay (MD = − 0.98; 95%CI: − 1.71 to − 0.26; P = 0.007; I2 = 40%). While other outcomes including anastomotic leakage, duodenal stump leakage, pancreatic leakage, intra-abdominal abscess, surgical-site infection, pulmonary infection, need for additional drainage, reoperation rate, readmission rate, and mortality were not significantly different between the two groups. Meta-analyses on observational studies showed good agreement with the pooled results from RCTs, with higher statistical power.
Conclusion
The present meta-analysis suggests that routine use of PD may not be necessary and even harmful in GC patients following gastrectomy. However, well-designed RCTs with risk-stratified randomization are still needed to validate the results of our study.
Similar content being viewed by others
Background
Gastric cancer (GC) is one of the most common causes of cancer-related deaths worldwide [1, 2]. Despite encouraging advances in chemoradiotherapy, targeted therapy, and immunotherapy, surgery remains the cornerstone of treatment for GC. Gastrectomy is regarded as a technically demanding abdominal surgery, with considerable postoperative complications rate, such as anastomotic leakage, bleeding, and intra-abdominal abscess [3, 4].
Prophylactic drainage (PD) has long been routinely performed in abdominal surgery for the purpose of preventing and managing potential postoperative abdominal complications [5, 6]. However, as related research advances, evidence is accumulating that PD may not be as clinically valuable as thought [7, 8]. A previous study involving 17 randomized controlled trials (RCTs) demonstrated that PD did not contribute to the reduction of morbidities following colorectal surgery, appendectomy, hepatectomy, and cholecystectomy [9]. In this context, avoidance of PD is strongly recommended for inclusion in the enhanced recovery after surgery (ERAS) pathway for GC surgery.
However, evidence for avoiding routine PD after surgery in patients with gastric cancer is sparse. In 2020, a meta-analysis based on 10 studies concluded that PD avoidance may favor a reduction in morbidities and a trend towards a decrease in length of stay [10]. Nevertheless, these pooled results were based on only 3 RCTs, which were not in line with those derived from observational studies. Also, the role of PD in other important perioperative outcomes was not well elucidated due to limited data. As a series of new RCTs and observational studies have been published over the years, we aim to perform an updated meta-analysis based on existing evidence to investigate the role of PD in GC patients after gastrectomy.
Methods
Our meta-analysis was performed in line with the requirements from PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [11] and assessing the methodological quality of systematic reviews (AMSTAR) Guidelines [12]. The meta-analysis was registered in PROSPERO (CRD42022371102).
Search strategy
Relevant studies from electronic datasets including PubMed, Embase, Web of Science, the Cochrane Central Register of Controlled Trials, and China National Knowledge Infrastructure were systematically examined up to December 15, 2022. The following key words (limited to title or abstract) were combined with Boolean operators AND or OR, to comprehensively capture potential articles: “drainage,” “drain,” “gastric cancer,” “gastric carcinoma,” “stomach cancer,” and “stomach neoplasm”. The search strategy was applied to suit each database, and the complete search strategy was reported in the supplementary file: Table S1. During the search process, language restrictions were not applied. In addition, the references of the included studies were manually searched for additional reports. The search was performed by two investigators independently (HY-P and LH-C).
Inclusion and exclusion criteria
The inclusion criteria were determined according to the PICOS approach as follows. P: Patients were pathologically diagnosed with GC and underwent gastrectomy; I: non-drainage; C: prophylactic drainage; O: perioperative outcomes; S: Comparative studies including RCTs, cohort and case-controlled studies.
The exclusion criteria were studies (1) reported as case reports, reviews, letters, and abstracts and (2) with overlapping data.
Data extraction
Two independent reviewers (HY-P and LH-C) conducted the data extraction and cross-checked all the results, and any discrepancies were resolved by a third reviewer (H S). The following data were extracted from each study: first author, publication year, study interval, country, study design and sample size, age, sex, neoadjuvant therapy, surgical approach, gastrectomy extent, combined organ resection, surgical margin, lymphadenectomy extent, TNM stage, time of drainage removal, and a series of perioperative outcomes.
Quality assessment and certainty of evidence assessment
The Cochrane Risk-of-Bias 2.0 (RoB 2.0) [13] tool was used to assess the risk of bias for RCTs, from five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. While the Risk of Bias in Non-Randomized Studies-of Interventions (ROBINS-I) [14] tool was used to assess the risk of bias for observational studies, from seven domains: confounding factors, selection of participants into the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported results. Regarding the quality of evidence of each outcome, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [15] approach was applied, which scores each endpoint from very low to high.
