Abstract
Background
Primary lymphoma of the liver, gallbladder, and extrahepatic bile ducts or secondary involvement of these organs by leukemia is exceedingly rare. Patients with primary lymphoma or leukemic involvement of the biliary tract and liver often present with symptoms and signs of biliary tract obstruction or inflammation.
Case presentation
We present a case of a 24-year-old male with biliary tract symptoms who underwent laparoscopic cholecystectomy. His precholecystectomy complete blood count performed on the same morning showed 72% lymphocytes while peripheral blood smears showed approximately 15% blasts. Surgeon went ahead with the procedure. Imaging done prior to surgery showed thickened gallbladder, while the liver, biliary tract, and pancreas did not show any thickening or mass lesion. However, the liver was enlarged. Grossly, the gallbladder wall did not show any stones or discrete mass involving the wall. Instead, there was subtle thickening of the gallbladder wall due to diffuse infiltration by the leukemic infiltrate. This lymphoid population reacted with PAX-5 and TdT immunohistochemical antibodies in a diffuse manner confirming precursor B-cell origin. This patient was found to have B-lymphoblastic leukemia involving his bone marrow on further clinical and diagnostic workup. Patient responded well to chemotherapy and is currently on maintenance treatment. He is well 1.5 years after his diagnosis.
Conclusion
This case highlights a unique and rare scenario where a previously undiagnosed and unsuspected hematologic malignancy initially presented with clinical features of a chronic inflammatory condition involving an abdominal organ owing to secondary involvement by the malignant infiltrate.
Similar content being viewed by others
Introduction
Primary lymphoma of the liver, gallbladder, and extrahepatic bile ducts or secondary involvement of these organs by leukemia is exceedingly rare. Both lymphoid and myeloid leukemia have been reported to involve the gallbladder. Most patients with primary or secondary involvement of the gallbladder present with symptoms mimicking cholecystitis. Patients with lymphoma/leukemic involvement of biliary tract and liver often present with symptoms and signs of biliary tract obstruction such as cholestatic jaundice and liver failure. Clinical presentation, radiological findings, and pre-operative findings are not often helpful in distinguishing primary or secondary involvement of these organs by hematolymphoid malignancies and preoperative diagnosis is extremely difficult [1,2,3,4,5]. A gallbladder with primary lymphoma or secondarily involved by a leukemic infiltrate often shows diffuse thickening of the wall mimicking cholecystitis [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20].
Herein, we report a case of acute lymphoblastic leukemia (ALL) in a young male involving the gallbladder secondarily. We describe the clinical, radiological, gross, microscopic, and immunohistochemical (IHC) findings and present a detailed review of the published literature.
Case presentation
Clinical presentation
A 24-year-old male presented with severe right upper quadrant abdominal pain for 3 months. There was no significant past medical history. He was vitally stable and general examination was unremarkable except for epigastric tenderness. His laboratory findings revealed abnormal liver function tests. Imaging studies were suggestive of cholelithiasis with an evolving cholecystitis. The liver was enlarged. An elective laparoscopic cholecystectomy was planned. His initial peripheral blood smears done on the day of the surgery showed 72% lymphocytes on differential leukocyte count, although total white cell count was 3.9 × 109E/L. Peripheral smear showed approximately 15% blasts. Hemoglobin was 9.4 gm/dL, while platelet count was low 100 × 109E/L. The surgeon decided to proceed with the procedure as he had no suspicion that the gallbladder was infiltrated by a neoplastic lesion and believed that the blast cells seen on complete blood count were an independent finding from cholelithiasis and cholecystitis.
