Abstract
Background
According to guidelines, every soft tissue tumor (STT) larger than 3 cm should be biopsied before definitive resection. Advances in magnetic resonance imaging (MRI) improve the possibility to give a provisional diagnosis of the tumor’s entity. Can lipomas and atypical lipomatous tumors (ALTs) of the extremities therefore be primarily marginally resected based on interpretation of MR images without a previous biopsy?.
Methods
In this retrospective, single-center study, 240 patients with the suspicion of a lipomatous tumor in MRI and surgical treatment in our institution between 2011 and 2020 were included. MR imaging was performed before surgery. All resected specimens underwent histopathological analysis.
Results
The collective comprised 142 tumors that were suspected as lipoma or ALT by the radiologist and underwent primary marginal resection (PMR). One case had myxoid liposarcoma that was underestimated on MRI and needed radical follow-up resection. One-hundred forty-one patients were cured after PMR. Ninety-eight patients were biopsied initially and in 93 cases resected afterwards according to the necessary oncological margins.
Conclusion
In our institution, PMR is performed if a lipoma or ALT is suspected on MR imaging. Our treatment method and the diagnostic algorithm are presented. Primary resection spares patients from one surgical procedure, but a slight risk for underestimation of the tumor remains.
Similar content being viewed by others
Background
Lipomatous tumors are the most common among soft tissue tumors (STTs) [1]. They can be found superficially just below the skin or deeper in the soft tissue [2]. In this very heterogeneous group of tumors, the most frequent are lipomas with an incidence of 2100 per 100,000 persons [3]. Lipomas are benign STTs.
Another frequent entity of adipocytic tumors is the atypical lipomatous tumor (ALT). Atypical lipomatous tumor and well-differentiated liposarcoma (WDLPS) are morphologically and genetically identical. However, for neoplasms that arise in the limbs or on the trunk, the term ALT is used, because complete excision is usually curative and achievable. ALT and WDLPS do not have potential for metastases unless they undergo dedifferentiation [4]. When located in deep anatomical regions like the retroperitoneum, spermatic cord, or mediastinum, complete resection can be difficult. The residual tumor potentially relapses and undergoes dedifferentiation, which results in a significantly poorer prognosis [5]. Therefore, in these locations, the tumors are named WDLPS. The treatment method for lipomas and ALTs of the trunk and in the extremities is alike: marginal resection of the tumor with its pseudocapsule [6].
Malignant STTs have an incidence of 4.7 per 100,000 persons [7]. Thus, they only account for 1% of all malignant tumors in adults [8]. At least 70 subtypes of soft tissue sarcomas exist [9]. The three most frequent are undifferentiated pleomorphic sarcoma, liposarcoma (LPS), and leiomyosarcoma [10]. LPS can further be subclassified in well-differentiated liposarcoma (WDLPS), myxoid LPS, pleomorphic LPS, dedifferentiated LPS, and myxoid pleomorphic liposarcoma [11]. Five-year survival of LPS ranges from 50 to 65% in Europe [12]. The wide range is in respect to the various subentities. LPS are treated with wide resection. Their response to chemotherapy is rather poor [13]. However, before resection, an interdisciplinary tumor conference is advised to discuss the individual therapy and a multimodal approach [14, 15].
Magnetic resonance imaging (MRI) is the most effective diagnostic tool for soft tissue imaging [16]. Figure 1 gives an exemplary overview of the discussed lipomatous tumors on MRI. MRI-based criteria have been developed to assess the tumor’s degree of malignancy [17, 18]. Homogeneity of the MRI signal, especially in T1-weighted sequences with fat suppression, is an indicator for lipomas [19]. The main MRI characteristic that helps to distinguish between lipoma and ALT is the broader and more nodular fibrous septa in the latter [20]. However, neither a clinical nor a radiologic differentiation between lipoma and ALT is safely possible [21]. The state-of-the-art method to distinguish benign lipomas from ALTs is fluorescence in situ hybridization (FISH). In contrast to lipomas, ALTs show mouse double minute 2 (MDM2) amplification [22]. As in all cancerous disease, the final diagnosis is provided by histologic analysis (Fig. 2). Contrast medium (gadolinium) should be applied for every patient who undergoes MRI for soft tissue tumor diagnostics. Malignant lipomatous tumors usually show heterogeneous contrast-medium enhancement [23].