Outcomes of interest and definitions
Perioperative outcomes that occur during hospitalization or within 30 days after surgery were assessed in this study, including total complications, anastomotic leakage, duodenal stump leakage, pancreatic leakage, intra-abdominal abscess, surgical-site infection, pulmonary infection, need for additional drainage, time to first soft diet, length of hospital stay, reoperation, readmission, mortality and drain-related complications.
Statistical analysis
The odds ratios (ORs) and mean differences (MDs) with their 95% confidence intervals (CIs) were used as the effect sizes for dichotomous variables and continuous variables, respectively. For studies that reported median with range or inter-quartile range, data were converted into mean with standard deviation (SD) using the method reported by McGrath et al. [16]. Heterogeneity among studies was assessed using I2 statistic. In the present study, all meta-analyses were performed assuming the random-effects model, which accounts for variance across included studies. Subgroup group analysis and meta-regression analysis were performed to investigate the sources of heterogeneity. Publication bias was tested using Begg’s funnel plot when there were at least 10 studies included. A two-tailed P value < 0.05 was considered statistically significant. All of these statistical analyses were performed by Review Manager Software, version 5.3 (Cochrane, London, UK), and Stata, version 12.0 (Statacorp, College Station, TX).
Results
Study characteristics
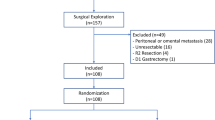
A flow chart of the selection process was shown in Fig. 1. The search strategy yielded 1886 potential studies. After the title, abstract, and full text assessment, 7 RCTs [17,18,19,20,21,22,23] and 14 observational [24,25,26,27,28,29,30,31,32,33,34,35,36,37] studies were finally included in the present study. The basic features of the 21 studies involved were shown in Table 1. A total of 5142 GC patients were included in this study. These studies were from 8 countries and published between 2004 and 2022, with a sample size ranging from 21 to 1989. Among these studies, 5 of them included patients who underwent neoadjuvant therapy. Open and minimally invasive gastrectomy were both performed in these patients. Based on the extent of tumor involvement, these patients underwent either distal, proximal, subtotal, or total gastrectomy regardless of curative resection or not. Combined organ resection was performed when necessary. The criteria of removing the drainage tubes also varied a lot among included studies. Additionally, the incidence of drain-related complications of included studies ranged from 1.5% to 9.4%, mainly including drain site infection, continuous leakage, and omentum coming out.
Meta-analysis of outcomes
Adverse event outcomes
All seven RCTs including 783 patients and 11 observational studies including 2146 patients contributed data for total complications (Table 2 and Fig. 2). The pooled analysis deriving from RCTs demonstrated that patients in the ND group had a 32% lower risk of total complications than the PD group (OR = 0.68; 95%CI:0.47–0.98; P = 0.04; I2 = 0%); observational data showed concordant result, however, without significant difference (OR = 0.87; 95%CI:0.69–1.11; P = 0.26; I2 = 0%). In addition, we compared the incidence of specific complications between the ND and PD groups. As shown in Table 2 and Fig. S1, the distribution of all reported specific complications was not significantly different between the two groups, either in the RCT subset or the observational subset.
Data on mortality were reported in 13 studies (4 RCTs involving 416 patients and 9 observational studies comprising 1881 patients). The pooled result from RCTs suggested that there was no evidence that ND increased the risk of early death (OR = 1.30; 95%CI:0.14–11.92; P = 0.82; I2 = 25%; Table 2 and Fig. S1). Besides, pooled data from observational studies indicated a trend towards lower mortality in the ND group (OR = 0.56; 95%CI:0.17–1.29; P = 0.17; I2 = 0%; Table 2 and Fig. S1).
Postoperative recovery outcomes
A total of 4 RCTs involving 438 patients and 6 observational studies involving 698 patients reported on time to first soft diet (Table 2 and Fig. 3). Pooled data from RCTs showed an earlier time to soft diet in the ND group (MD = − 0.27; 95%CI: − 0.55 to 0.00; P = 0.05; I2 = 0%), which was in line with the results of observational studies (MD = − 0.78; 95%CI: − 1.32 to − 0.24; P = 0.005; I2 = 74%). While the results of the heterogeneity test demonstrated a high heterogeneity among the observational studies.