Radiological findings
Pre-cholecystectomy ultrasound of liver and gallbladder showed moderately distended and thickened gallbladder measuring 80 × 35 mm and containing tiny stones mixed with sludge. Wall thickness was 7 mm. The liver was enlarged and showed mild increase in parenchymal echogenicity suggesting mild fatty infiltration. No focal lesion was seen. No intra- or extrahepatic biliary dilation was present. The common bile duct (CBD) and portal vein were unremarkable. Magnetic resonance cholangiopancreatography (MRCP) showed slightly edematous, distended, and thick-walled gallbladder with sludge and stones. Ultrasound, MRI, and MRCP findings were suggestive of cholelithiasis with evolving cholecystitis. There was no evidence of any intra or extra hepatic biliary dilatation. The right and left hepatic ducts and cystic duct appeared unremarkable. Post-cholecystectomy ultrasound, MRI, and MRCP showed no focal lesion or distortion in liver, biliary tract, or spleen. However, the liver appeared enlarged. No evidence of bilateral pleural effusion or ascites was noted. He underwent laparoscopic cholecystectomy.
Operative findings
On laparoscopic cholecystectomy, the gallbladder was distended and was adherent to the liver. The cystic duct was short and wide. Dissection was difficult due to adhesions and hepatomegaly. The liver appeared inflamed and friable; peri-hepatic fluid was present. Adhesiolysis was performed and dissection was continued over the liver bed. The gallbladder was dissected from the gallbladder fossa. Some bleeding was encountered due to traction on the liver.
Pathological analysis
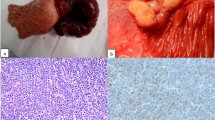
Grossly, the gallbladder measured 7 cm in length and 2 cm in diameter. The serosal surface was smooth. The fundal wall showed an average thickness of 5 mm, with very subtle thickening at some places (Fig. 1, red arrow shows thickening of the wall as compared to uninvolved area marked by yellow arrow. Curved arrow points at cystic duct margin). No stones were present in the lumen.
Microscopic examination of the resected specimen revealed diffuse, densely cellular atypical lymphoid cell infiltrate involving the full thickness of the gallbladder wall. Cystic duct resection margin was involved. The infiltrate was composed of small- to medium-sized cells with round to oval, convoluted nuclei, condensed nuclear chromatin, indistinct nucleoli, and scant cytoplasm (Fig. 2, medium power view of H&E-stained section). Nuclear to cytoplasmic ratio was high (Fig. 3, high power view of H&E-stained section showing cellular details). Numerous mitotic figures were seen. IHC studies were performed, and the neoplastic lymphoid cells demonstrated strong diffuse positivity for PAX-5 (Fig. 4) and terminal deoxynucleotidyl transferase (TdT) (Fig. 5). Ki-67 (Mib-1) proliferative index was raised up to approximately 60% (not shown). The neoplastic cells were negative for Keratin cocktail AE1/AE3, CD20, CD3, CD5, myeloperoxidase (MPO), CD34, and IgG4. Liver biopsy was not performed.
Final diagnosis and further workup
Based on these findings, a diagnosis of B-lymphoblastic lymphoma/ leukemia (B-LL) was rendered. Peripheral blood film was repeated and bone marrow biopsy was performed post-resection. Peripheral blood film showed normochromic, microcytic red blood cells. Hemoglobin was 7.8 gm/dl. Approximately 80% blast cells were seen. Platelets were low on film. Bone marrow aspirate revealed diffuse infiltration with blast cells which constituted approximately 85% of the total nucleated non-erythroid cell population. Similarly, section of bone trephine also showed hypercellularity (approximately 80 to 85%) and diffuse infiltration by blast cells. Immunophenotyping by flow cytometry performed on bone marrow showed reactivity of blast cell population to pan B-cell markers i.e., CD10, CD19, CD22, and CD79a along with HLA-DR and CD45. This population also showed positivity to CD9, CD58, CD34, and TdT. Overall findings were consistent with B-LL.
Molecular analysis
ABL1 by FISH/PCR was subsequently performed and was negative. Conventional cytogenetics showed 46 XY chromosomes.
Clinical course
Our patient responded well to treatment. He received treatment with MRC UK ALL XII protocol. His minimal residual disease after induction phase I was less than 0.01%. He subsequently successfully completed all blocks of treatment till consolidation. During induction phase I, he developed steroid-induced hyperglycemia which reverted with insulin and oral hypoglycemics. Since then, he has not developed any other chemotherapy-related complications. He is currently on maintenance treatment with 6-mercaptopurine and methotrexate with prophylactic trimethoprim/sulphamethoxazole. His last complete blood count (CBC) showed hemoglobin 13.2 gm/dl, white blood cells (WBC) 5.1 × 109E/L, platelets 450 × 109E/L. He is well 2 years after the diagnosis.