Examples for lipomatous tumors in MRI. A Lipoma in a T1-weighted sequence in transversal plane. B Corresponding T2-weighted coronal image. C Atypical lipomatous tumor (ALT) in a contrast-enhanced fat-saturated T1-weighed sequence in transversal plane demonstrating inhomogeneous contrast enhancement. D Corresponding T2-weighted coronal image. E Pleomorphic liposarcoma in a T1-weighted-sequence in axial plane. F Corresponding T2-weighted coronal image
Histologic images of lipomatous tumors. A The image shows the characteristic histology of lipoma: a homogeneous proliferation of mature adipocytes without cellular atypia. B The adipocytic (lipoma-like) type of atypical lipomatous tumor (ALT) consists of adipocytes with variation in size and shape and a typical component of stromal spindle cells with hyperchromatic atypical nuclei. C Liposarcomas with low-grade dedifferentiation are rare but recognized increasingly. The tumor frequently shows dense proliferation of uniform spindle cells with mild nuclear atypia arranged in a fascicular pattern. D The dedifferentiated areas of high-grade dedifferentiated liposarcoma usually resemble undifferentiated pleomorphic sarcoma or myxofibrosarcoma. However, any type of high-grade sarcoma can be present. The actual image shows high-grade spindle cell sarcoma with features of fibrosarcoma. All images are in hematoxylin and eosin stain. The black bar indicates 100 μm
According to guidelines, every tumor should be biopsied before its definitive resection. An exclusion are tumors smaller than 3 to 5 cm in diameter, which can be safely excised [24]. The range in tumor’s diameter is in respect to the various national guideline, which differs to some extent [25]. Yet, these small, unnamed tumors need to be resected in sano, to achieve an R0 resection margin. This general recommendation has improved the oncologic outcome of patients and allows to give patients the advantages of targeted, neoadjuvant chemotherapy [26]. Over the course of the past decade, continuous progression of MRI quality and improved prognostication of tumor entity has been made. This raises the question whether tumors with a homogeneous, lipomatous appearance in MRI, in the extremities, can safely be resected without previous biopsy.
In this article, we describe our treatment method for lipomas as well as ALTs and analyze our collective of treated patients. MRI and histological reports were compared to each other. The patients’ charts were searched for the necessity of a follow-up resection after primary marginal resection of a lipomatous tumor.
Methods
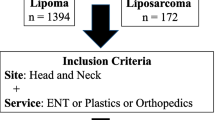
The study had a retrospective, single-center design. Approval was obtained from the institutional review board (WF-071/20). The patients analyzed were surgically treated between 2011 and 2020 at our institution. The inclusion criteria are outlined in a flow chart (Fig. 3). All biopsies were performed as open incisional biopsies. According to the working procedure for STT in our institution, every patient with an STT receives an MRI of the tumorous region, which should be supported by the application of gadolinium [27]. In our patient collective, 93% of the patients received contrast medium. All MRI reports were written by fellowship-trained radiologists. A total of 86% of the MRI scans were not performed in our institution. Consequently, the evaluation of the MRI was done by external radiologists. Their MRI reports were scanned into the patients’ electronic medical charts at our institution. Most patients contacted the sarcoma outpatient clinic of our institution after referral by a resident doctor. Patients were clinically examined, and the MR images were analyzed by two senior surgeons who both have more than 10 years of experience in musculoskeletal tumor surgery. Then, patients received a recommendation for PMR or incisional biopsy of the lipomatous tumors.