Data regarding postoperative hospital stay were available from 6 RCTs and 12 observational studies, including 716 and 2303 patients, respectively (Table 2 and Fig. 4). In the RCT subset, patients in the ND group showed a 0.98-day lower length of hospital stay than patients in the PD group (95%CI: − 1.71 to − 0.26; P = 0.007; I2 = 40%). The observational data were consistent, although the statistical difference threshold was not reached (MD = − 0.43; 95%CI: − 1.08 to 0.21; P = 0.19; I2 = 61%). The results of the heterogeneity test demonstrated a moderate and high heterogeneity among the RCTs and observational studies, respectively.
Need for additional drainage, reoperation, and readmission
As shown in Table 2 and Fig. S1, there were 2 RCTs involving 278 patients and 3 observational studies including 2411 patients reporting the need for additional drainage. Pooled results from both the RCTs (OR = 1.07; 95%CI:0.15–7.38; P = 0.95; I2 = 0%) and observational studies (OR = 1.67; 95%CI:0.38–7.23; P = 0.49; I2 = 85%) demonstrated a similar rate of additional drainage between the ND and PD patients. However, the results of the heterogeneity test showed a high heterogeneity among the observational studies.
Two RCTs comprising 168 patients reported on reoperation. Pooled data from RCTs showed no significant difference in reoperation rate between the ND and PD groups (OR = 0.64; 95%CI:0.23–1.84; P = 0.41; I2 = 25%; Table 2 and Fig. S1). Across 5 observational studies comprising 1146 patients, the reoperation rate tended to be lower in the ND group (OR = 0.57; 95%CI:0.29–1.14; P = 0.11; I2 = 0%; Table 2 and Fig. S1).
No RCT was found eligible for evaluating the readmission rate in this study. Three observational studies, with 950 patients involved, demonstrated that there was no significant difference in terms of readmission rate between the two groups (OR = 1.47; 95%CI:0.87–2.47; P = 0.15; I2 = 0%; Table 2 and Fig. S1).
Subgroup analysis and meta-regression analysis
Subgroup analyses stratified by the sample size (≥ 100 vs. < 100) and academic institution (Yes vs. No) were performed to explore the potential discrepant treatment effect of different subgroups. Moreover, the efficacy of PD was also explored in GC patients who underwent laparoscopic surgery or a total gastrectomy. As shown in Fig. S2–5, the findings of all subgroup analyses demonstrated that the perioperative outcomes of the ND group were not inferior to the PD group. In addition, among patients undergoing laparoscopic gastrectomy, the incidence of anastomotic leakage and pancreatic leakage was slightly lower in the ND group. And in patients undergoing total gastrectomy, the reoperation rate (P = 0.06) tended to be lower in the ND group than in the PD group.
For pooled outcomes with significant heterogeneity (time to first soft diet and postoperative hospital stay), meta-regression analyses based on the following covariates were also performed to investigate the sources of heterogeneity: study design (RCT vs. non-RCT), sample size (≥ 100 vs. < 100), academic institution (Yes vs. No), surgical approach (laparoscopic gastrectomy or not) and surgical procedure (total gastrectomy or not). As shown in Table S2, for these pooled results, none of these variables contributed to the source of heterogeneity (all P values > 0.05).
Risk of bias and certainty of evidence assessment
As shown in Fig. 5A, all 7 RCT studies were evaluated using the RoB 2.0 tool and were of some concerns in the overall risk of bias. To be specific, three RCTs had some concerns in the domain of measurement of outcome, and all of them had some concerns in the domain of the randomization process because none of the studies reported specific implementation methods of randomization and allocation concealment. The 14 observational studies were evaluated using the ROBINS-I tool, and 7 of them were moderate risk in the overall risk of bias due to 3 studies had a moderate risk in the domain of confounding factors and 4 studies had a moderate risk in the domain of missing data (Fig. 5B). According to the GRADE approach, the overall certainty of the of evidence of each outcome was low or very low (Table 2).
Publication bias
The Begg’s funnel plot was used to assess the potential publication bias of the pooled outcomes including at least 10 studies. As shown in Table 2 and Fig. S6, all of the P values were greater than 0.05, indicating that these pooled outcomes had a low risk of publication bias.