Discussion
Secondary involvement of gallbladder by malignancies is extremely rare and malignant melanomas constitute the most common tumor type [21]. In more than 90% cases, diagnosis is late, often at the terminal stage of malignancy with involvement of multiple organs [22]. Although most cases present as diffuse infiltration, involvement by lymphoma/leukemia may rarely present in the form of true polyps [23].
Over the years, several case reports have documented primary or secondary involvement of liver, gallbladder, and biliary tract by acute and chronic lymphoid [5,6,7,8,9,10,11,12,13,14,15] or acute and chronic myeloid [16,17,18,19] leukemia. These are listed in Table 1.
Most cases of leukemic infiltration of gallbladder present with clinicopathological features closely resembling acute and/or chronic cholecystitis [6, 11,12,13, 16, 17]. Azin et al reported a patient with known AML who received chemotherapy and achieved a morphological free state. He developed signs and symptoms of cholecystitis 2 years later for which he underwent cholecystectomy. Histological examination revealed extensive infiltration by AML [19]. Liver involvement by CLL leading to liver failure has also been reported [10, 14, 15].
Constitutional symptoms in such patients include nausea, vomiting, right upper quadrant and epigastric abdominal pain and cramps, bloating, abdominal swelling, and mass due to enlargement of the liver, spleen and regional lymph nodes, and/or fluid accumulation in the abdominal cavity, persistent weakness and fatigue, weight loss, obstructive jaundice, and gall stones [1,2,3,4,5,6,7, 9, 12,13,14, 16, 17, 19, 20, 22].
In cases where cholecystectomy was performed with suspicion of cholelithiasis and acute or chronic cholecystitis reveals infiltration by lymphoma or leukemia, blood tests (leukocytosis, abnormal liver function tests, raised CA 19-9), bone marrow aspirate, fine needle aspiration cytology (FNAC) of abdominal fluid for malignant cells, and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for evaluation of lymph nodes in porta hepatic region should be carried out. In addition, abdominal ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT) scan should be performed to investigate systemic involvement [24].
Leukemic infiltration only rarely produces a discrete focal mass. Even the gross appearance at cholecystectomy is often strongly suggestive of acute and/or chronic cholecystitis [11, 12, 16, 17]. In a series of 19 cases of lymphoma and leukemia involving the gallbladder and extrahepatic bile ducts, 14 were primary and five were secondary. In case of the latter, there was no prior diagnosis of lymphoma and it was diagnosed post-cholecystectomy. These five patients were found to have widespread disease on additional workup. However, these patients came to clinical attention due to involvement of the gallbladder [3]. In most cases of leukemic infiltration, histopathology shows diffuse infiltration of the gallbladder wall by the atypical lymphoid or myeloid infiltrate. IHC is required to rule out poorly differentiated carcinoma [8]. If there is known history of lymphoid or myeloid leukemia, appropriate IHC will resolve the issue. However, when there is no known history of leukemia and gallbladder involvement is the first manifestation of the disease, an extensive IHC panel may need to be performed. However, a CBC may help enormously in such cases in alerting the clinician if the lymphoid or myeloid cell counts are abnormal. A subsequent bone marrow examination supplemented by IHC will confirm the diagnosis. CD15 positivity indicates a myeloid lineage [17]. CD4, CD43, CD45, CD68, MPO, and lysozyme positivity also indicate a myelomonocytic derivation [18]. Positivity for IHC stains CD79a, CD10, CD 4, and TdT is also seen in B lymphoblasts in cases of B-ALL. However, cyclin D1 is negative [9, 15]. T-cell lineage is indicated by positivity for CD1a, CD3, CD4, CD5, CD68, and CD43 [7]. B-cell lineage is indicated by positivity for CD20, BCL2, CD5, CD43, and CD23. Positivity for CD38 may be associated with poor prognosis in cases of CLL if expressed by more than 30% of the atypical B lymphoid cells [14, 20].