All surgical specimen were analyzed by fellowship-trained pathologists in our institution. In cases of doubt, a reference pathology institution was contacted. Lipomas were distinguished from ALTs by FISH for the MDM2 gene [28]. In cases of doubt, tissue samples were sent to an appropriate reference pathology institution. All intrathoracic and intra-abdominal lesions were excluded from the study since different oncology treatment applies to them.
A retrospective comparison of the provisional diagnosis given in the MRI report with the final histopathologic diagnosis was performed. In the case of a biopsy before resection, the histopathologic result from the biopsy was compared to the final analysis of the completely resected tumor.
The study data were statistically analyzed (GraphPad Software 9 Los Angeles, CA, USA). Parametric distribution of the data was tested with the Shapiro-Wilk test. For all data, nonparametric distribution was applied; thus, comparisons were done with Whitney-Mann U-test (confidence interval of 95%). A p-value less than 0.05 was regarded as significant and less than 0.005 as highly significant.
Results
Overall, 240 lesions in 240 patients with a suspicion of a lipomatous tumor on MRI were included in the study. One-hundred forty-two (59%) were selected for primary marginal resection. In 98 (41%) of the 240 lesions, an open incisional biopsy was favored instead of primary resection (Fig. 3).
In the cohort of PMR, patients’ average age was 54.0 ± 13.4 years. There were 71 women and 71 men in the cohort of PMR. After resection, histopathologic analysis of the specimens revealed 116 (81.7%) lipomas, 25 (17.6%) ALTs, and one myxoid LPS (0.7%) (Table 1). For these 142 tumors, the concordance of the provisional diagnosis in the radiologists’ MRI report with the final histopathologic diagnosis was 74%. In 25%, the radiologist’s provisional diagnosis overestimated the tumor’s degree of malignancy. Only in one case a tumor was falsely diagnosed as ALT in the MRI report, but histology revealed a myxoid LPS. MRI was performed with use of contrast medium. This malignant tumor needed a radical follow-up resection. The affected patient was a 65-year-old male. The myxoid liposarcoma measured 200.7 cm3, which is smaller than the average LPS in the study (706.6 cm3). It was located in close relation to the musculus latissimus dorsi. Two weeks after the first surgery, a radical oncological follow-up resection was performed.
Regarding the tumors that were treated by primarily marginal resection (PMR), 33 tumors were located in the neck and trunk, 57 were in the upper extremities, and 52 were in the lower extremities (Fig. 4).
Patients who received a biopsy of the lesion were on average 57.5 ± 17.9 years old. Thirty-six women and 62 men were in the biopsy cohort (Fig. 3). All biopsies were performed as open incisional biopsies and led to a histopathologic diagnosis. In 84 (86%) of the 98 lesions, the radiologist suspected malignant neoplasia. Sixty-seven (68%) malignant tumors were confirmed in the histopathological analysis. Seventeen (17.5%) lesions were benign, and 14 (14.5%) were ALTs. The exact entities of the lesions are listed in detail in Table 2. All but five tumors were later resected by required oncologic margins. Comparison of the histopathologic diagnosis from the biopsies (n = 98) to the histopathologic result after analysis of the complete tumor (n = 93) showed no change in diagnosis and a diagnostic accuracy of incisional tissue biopsy of 100%.
In 14 cases, a tumor was biopsied, which was rated as benign or intermediate by the radiologist. The decision to do a biopsy instead of a PMR was made after the MR images have been viewed by a musculoskeletal surgeon. The reasons were doubts about the benign character of the lesion by the surgeon or the patient’s explicit wish for a biopsy. In these 14 cases, the biopsy lead to the histopathologic diagnosis of the following entities: lipoma arborescence (1×), hibernoma (2×), lipoma (2×) spindle cell lipoma (1×), and ALT (3×), pleomorphic LPS (2×), and myxoid LPS (3×).
The lipomatous tumors were distributed throughout the whole body (Fig. 4). In the biopsy cohort, 22 tumors in the neck and trunk were biopsied before resection. In the same way, treated were 61 tumors in the upper extremity and 15 tumors in the lower extremity.
