Abstract
Background
We evaluated the influence of infectious complications, particularly surgical site infection (SSI), on long-term oncological results after elective laparoscopic resection of colorectal cancer.
Methods
A total of 199 patients who underwent laparoscopic elective resection with negative resection margins for stage I–III colorectal cancer were retrospectively examined. The postoperative course was recorded based on hospital records, and cancer relapse was diagnosed based on radiological or pathological findings under a standardized follow-up program. The severity of complications was graded using Clavien-Dindo (CD) classification.
Results
SSI was found in 25 patients (12.6%), with 12 (6.0%) showing anastomotic leak. The postoperative relapse-free survival (RFS) rate was significantly lower in patients with SSI (49.2%) than in patients without SSI (87.2%, P<0.001). Differences in RFS were found after both colectomy and rectal resection (P<0.001 and P<0.001, respectively). RFS did not differ between patients who had major SSI CD (grade III) and those who had minor SSI CD (grades I or II). Multivariate Cox regression analysis identified the occurrence of SSI and pathological stage as independent co-factors for RFS (P<0.001 and P=0.003).
Conclusion
These results suggest that postoperative SSI compromises long-term oncological results after laparoscopic colorectal resection. Further improvements in surgical technique and refinements in perioperative care may improve long-term oncological results.
Similar content being viewed by others
Introduction
Laparoscopic surgery has commonly been applied for colorectal cancer in recent years. Postoperative complications are reportedly less frequent after laparoscopic surgery than after open surgery [1,2,3]. Long-term oncological results have been shown to be comparable between laparoscopic and open surgeries [4,5,6].
Infectious complications after surgery have been reported to compromise long-term oncological results in gastric cancer [7,8,9], esophageal cancer [10], and breast cancer [11]. With regard to colorectal cancer, although anastomotic leak (AL) has been suggested as a risk factor for local recurrence [12,13,14,15], the impact of infectious complications including surgical site infections (SSIs) such as AL on long-term oncological outcome after resection of colorectal cancer has been controversial [12,13,14,15,16,17,18,19,20,21,22,23,24]. In particular, studies limited to patients after laparoscopic colorectal resection remain scarce [25]. The present study explored the relationship between SSI and long-term oncological results in patients who underwent laparoscopic curative resection for stage I–III colorectal cancer in our surgical department.
Patients and methods
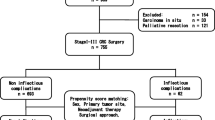
A total of 405 patients underwent elective resection for colorectal cancer between January 2013 and May 2015 in the Department of Surgery at Saitama Medical Center, Dokkyo Medical University. Of those 405 patients, 239 patients underwent laparoscopic resection. Fifteen patients with pathological pT0 lesions after neoadjuvant treatment or pTis lesion and 14 patients with clinically confirmed stage IV lesion were excluded. The tumor stage was based on the classification by the Japanese Society for Cancers of the Colon and Rectum [26]. Eleven patients with positive or unclear resection margins were also excluded. As a result, a total of 199 patients were included in the study.
Diabetes mellitus (DM) which is a well-established risk factor for colorectal cancer was recorded as present in patients receiving oral medication or insulin injection or in those with hemoglobin (Hb)A1c level ≥6.5% without treatment for DM [27]. Systemic co-morbidities including DM were evaluated using the American Society of Anesthesiology physical status. The surgical procedures performed were colectomy including anterior resection for rectosigmoid cancer in 138 patients and rectal resection in 61 patients. Adjuvant chemotherapy was carried out mainly in stage III patients for 6 months using a regimen of oral 5-fluorouracil-leucovorin or capecitabine alone, or regimens including oxaliplatin (FOLFOX or CAPOX). Neoadjuvant treatment including neoadjuvant chemoradiotherapy was performed mainly for patients with rectal cancer.
Patients received mechanical bowel preparation comprising magnesium citrate solution and laxatives the day before surgery. Preoperative oral antibiotics were not applied. Prophylactic intravenous antibiotics were administered at the time of skin incision and every 4 h thereafter during surgery, in the evening of the day of surgery, and twice on the first postoperative day.