Prognosis of B-LL has improved with new chemotherapy. There is a >95% complete remission rate in children compared to 60–85% in adults. Approximately 80% children are cured while cure rate in adults is <50% [25]. T-LL is associated with a higher risk for induction failure and early relapse compared to B-LL [26]. Chronic lymphocytic leukemia (CLL) has a better prognosis than LL [27,28,29]. Prognosis of AML is poor despite chemotherapy with survival times of only a few months [30, 31]. NK lymphoblastic leukemia /lymphoma is considered indistinguishable from AML both in terms of treatment and prognosis. Prognosis of CML is excellent with tyrosine kinase inhibitor (TKI) therapy. Mortality rates have been reduced to only 2 to 3% per year while 5-year survival rates have increased to 80–95% [32, 33].
The usual chemotherapy regimen for patients with LL includes vincristine, dexamethasone or prednisolone, and an anthracycline drug such as doxorubicin (Adriamycin) or daunorubicin. Some regimens may also include cyclophosphamide, L-asparaginase, and/or high doses of methotrexate or cytarabine (Ara-C) as part of the induction phase. In LL patients whose leukemia cells have the Philadelphia chromosome, a targeted drug such as imatinib (Gleevec) or dasatinib (Sprycel) is often included. For AML, combination of cytarabine with anthracycline (Daunorubicin) has been used for decades. However, several novel targeted therapies are now becoming available. These include hypomethylating agents, drugs inhibiting Hedgehog pathway and drugs modulating TP53 pathway, etc. In addition, allogenic hematopoietic stem cell transplant can be considered for post-remission therapy in patients with adverse risk of relapse. In CML, targeted therapy with imatinib, dasatinib, and nilotinib (Tasigna) is given. In a group of patients with CLL (young, fit with mutated IGHV, without TP53 mutations or deletions in chromosomes 11 or 17), a defined course of therapy with fludarabine, cyclophosphamide, and rituximab has been shown to be of great benefit and many patients achieve durable remissions [28, 34,35,36,37,38,39,40,41,42,43].
Conclusion
Involvement of the gallbladder, biliary tract, or liver by leukemic infiltrate is very rare and documented cases mostly present with symptoms strongly mimicking acute and/or chronic inflammation of the involved organs. Gallbladder involvement usually presents with features suggestive of acute or chronic cholecystitis and diffuse thickening of the wall rather than a discrete mass. When there is no previous diagnosis of leukemia, it is not possible in most cases to make a pre-operative diagnosis and leukemia is only diagnosed on histopathological examination of the resected gallbladder.
Availability of data and materials
All data generated are included in this article.
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- CBC:
-
Complete blood count
- CBD:
-
Common bile duct
- CLL:
-
Chronic lymphoid leukemia
- CT:
-
Computed tomography
- IHC:
-
Immunohistochemical
- MPO:
-
Myeloperoxidase
- MRCP:
-
Magnetic resonance cholangiopancreatography
- TdT:
-
Terminal deoxynuleotidyl transferase
- WBC:
-
White blood cells
References
Fidias P, Carey RW, Grossbard ML. Non-Hodgkin’s lymphoma presenting with biliary tract obstruction. A discussion of seven patients and a review of the literature. Cancer. 1995;75(7):1669–77.
Dellon ES, Morris SR, Tang W, Dunphy CH, Russo MW. Acute liver failure due to natural killer-like T-cell leukemia/lymphoma: a case report and review of the literature. World J Gastroenterol. 2006;12(25):4089–92.
Mani H, Climent F, Colomo L, Pittaluga S, Raffeld M, Jaffe ES. Gall bladder and extrahepatic bile duct lymphomas: clinicopathological observations and biological implications. Am J Surg Pathol. 2010;34(9):1277–86.