The mean volume of the primary, marginally resected tumors in pathology was highly significantly smaller (566 ± 974 cm3) than the volume of tumors that were chosen for tissue biopsy first (799 ± 1055 cm3; p < 0.0001) (Fig. 5a).
When considering ALTs separately, ALTs that were chosen for PMR had an average volume of 890.9 ± 628.8 cm3 in MRI. In contrast, ALTs that were biopsied before resection had an average volume of 1410.9 ± 1133.8 cm3 in MRI (Fig. 5b). The difference was not statistically significant (p = 0.197). In both cohorts, ALTs were located in similar regions — for PMR, neck/trunk 2, upper extremity 5, and lower extremity 18 — for biopsy, neck/trunk 1, upper extremity 1, and lower extremity 12.
Discussion
Due to the low incidence of soft tissue sarcomas, radiologists are rarely confronted by them [1]. This makes their differentiation from other lipomatous STTs even more difficult. Thus, musculoskeletal surgeons need to view MR images themselves and decide whether to marginally resect the tumor primarily or to biopsy it first. In cases of doubt or technical difficulties to perform a surgical biopsy, an a priori presentation of the case in an interdisciplinary tumor conference can be helpful.
The study’s data suggests that there is a trend towards biopsy for tumors with large volume. One must keep in mind that the tumor’s volume is unrelated to its dignity. Even larger lipomatous tumors with homogeneous appearance in MRI should be considered for PMR.
There are various guidelines with diagnostic and therapeutic algorithms for soft-tissue tumors, which are frequently followed insufficiently, especially in nonspecialized hospitals [18, 29, 30]. As MRI is the most important tool for diagnostic imaging, imaging guidelines are presented from a radiological point of view. In cases of radiological signs of malignancy and/or indeterminate lesions, tissue biopsy is recommended [31, 32].
From the surgeon’s point of view, a tumor with potential to local recurrence after incomplete resection requires identical treatment like a benign tumor in cases of lipomatous tumors because marginal resection of the tumor with its pseudocapsule is sufficient in both lipoma and ALT. Radical wide oncological resection is reserved for high-grade malignant tumors (G2 and G3 gradings). Therefore, radiological guidelines for soft tissue tumors and their adherence might not be adequate in cases of lipomatous tumors. Although diagnostic algorithms that are presented (especially recommended biopsies) are valid for most soft tissue tumors, they should be questioned in lipomatous tumors even if signs of malignancy might be present.
Coran et al. reported a correct detection rate of malignant LPS of 100% in 54 cases by radiologists in MRI [19]. In our reported findings, only one LPS was misinterpreted as an intermediate lesion. But 14 biopsies were performed of tumors, which had been rated as benign or intermediate by the radiologist. The reasons were doubts about the benign character of the lesion by the surgeon or the patient’s explicit wish for a biopsy. Out of these 14 tumors, five were malignant LPS.
We recommend primary marginal resection for small (< 3cm), epifascial lesions and deeper or larger lipomatous tumors with a truly homogeneous appearance in a T1-weighted sequence and with only very narrow or without septa [2]. The surgeon who performs the biopsy should be experienced in musculoskeletal tumor surgery and be prepared to do the final resection as well [33]. If a biopsy is needed, it should be an incisional biopsy, and it should be undertaken under general or plexus anesthesia. There is a tendency in the literature towards the use of core needle biopsies [26]. Core needle biopsies reveal the correct diagnosis in 68 to 84.9% of sarcoma patients, depending on the literature [34,35,36]. The rate of accuracy might even be higher in the combination of a fine needle aspiration biopsy [37], but incisional biopsy remains the gold standard [38, 39]. Our data supports this approach since all 98 biopsies produced a reliable histopathologic diagnosis. The surgical approach to the tumor is of utmost importance. It should be chosen with care, and it should always be kept in mind that further resection may eventually be necessary [39]. The surgical approach should be as direct as possible without crossing other compartments to avoid any tumorous contamination. If drainage is used, it should be let out through the wound.