Discussion
Currently, the routine placement of abdominal drainage tubes after gastrectomy is still widely used worldwide for the early diagnosis and management of critical abdominal complications such as post-operative bleeding, anastomotic leakage, and intra-abdominal infections [5]. Successive studies, however, have shown no clear benefit from prophylactic abdominal drainage [10, 21]. In addition, the placement of drainage tubes increases the patient’s postoperative pain, prolongs the use of analgesics and leads to the occurrence of drainage-related complications [20]. As a result, some institutions no longer routinely perform PD after GC surgery. Nevertheless, as these studies are limited by relatively small sample sizes and underpowered statistics, the conclusions are unclear.
To our knowledge, this is the largest meta-analysis (21 studies including 5142 patients) to evaluate the role of PD in perioperative outcomes of GC surgery. In this study, we found that the routine use of PD after surgery did not reduce the incidence of abdominal complications such as anastomotic leakage and pancreatic leakage. In contrast, the overall complication rate was significantly higher in the PD group. In addition, the length of hospital stay and the time to soft diet were much longer in the PD group than in the ND group. Moreover, PD did not also show any benefit in reducing readmission, reoperation, or mortality in GC surgery.
Several previously published meta-analyses [10, 38,39,40] have demonstrated the potential benefits of PD avoidance in GC patients, which were largely in line with our results. However, those studies were only able to achieve reliable conclusions in a few variables due to a limited number of included studies. At variance, by integrating all applicable RCTs and observational studies, the present study highlighted a faster recovery in the ND group, except for a reduced morbidity and hospital stay, while the previous studies did not find this difference between the two groups. Moreover, benefiting from the increased sample size, nearly all the results in our study showed low heterogeneity and good agreement across the RCT subset and observational subset, further convincing us of the efficacy of ND in GC surgery.
In recent years, laparoscopic surgery has been widely performed in GC, but the role of PD in laparoscopic gastrectomy is still unclear. Therefore, we performed a subgroup analysis for laparoscopic resections. Based on the results from 567 patients who underwent laparoscopic gastrectomy, our finding of the benefit of ND in these patients remained unchanged. Besides, we found that in this subgroup, the incidence of anastomotic leakage (P = 0.11) and pancreatic leakage (P = 0.07) was slightly lower in the ND group, although there was no strong evidence at the pooled analyses that routine ND has an effect on reducing these adverse outcomes. With advances in surgical techniques and laparoscopic equipment, laparoscopic surgery has been shown to be less likely to result in serious postoperative complications in experienced centers, due to its minimally invasive nature [41,42,43]. Consequently, we believe that routinely using PD following laparoscopic gastrectomy is not necessary.
The avoidance of drainage tubes in simple and routine surgery is well understood, but its feasibility in the context of complex surgery is uncertain. Total gastrectomy is a highly complex and challenging surgical procedure in GC patients. Its operation time, intraoperative blood loss, and postoperative complications are much higher than other surgical methods [44, 45]. However, in our present analysis based on 1049 patients, we found that PD did not show any advantage over ND in patients undergoing total gastrectomy. Unexpectedly, several recent meta-analyses demonstrated that even pancreaticoduodenectomy and major liver resection can safely avoid abdominal drainage, which indicated that PD is not a substitute for a meticulous surgical procedure in complex operations [8, 46]. In view of this, avoiding routine drainage should also be recommended during total gastrectomy.
To further clarify the reliability and generalizability of our study, we also analyzed the effect of sample size (≥ 100 vs. < 100) and hospital nature (academic institution vs. non-academic institution) on the perioperative outcomes of PD in GC patients. As shown in Fig. S2–3, the pooled results of these subgroup analyses remained consistent with our previous meta-analyses. These results further convinced us that routine drainage after gastrectomy was not indispensable, even in non-academic hospitals where the surgeons’ expertise and the back system are relatively insufficient compared to academic hospitals.
Nevertheless, our findings are based on literature, some uncertainties exist in the evidence included in this meta-analysis. The lack of stratified information in the original literature prevented us from analyzing the applicability of ND in certain specific subgroups, such as patient demographics (age, BMI, co-morbidity, and history of abdominal surgery), surgical parameters (combined organ resection, extended lymphadenectomy, intra-operative blood loss and sterility of surgery) and oncological variables (neoadjuvant therapy and TNM stage). Therefore, the current evidence does not mean that abdominal drainage should be discontinued in all patients after GC surgery. What we can conclude is the avoidance of routine drainage of a prophylactic nature. Drainage is strongly recommended in some cases, such as abdominal contamination due to perforation and obvious iatrogenic organ injury [40, 47]. In addition, there is evidence demonstrating that PD may be useful in high-risk patients with long operative time or massive intraoperative bleeding [31, 34].