Hwang DW, Lim CS, Jang JY, Lee SE, Yoon SO, Jeon YK, et al. Primary hematolymphoid malignancies involving the extrahepatic bile duct or gallbladder. Leuk Lymphoma. 2010;51(7):1278–87.
Psarras K, Symeonidis N, Vlachaki E, Baltatzis M, Papatolios G, Pavlidis E, et al. Primary gallbladder small lymphocytic lymphoma as a rare postcholecystectomy finding. Case Rep Hematol. 2014;2014:716071.
Finlay DE, Mitchell SL, Letourneau JG, Longley DG. Leukemic infiltration of the gallbladder wall mimicking acute cholecystitis. AJR Am J Roentgenol. 1993;160(1):63–4.
Patel KJ, Latif SU, de Calaca WM. An unusual presentation of precursor T cell lymphoblastic leukemia/lymphoma with cholestatic jaundice: case report. J Hematol Oncol. 2009;2:12.
Ozawa T, Kosugi S, Noda T, Take H. Gall bladder B-lymphoblastic lymphoma showing carcinoma-like appearance. Intern Med. 2012;51(6):663–4.
Sayyed AH, Aleem A, Al-Katari MS, Algahtani F, Aljerian K, Aleem TA, et al. Acute lymphoblastic leukemia presenting with liver infiltration and severe lactic acidosis. Am J Case Rep. 2018;19:453–7.
Costa F, Choy CG, Seiter K, Hann L, Thung SN, Michaeli J. Hepatic outflow obstruction and liver failure due to leukemic cell infiltration in chronic lymphocytic leukemia. Leuk Lymphoma. 1998;30(3-4):403–10.
Chim CS, Loong F, Chung LP. Chronic lymphocytic leukemia involving the gallbladder. Br J Haematol. 2001;115(4):717.
Dasanu CA, Mesologites T, Homsi S, Ichim TE, Alexandrescu DT. Chronic lymphocytic leukemia presenting with cholecystitis-like symptoms and gallbladder wall invasion. South Med J. 2010;103(5):482–4.
Rao V, Watkins R, Kaleem A, Cooke J, Wedgwood K. Leukaemic infiltration of gall bladder - unusual presentation of occult chronic lymphocytic leukaemia. J Surg Case Rep. 2011;2011(1):7. https://doi.org/10.1093/jscr/2011.1.7.
Esfahani K, Gold P, Wakil S, Michel RP, Solymoss S. Acute liver failure because of chronic lymphocytic leukemia: case report and review of the literature. Curr Oncol. 2011;18(1):39–42.
Jafroodifar A, Thibodeau R, Goel A, Coelho M, Bryant S, Nguyen Q, et al. Chronic lymphoid leukemia metastasis to the gallbladder as a focal mass: a case report. Radiol Case Rep. 2021;16(6):1477–84.
Bloom SH, Coad JE, Greeno EW, Ashrani AA, Hammerschmidt DE. Cholecystitis as the presenting manifestation of acute myeloid leukemia: report of a case. Am J Hematol. 2002;70(3):254–6.
Shimizu T, Tajiri T, Akimaru K, Arima Y, Yokomuro S, Yoshida H, et al. Cholecystitis caused by infiltration of immature myeloid cells: a case report. J Nippon Med Sch. 2006;73(2):97–100.
Bartley AN, Nelson CL, Nelson DH, Fuchs DA. Disseminated extramedullary myeloid tumor of the gallbladder without involvement of the bone marrow. Am J Hematol. 2007;82(1):65–8.
Azin A, Racz JM, Carolina Jimenez M, Sunil S, Porwit A, Jackson T, et al. Relapse of acute myeloid leukemia manifested by cholecystitis: a case report and review of the literature. Int J Surg Case Rep. 2014;5(6):302–5.
Mitropoulos FA, Angelopoulou MK, Siakantaris MP, Rassidakis G, Vayiopoulos GA, Papalampros E, et al. Primary non-Hodgkin’s lymphoma of the gall bladder. Leuk Lymphoma. 2000;40(1-2):123–31.