All surgical specimens must be marked according to their anatomical location and sent to pathology for histological analysis [40]. After pathological analysis, all cases with malignant tumors should be discussed in an interdisciplinary tumor conference after radiological tumor staging of the patient [15]. Complete resection should always be favored in all tumors [41]. Each wrong diagnosis could bear extreme consequences for the individual patient.
All general limitations of a retrospective analysis apply to this study. Resolution of the MR tomographs (slice thickness and magnetic field strength) was not considered since they were not completely reported. Surgical treatment was performed by two surgeons, but a heterogeneous group of radiologists and pathologists performed imaging/tumor analysis.
Conclusions
Our study shows that a very exact prediction of the tumor’s entity can be given with diagnostic support from MRI. Improved MRI quality allows an exact evaluation of tumor size, location, and depth within the tissue and a provisional diagnosis of the tumor’s entity. Therefore, we suggest a four-eye concept: when the radiologist assesses the tumors degree of malignancy as benign or intermediate. An experienced musculoskeletal surgeon views the MR images again. When the tumor appears lipomatous and truly homogeneous, it can be chosen for PMR. This results in faster and more comfortable treatment for the patient, especially with lipomas or ALTs.
However, the data supporting these results was retrospectively collected in one specialized institution. Prospective, multicenter studies are needed with an analysis for intra-observer and inter-observer comparisons, to recommend a true change in the standard of care for soft tissue tumors.
Availability of data and materials
The raw data are available upon reasonable request from the corresponding author.
Abbreviations
- ALT:
-
Atypical lipomatous tumor
- FISH:
-
Fluorescence in situ hybridization
- MDM2:
-
Mouse double minute 2
- MRI:
-
Magnetic resonance imaging
- LPS:
-
Liposarcoma
- PMR:
-
Primary marginal resection
- STT:
-
Soft tissue tumor
- WDLPS:
-
Well-differentiated liposarcoma
References
Gupta P, Potti TA, Wuertzer SD, Lenchik L, Pacholke DA. Spectrum of fat-containing soft-tissue masses at MR imaging: the common, the uncommon, the characteristic, and the sometimes confusing. Radiographics. 2016;36(3):753–66.
Mashima E, Sawada Y, Saito-Sasaki N, Yamamoto K, Ohmori S, Omoto D, et al. A retrospective study of superficial type atypical lipomatous tumor. Front Med (Lausanne). 2020;7:609515.
Rydholm A, Berg NO. Size, site and clinical incidence of lipoma. Factors in the differential diagnosis of lipoma and sarcoma. Acta Orthop Scand. 1983;54(6):929-934.
Papanastassiou ID, Piskopakis A, Gerochristou MA, Chloros GD, Savvidou OD, Issaiades D, et al. Dedifferentiation of an atypical lipomatous tumor of the thigh - a 6 year follow-up study. J Musculoskelet Neuronal Interact. 2019;19(1):123–6.
Creytens D. What's new in adipocytic neoplasia? Virchows Arch. 2020;476(1):29–39.
Sato D, Suga H, Takushima A. Liposarcoma preoperatively diagnosed as lipoma: 10-year experience at a single institution. Dermatol Surg. 2018;44(8):1065–9.
Trama A, Badalamenti G, Baldi GG, Brunello A, Caira M, Drove N, et al. Soft tissue sarcoma in Italy: from epidemiological data to clinical networking to improve patient care and outcomes. Cancer Epidemiol. 2019;59:258–64.
Gage MM, Nagarajan N, Ruck JM, Canner JK, Khan S, Giuliano K, et al. Sarcomas in the United States: recent trends and a call for improved staging. Oncotarget. 2019;10(25):2462–74.
Younger E, Husson O, Bennister L, Whelan J, Wilson R, Roast A, et al. Age-related sarcoma patient experience: results from a national survey in England. BMC Cancer. 2018;18(1):991.