The duration of operation and intraoperative bleeding was recorded from the hospital records, which were completed by residents, ward nurses, and attending surgeons. Perioperative morbidity up to 30 days after surgery was also retrieved by two of the authors (NS and MO) from the hospital records. Infectious complications were recorded mainly based on clinical symptoms such as fever, pain and tenderness, radiological findings, and urinalysis. SSI was defined according to the Centers for Disease Control and Prevention (CDC) guidelines [28]. The severity of postoperative complications was graded according to Clavien-Dindo (CD) classification [29].
Postoperative follow-up was standardized as follows: blood testing, including measurement of carcinoembryonic antigen (CEA) every 3 months; and CT from the chest to the pelvis every 6 months until 5 years after surgery. Surveillance colonoscopy was usually performed within 1 year after surgery and annually thereafter until no neoplastic lesion was detected. If no neoplastic lesions were detected on surveillance colonoscopy, the next colonoscopy was performed 3 years later. When CEA concentration was elevated without accompanying CT, CT was usually performed. Relapse was diagnosed based on the findings from CT or biopsy at colonoscopy. Positron-emission tomography (PET) was occasionally performed to confirm relapse.
Relapse-free survival (RFS) time was calculated as the duration between surgery and diagnosis of relapse. If a patient died without a diagnosis of relapse, patient data were censored as of the time of death. Overall survival (OS) was also recorded as the duration between surgery and death from any cause, including other causes than colorectal cancer. However, since OS is strongly affected by the treatment after relapse, OS was not analyzed as an endpoint of the present study. The median duration of follow-up for RFS was 60.8 months.
Statistical analysis
Postoperative RFS was compared between patients who did and did not develop infectious complications using Kaplan-Meier methods with log-rank testing. The influence of various factors including SSI on RFS was examined using uni- and multivariate Cox proportional hazard regression modeling. Multivariate analysis was performed using a forward stepwise selection method. Factors that trended toward significance (P<.10) on univariate analysis were included as candidates for independent variables affecting disease-free survival (DFS). A two-sided value of P <.05 was considered significant. All statistical analyses were performed using the Dr. SPSS software package (SPSS Japan, Tokyo, Japan).
Results
Postoperative complications
Patient characteristics and the details of adjuvant chemotherapy and neoadjuvant treatment are shown in Tables 1 and 2. Postoperative SSIs in a total of 199 patients are summarized in Table 3. SSI occurred in 25 patients (12.6%, Table 4), including in 14 patients (10.1%) after colectomy and 11 patients (18.0%) after rectal resection. Of the 25 patients who developed SSI, 18 patients showed minor SSI (CD grades I or II), whereas 7 patients developed major SSI (grades III). Major SSI occurred in only 1 patient after colectomy and 6 patients after rectal resection. Although AL occurred in 4 patients after colectomy and 8 patients after rectal resection, CD grades were II in 7 patients and III in 5 patients. No postoperative deaths occurred within 90 days after surgery.
Comparisons of RFS according to infectious complications
The 3-year RFS rate was significantly lower in the 25 patients with SSI (49.2%) than in the 174 patients without SSI (87.2%, P<0.001, Fig. 1). When patients after colectomy and patients after rectal resection were analyzed separately, a 3-year RFS rate was significantly worse in patients who developed SSI than in those who did not in both colectomy and rectal resection (colectomy: 47.1% in 14 patients with SSI versus 86.3% in 124 patients without SSI, P<0.001; rectal resection: 53.0% in 11 patients with SSI versus 91.4% in 50 patients without SSI, P<0.001, Figs. 2 and 3). RFS rate did not differ between patients with major SSI (CD grade III/IV) and those with minor SSI (CD grade I/II) (P=0.689, Fig. 4).
Factors associated with RFS
Table 5 shows the results of univariate Cox proportional regression modeling for RFS. SSI was associated with shorter RFS. Other factors associated with shorter RFS were pathological stage II/III compared with stage I and intravenous adjuvant chemotherapy. In multivariate Cox proportional regression modeling, pathological stage and the presence of SSI were significantly associated with shorter RFS (Table 6). In the sub-analysis by stage, stage I cases showed no significant difference in a 3-year RFS rate according to the occurrence of SSI (SSI− vs. SSI+, in stage I: 100 vs. 100%). By contrast, significant differences in a 3-year RFS rate were seen in each analysis for stage II and stage III (SSI− vs. SSI+, in stage II: 53.3 vs. 89.2%, P=0.0001; in stage III: 26.7 vs. 75.4%, P<0.0001).