Recently, the first nomogram for predicting the risk of postoperative percutaneous drain placement has been constructed [31]. This prediction model encompassed sex, age, surgical approach, and operative time, which may enable the surgeons to identify high-risk patients, so that PD can be performed selectively. However, this model was derived from a retrospectively study without external validation. Future multicenter RCTs including risk-stratified randomization are urgently needed before final conclusions can be drawn.
The present study has some limitations that should be acknowledged. First, although 7 RCTs were included in our study, the quality of these RCTs was not high and did not also perform stratified analyses in specific populations, which had a certain impact on the reliability of the results of this study. Therefore, more well-designed RCTs with large sample sizes are expected to provide more credible evidence on this issue. Second, several included studies [24, 26, 33] were published over a large time frame, so improvements in gastric surgery and perioperative management during this time could potentially influence the results. Third, there was considerable heterogeneity between studies, including the type of drain used and the period of drain placement, which could also have an impact on the reliability of our results.
Conclusions
The present meta-analysis suggests that the routine use of PD after GC surgery is not beneficial, and even harmful with increased morbidities, and prolonged time to soft diet and hospital stay. However, based on the abovementioned limitations and low level of evidence of the comparisons, more multicenter RCTs with risk-stratified randomization are needed to confirm these questions.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- GC:
-
Gastric cancer
- PD:
-
Prophylactic drainage
- ND:
-
Non-drainage
- RCTs:
-
Randomized controlled trials
- ERAS:
-
Enhanced recovery after surgery
- GRADE:
-
Grading of Recommendations Assessment, Development, and Evaluation
- OR:
-
Odds ratio
- MD:
-
Mean difference
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- RoB:
-
Cochrane Risk-of-Bias
- ROBINS-I:
-
Risk of Bias in Non-Randomized Studies-of Interventions
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Pang H, Zhang W, Liang X, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric cancer: a two-center cohort study. Adv Ther. 2021;38(9):4917–34.
Pang HY, Zhao LY, Wang H, et al. Impact of type of postoperative complications on long-term survival of gastric cancer patients: results from a high-volume institution in China. Front Oncol. 2021;11:587309.
Ojima T, Hayata K, Kitadani J, et al. Risk factors of postoperative intra-abdominal infectious complications after robotic gastrectomy for gastric cancer. Oncology. 2022;100(11):583–90.
Mengardo V, Weindelmayer J, Veltri A, et al. Current practice on the use of prophylactic drain after gastrectomy in Italy: the Abdominal Drain in Gastrectomy (ADiGe) survey. Updates Surg. 2022;74:1839–49.
Vissers FL, Balduzzi A, van Bodegraven EA, et al. Prophylactic abdominal drainage or no drainage after distal pancreatectomy (PANDORINA): a binational multicenter randomized controlled trial. Trials. 2022;23:809.
He S, Xia J, Zhang W, et al. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database Syst Rev. 2021;12:Cd010583.
Dezfouli SA, Ünal UK, Ghamarnejad O, et al. Systematic review and meta-analysis of the efficacy of prophylactic abdominal drainage in major liver resections. Sci Rep. 2021;11:3095.
Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240:1074–84 (discussion 1084-1075).
Weindelmayer J, Mengardo V, Veltri A, et al. Should we still use prophylactic drain in gastrectomy for cancer? a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:1396–403.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
McGrath S, Zhao X, Steele R et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res 2020;29(9):2520-37.
Alvarez Uslar R, Molina H, Torres O, Cancino A. Total gastrectomy with or without abdominal drains. a prospective randomized trial. Rev Esp Enferm Dig. 2005;97:562–9.
Chen BH. Clinical application of abdominal drainage after radical gastrectomy. Guide China Med. 2011;9:242–3.
Jiang ZW, Li JS, Wang ZM et al. Prospective randomized study of abdominal drains in gastric cancer surgery. Chinese Journal of Practical Surgery 2008;28(9):0761-2.