Society AC. Cancer Statistics Center http://cancerstatisticscenter.cancer.org.2018 [
Manji GA, Schwartz GK. Managing liposarcomas: cutting through the fat. J Oncol Pract. 2016;12(3):221–7.
Baili P, Di Salvo F, Marcos-Gragera R, Siesling S, Mallone S, Santaquilani M, et al. Age and case mix-standardised survival for all cancer patients in Europe 1999-2007: results of EUROCARE-5, a population-based study. Eur J Cancer. 2015;51(15):2120–9.
Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786–93.
Leporq B, Bouhamama A, Pilleul F, Lame F, Bihane C, Sdika M, et al. MRI-based radiomics to predict lipomatous soft tissue tumors malignancy: a pilot study. Cancer Imaging. 2020;20(1):78.
Pan M, Seto T, Yu J, Sidhu M, Kim B, McCormick C, et al. Feasibility and value of establishing a community-based virtual multidisciplinary sarcoma case conference. JCO Oncol Pract. 2020;16(10):e1143–e50.
Ballhause TM, Reiter A, Korthaus A, Frosch KH, Schlickewei CW, Priemel MH. Diagnostic delay in soft tissue tumors: a single-center study of a university cancer center with a focus on health services research. BMC Health Serv Res. 2022;22(1):452.
Gaskin CM, Helms CA. Lipomas, lipoma variants, and well-differentiated liposarcomas (atypical lipomas): results of MRI evaluations of 126 consecutive fatty masses. AJR Am J Roentgenol. 2004;182(3):733–9.
Weiss S, Korthaus A, Baumann N, Yamamura J, Spiro AS, Lubke AM, et al. Musculoskeletal soft-tissue sarcoma: quality assessment of initial MRI reports shows frequent deviation from ESSR guidelines. Diagnostics (Basel). 2021;11(4).
O'Donnell PW, Griffin AM, Eward WC, Sternheim A, White LM, Wunder JS, et al. Can experienced observers differentiate between lipoma and well-differentiated liposarcoma using only MRI? Sarcoma. 2013;2013:982784.
Munk PL, Lee MJ, Janzen DL, Connell DG, Logan PM, Poon PY, et al. Lipoma and liposarcoma: evaluation using CT and MR imaging. AJR Am J Roentgenol. 1997;169(2):589–94.
Brisson M, Kashima T, Delaney D, Tirabosco R, Clarke A, Cro S, et al. MRI characteristics of lipoma and atypical lipomatous tumor/well-differentiated liposarcoma: retrospective comparison with histology and MDM2 gene amplification. Skeletal Radiol. 2013;42(5):635–47.
Clay MR, Martinez AP, Weiss SW, Edgar MA. MDM2 amplification in problematic lipomatous tumors: analysis of FISH testing criteria. Am J Surg Pathol. 2015;39(10):1433–9.
Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25(5):1371–95.
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Adulte Weichgewebesarkome, Langversion Version 1.0, 2021, AWMF-Registernummer: 032/044OL, https://www.leitlinienprogrammonkologie.de/leitlinien/adulte-weichgewebesarkome/. Accessed 1 Apr 2022.
Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182.
Gilbert NF, Cannon CP, Lin PP, Lewis VO. Soft-tissue sarcoma. J Am Acad Orthop Surg. 2009;17(1):40–7.
Sung MS, Kang HS, Suh JS, Lee JH, Park JM, Kim JY, et al. Myxoid liposarcoma: appearance at MR imaging with histologic correlation. Radiographics. 2000;20(4):1007–19.
Sirvent N, Coindre JM, Maire G, Hostein I, Keslair F, Guillou L, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol. 2007;31(10):1476–89.
Nijhuis PH, Schaapveld M, Otter R, Hoekstra HJ. Soft tissue sarcoma--compliance with guidelines. Cancer. 2001;91(11):2186-2195.