Muszynska C, Lundgren L, Andersson R, Søland T, Lindell G, Sandström P, et al. Incidental metastases and lymphoma of the gallbladder–an analysis of ten rare cases identified from a large national database. Scand J Gastroenterol. 2019;54(3):350–8.
Cocco G, Delli Pizzi A, Basilico R, Fabiani S, Taraschi AL, Pascucci L, et al. Imaging of gallbladder metastasis. Insights Imaging. 2021;12(1):1–8.
Cocco G, Basilico R, Delli Pizzi A, Cocco N, Boccatonda A, D’Ardes D, et al. Gallbladder polyps ultrasound: what the sonographer needs to know. J Ultrasound. 2021;24(2):131–42.
Jones K, Biederman L, Draganova-Tacheva R, Solomides C, Bibbo M. Diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration cytology of porta hepatis lesions: a retrospective study. Acta Cytol. 2016;60(2):154–60.
Hallböök H, Gustafsson G, Smedmyr B, Söderhäll S, Heyman M. Swedish Adult Acute Lymphocytic Leukemia Group; Swedish Childhood Leukemia Group. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer. 2006;107(7):1551–61. https://doi.org/10.1002/cncr.22189 PMID: 16955505.
Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21(19):3616–22. https://doi.org/10.1200/JCO.2003.10.116 PMID: 14512392.
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198–206. https://doi.org/10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. PMID: 7237385.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood, J Am Soc Hematol. 2018;131(25):2745–60.
Van Bockstaele F, Verhasselt B, Philippé J. Prognostic markers in chronic lymphocytic leukemia: a comprehensive review. Blood Rev. 2009;23(1):25–47.
Suvajdzic N, Marisavljevic D, Kraguljac N, Pantic M, Djordjevic V, Jankovic G, et al. Acute panmyelosis with myelofibrosis: clinical, immunophenotypic and cytogenetic study of twelve cases. Leuk Lymphoma. 2004;45(9):1873–9. https://doi.org/10.1080/10428190410001683688 PMID: 15223649.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood, J Am Soc Hematol. 2013;122(6):872–84.
Orazi A, O'Malley DP, Jiang J, Vance GH, Thomas J, Czader M, et al. Acute panmyelosis with myelofibrosis: an entity distinct from acute megakaryoblastic leukemia. Mod Pathol. 2005;18(5):603–14. https://doi.org/10.1038/modpathol.3800348 PMID: 15578075.
Hehlmann R. CML—where do we stand in 2015. Ann Hematol. 2015;94(2):103–5.
Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524.
Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018;60(1):4–12.
Vrooman LM, Silverman LB. Treatment of childhood acute lymphoblastic leukemia: prognostic factors and clinical advances. Curr Hematol Malig Rep. 2016;11(5):385–94.
Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94(11):1266–87.
Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–705.
Sharma S, Rai KR. Chronic lymphocytic leukemia (CLL) treatment: so many choices, such great options. Cancer. 2019;125(9):1432–40.
Parikh SA. Chronic lymphocytic leukemia treatment algorithm 2018. Blood Cancer J. 2018;8(10):1.
Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026. https://doi.org/10.1136/bmj.n2026.
Medinger M, Heim D, Halter JP, Lengerke C, Passweg JR. Diagnosis and therapy of acute myeloid leukemia. Therapeut Umschau Rev Therapeut. 2019;76(9):481–6.
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93(3):442–59.
Acknowledgments
Not applicable.
Funding
The authors received no funding for the writing.
Author information
Authors and Affiliations
Contributions
SR, AA, and NA were involved in case diagnoses. ZA conceived the idea. SR and QC wrote and involved in writing of the first draft of the manuscript. ZA and QC reviewed the manuscript. JAG was a major contributor to the critically revising of the manuscript and important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rahim, S., Ahmad, Z., Chundriger, Q. et al. Secondary involvement of gallbladder by acute lymphoblastic leukemia presenting clinically as cholecystitis in a young patient: a case report. World J Surg Onc 21, 63 (2023). https://doi.org/10.1186/s12957-023-02956-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-02956-4