Kim J, Lee J, Hyung WJ, et al. Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg. 2004;8:727–32.
Muduly DK, Imaduddin M, Sultania M, et al. Prophylactic drain versus no drain in curative gastric cancer surgery-a randomized controlled trial. J Gastrointest Surg. 2022;26:2470–6.
Song YF, Ma Y. Necessity of abdominal drainage aft er radical gastrectomy. China Modern Doctor. 2011;49:93–4.
Zhang CK, Zhang HY. The study of necessary of prophylactic drainage in postoperative patients with gastric cancer. Chin Foreign Med Res. 2010;8:3.
Akira S, Masahiko M, Koji O et al. The efficacy of prophylactic drain placement in laparoscopic total gastrectomy: a retrospective study. International surgery 2016; INTSURG-D-16–00111.00111-.
Cai XM. Significance of abdominal drainage after radical gastrectomy for gastric cancer. Contemp Med. 2013;19:25–6.
Dann GC, Squires MH 3rd, Postlewait LM, et al. Value of peritoneal drain placement after total gastrectomy for gastric adenocarcinoma: a multi-institutional analysis from the US gastric cancer collaborative. Ann Surg Oncol. 2015;22(Suppl 3):S888-897.
Fu JC, Cheng H. Retrospective analysis of abdominal drains in gastric cancer surgery. Chin J Mod Med. 2011;13:56–7.
Hirahara N, Matsubara T, Hayashi H, et al. Significance of prophylactic intra-abdominal drain placement after laparoscopic distal gastrectomy for gastric cancer. World J Surg Oncol. 2015;13:181.
Ishikawa K, Matsumata T, Kishihara F, et al. Laparoscopy-assisted distal gastrectomy for early gastric cancer with versus without prophylactic drainage. Surg Today. 2011;41:1049–53.
Kumar M, Yang SB, Jaiswal VK, et al. Is prophylactic placement of drains necessary after subtotal gastrectomy? World J Gastroenterol. 2007;13:3738–41.
Lee J, Choi YY, An JY, et al. Do All patients require prophylactic drainage after gastrectomy for gastric cancer? the experience of a high-volume center. Ann Surg Oncol. 2015;22:3929–37.
Li BH, Zhang CK, Qi XJ. Prophylactic placement of drainage tube after radical distal gastrectomy Discussion on Necessity. Chinese Journal of Gastrointestinal Surgery 2008;11(6):590-1.
Lim SY, Kang JH, Jung MR, et al. Abdominal drainage in the prevention and management of major intra-abdominal complications after total gastrectomy for gastric carcinoma. J Gastric Cancer. 2020;20:376–84.
Liu H, Jin P, Quan X, et al. Feasibility of totally laparoscopic gastrectomy without prophylactic drains in gastric cancer patients. World J Gastroenterol. 2021;27:4236–45.
Schots JPM, Luyer MDP, Nieuwenhuijzen GAP. Abdominal drainage and amylase measurement for detection of leakage after gastrectomy for gastric cancer. J Gastrointest Surg. 2018;22:1163–70.
Shimoike N, Akagawa S, Yagi D, et al. Laparoscopic gastrectomy with and without prophylactic drains in gastric cancer: a propensity score-matched analysis. World J Surg Oncol. 2019;17:144.
Zhang WD, Yao K, Qu Z, Fang SB. Retrospective analysis of abdominal drains in gastric cancer surgery. China Prac Med. 2009;4:18–20.
Liu HP, Zhang YC, Zhang YL, et al. Drain versus no-drain after gastrectomy for patients with advanced gastric cancer: systematic review and meta-analysis. Dig Surg. 2011;28:178–89.
Wang Z, Chen J, Su K, Dong Z. Abdominal drainage versus no drainage post gastrectomy for gastric cancer. Cochrane Database Syst Rev 2011;10(8):CD008788.
Wang Z, Chen J, Su K, Dong Z. Abdominal drainage versus no drainage post-gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2015;2015:Cd008788.
Lin JX, Lin JP, Wang ZK et al. Assessment of laparoscopic spleen-preserving hilar lymphadenectomy for advanced proximal gastric cancer without invasion into the greater curvature: a randomized clinical trial. JAMA Surg 2022.
Song JH, Han SU. Perspectives of laparoscopic surgery for gastric cancer. Chin J Cancer Res. 2022;34:533–8.