Jansen-Landheer ML, Krijnen P, Oostindier MJ, Kloosterman-Boele WM, Noordijk EM, Nooij MA, et al. Improved diagnosis and treatment of soft tissue sarcoma patients after implementation of national guidelines: a population-based study. Eur J Surg Oncol. 2009;35(12):1326–32.
Noebauer-Huhmann IM, Weber MA, Lalam RK, Trattnig S, Bohndorf K, Vanhoenacker F, et al. Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging. Semin Musculoskelet Radiol. 2015;19(5):475–82.
Expert Panel on Musculoskeletal I, Kransdorf MJ, Murphey MD, Wessell DE, Cassidy RC, Czuczman GJ, et al. ACR appropriateness criteria((R)) soft-tissue masses. J Am Coll Radiol. 2018;15(5S):S189–S97.
Paszat L, O'Sullivan B, Bell R, Bramwell V, Groome P, Mackillop W, et al. Processes and outcomes of care for soft tissue sarcoma of the extremities. Sarcoma. 2002;6(1):19–26.
Mitsuyoshi G, Naito N, Kawai A, Kunisada T, Yoshida A, Yanai H, et al. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol. 2006;94(1):21–7.
Dupuy DE, Rosenberg AE, Punyaratabandhu T, Tan MH, Mankin HJ. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am J Roentgenol. 1998;171(3):759–62.
Klein A, Fell T, Birkenmaier C, Fromm J, Jansson V, Knosel T, et al. Relative sensitivity of core-needle biopsy and incisional biopsy in the diagnosis of musculoskeletal sarcomas. Cancers (Basel). 2021;13(6).
Domanski HA, Akerman M, Carlen B, Engellau J, Gustafson P, Jonsson K, et al. Core-needle biopsy performed by the cytopathologist: a technique to complement fine-needle aspiration of soft tissue and bone lesions. Cancer. 2005;105(4):229–39.
Singh HK, Kilpatrick SE, Silverman JF. Fine needle aspiration biopsy of soft tissue sarcomas: utility and diagnostic challenges. Adv Anat Pathol. 2004;11(1):24–37.
Winkler D, Fritzsche H, Schaser KD, Hofbauer C. Biopsy of musculoskeletal tumors : complications and avoidable errors. Orthopade. 2020;49(2):88–97.
Dei Tos AP, Bonvalot S, Haas R. The key role of pathology, surgery and radiotherapy in the initial management of soft tissue sarcoma. Future Oncol. 2018;14(10s):15–23.
Pretell-Mazzini J, Barton MD Jr, Conway SA, Temple HT. Unplanned excision of soft-tissue sarcomas: current concepts for management and prognosis. The Journal of bone and joint surgery American volume. 2015;97(7):597–603.
Acknowledgements
We kindly thank Annika Ballhause for her help with the creation of the figures and Florian Barkmann for his advice in statistics.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support for the research, authorship, and publication of this article.
Author information
Authors and Affiliations
Contributions
Conceptualization, MHP; methodology, TMB; validation, SW, AR, and CW; investigation, TMB; data curation, TMB and AML; writing—original draft preparation, TMB and MHP; writing—review and editing, PB, AML, and KHF; visualization, TMB; supervision, CWS and MHP; project administration, MHP. The authors read and approved the final the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee (Ethikkommission der Hamburger Ärztekammer) approval was given for retrospective registration (Reference number: WF-071/20) on the 05-04-2020 by the Physicians Chamber of Hamburg (Hamburger Ärztekammer). We confirm that all methods were performed in accordance with the relevant guidelines and regulations. The need for informed consent was waived by the ethics committee (Ethikkommission der Hamburger Ärztekammer) in its decision from 05-04-2020.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ballhause, T.M., Weiss, S., Reiter, A. et al. Can homogeneous, lipomatous tumors be primarily resected without biopsy? A retrospective analysis of 240 tumors. World J Surg Onc 20, 184 (2022). https://doi.org/10.1186/s12957-022-02665-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02665-4