Yen HH, Yeh CC, Lai IR. Laparoscopic versus open distal gastrectomy for elderly patients with advanced gastric cancer: a retrospective comparative study. World J Surg Oncol. 2022;20:355.
Pang HY, Zhao LY, Zhang ZQ, et al. Comparisons of perioperative and survival outcomes of laparoscopic versus open gastrectomy for serosa-positive (pT4a) gastric cancer patients: a propensity score matched analysis. Langenbecks Arch Surg. 2021;406:641–50.
Liu F, Huang C, Xu Z, et al. Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: the CLASS02 multicenter randomized clinical trial. JAMA Oncol. 2020;6:1590–7.
Liu X, Chen K, Chu X, et al. Prophylactic intra-peritoneal drainage after pancreatic resection: an updated meta-analysis. Front Oncol. 2021;11:658829.
Shi J, Wu Z, Wu X, et al. Early diagnosis of anastomotic leakage after gastric cancer surgery via analysis of inflammatory factors in abdominal drainage. Ann Surg Oncol. 2022;29:1230–41.
Acknowledgements
Not applicable.
Research registration Unique Identifying Number (UIN)
(1) Name of the registry: PROSPERO database; (2) Registration ID: CRD42022371102; (3) Hyperlink: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=371102.
Funding
This study was funded by: (1) Chongqing Technology Innovation and Application Development Special General Project (cstc2019jscx-msxmX0194); (2) Research Institutions Performance Incentive Guidance Special Fund (cstc2022jxjl0234).
Author information
Authors and Affiliations
Contributions
HY-P wrote the manuscript. HY-P, LH-C, and XF-C performed the data search and data analysis. HY-P, LH-C, and XF-C prepared figures. All authors reviewed the manuscript. HS approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Detailed search strategies of each database. Table S2. Outcomes of the meta-regression analyses. Figure S1. Forest plots of perioperative outcomes including: A. anastomotic leakage; B. Duodenal stump leakage; C. Pancreatic leakage; D. Intra-abdominal abscess; E. Surgical-site infection; F. Pulmonary infection; G. Mortality; H. Need for additional drainage; I. Readmission; J. Reoperation. Figure S2. Subgroup analyses of perioperative outcomes based on sample size (≥100 vs. <100). A. total complications; B. anastomotic leakage; C. Duodenal stump leakage; D. Pancreatic leakage; E. Intra-abdominal abscess; F. Surgical-site infection; G. Pulmonary infection; H. Mortality; I. Time to first soft diet; J. Length of hospital stay; K. Need for additional drainage; L. Readmission; M. Reoperation. Figure S3. Subgroup analyses of perioperative outcomes based on academic institution (Yes vs. No). A. total complications; B. anastomotic leakage; C. Duodenal stump leakage; D. Pancreatic leakage; E. Intra-abdominal abscess; F. Surgical-site infection; G. Pulmonary infection; H. Mortality; I. Time to first soft diet; J. Length of hospital stay; K. Need for additional drainage; L. Readmission; M. Reoperation. Figure S4. Subgroup analyses of perioperative outcomes in GC patients who underwent laparoscopic surgery. A. total complications; B. anastomotic leakage; C. Duodenal stump leakage; D. Pancreatic leakage; E. Intra-abdominal abscess; F. Surgical-site infection; G. Pulmonary infection; H. Time to first soft diet; I. Length of hospital stay; J. Reoperation. Figure S5. Subgroup analyses of perioperative outcomes in GC patients who underwent total gastrectomy. A. total complications; B. anastomotic leakage; C. Duodenal stump leakage; D. Pancreatic leakage; E. Intra-abdominal abscess; F. Surgical-site infection; G. Pulmonary infection; H. Mortality; I. Time to first soft diet; J. Length of hospital stay; K. Need for additional drainage; L. Readmission; M. Reoperation. Figure S6. Begg’s funnel plot of perioperative outcomes including: A. total complications; B. anastomotic leakage; C. Intra-abdominal abscess; D. Surgical-site infection; E. Pulmonary infection; F. Time to first soft diet; G. Length of hospital stay. All P values >0.05.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pang, HY., Chen, LH., Chen, XF. et al. Prophylactic drainage versus non-drainage following gastric cancer surgery: a meta-analysis of randomized controlled trials and observational studies. World J Surg Onc 21, 166 (2023). https://doi.org/10.1186/s12957-023-03054-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03054-1