Site of relapse
Table 7 compares sites of relapse according to the occurrence of SSI. Liver, distant lymph node, and peritoneal dissemination recurrences were significantly more frequent in patients with SSI compared to those without (P=0.009, P=0.04, and P=0.04, respectively). In patients without SSI, lung metastasis was the most frequent site of relapse. The local recurrence occurred in 4 patients, comprising 2 patients with SSI and 2 patients without.
Adjuvant chemotherapy in patients with stage III lesion
Table 8 shows adjuvant chemotherapy in patients with stage III lesions. In 10 patients who had SSI, adjuvant chemotherapy was carried out in 5 patients (50%). By contrast, among the 59 patients who did not have SSI, adjuvant chemotherapy was performed in 45 patients (76.3%). The rate of patients who received adjuvant chemotherapy tended to be lower in patients with SSI than in patients without (P=0.09, chi-square test).
Discussion
The present study showed that postoperative SSI was significantly associated with inferior RFS after potentially curative laparoscopic resection for stage I–III colorectal cancer. The significance of the association was independent of the tumor stage and was present after both colonic resection and rectal resection.
Previous studies have usually included both patients after open surgery and those after laparoscopic surgery [14, 15, 19,20,21,22,23,24, 30,31,32]. However, laparoscopic surgery has been applied for the majority of elective resections for colorectal cancer in recent years. In our department, elective open surgery is selected on a limited basis for large lesions, those with suspected invasion into adjacent organs and those with extensive lymph node metastasis. These factors influence not only the pathological stage of the tumor, but also the technical difficulty of potentially curative resection. We therefore analyzed only those patients who underwent R0 resection by laparoscopic surgery in the present study.
The rates of AL after open rectal resection were around 10% in the literature [12, 13]. Akiyoshi et al. reported an AL rate of only 3.8% in 363 patients after laparoscopic anterior resection [33]. Others reported a lower incidence of SSI or LA after laparoscopic surgery than after open surgery [1,2,3]. In the present study, however, the LA rate in 51 patients after anterior resection for rectal cancer was 15.6% (8 of 51 patients, after excluding 10 patients who underwent abdominoperineal resection). The high incidence of AL after rectal resection in the present study may have been due to the affirmative application of very low anastomosis for low rectal lesions. The AL rate after colectomy (including anterior resection for rectosigmoid cancer) was low (only 4 of 138 patients, 2.9%). Moreover, AL in the present study was not always recorded as a major SSI (grade III or more in the CD classification).
SSI has been reported to be more frequent after rectal resection than after colectomy in both open surgery [34] and laparoscopic surgery [35]. In the present study, although SSI tended to be more frequent after rectal resection (11 of 61 patients) than after colectomy (14 of 138 patients), the difference between colectomy and rectal resection was not significant. This was probably due to the small number of patients.
Several previous studies, including meta-analyses, have shown that AL after rectal resection is a risk factor for local recurrence [12,13,14,15]. However, this association has not been found in other studies [18, 31]. In the present study, only one of the 12 patients who showed AL developed local recurrence. The overall incidence of local recurrence was low in the present study (only 4 of 199 patients, 2.0%).
The low incidence of local recurrence may be attributable to the exclusion from the present study of patients with positive or unclear resection margins. A positive circumferential resection margin is well established as a strong risk factor for local recurrence after mesorectal excision for rectal cancer [36]. For colonic cancer, recent studies have suggested the importance of negative resection margin for preventing local recurrence [37].
Reports exploring the impact of SSI on RFS or cancer-specific survival after laparoscopic surgery are limited. Park et al. reported that complications after laparoscopic low anterior resection for rectal cancer compromised RFS [25]. In the present study, the RFS rate was lower in patients with postoperative SSI than in those without, for both colectomy and rectal resection. Sub-analysis by stage found no significant difference in RFS rate according to the occurrence of SSI in patients with stage I, but a significant difference in patients with stages II or III. Further studies focused on these stages may support our results more strongly.
The impact of the severity of postoperative complications on long-term oncological results is still controversial. Odermatt et al. reported that increasing CD scores from grades I to IV was significantly associated with progressive decreases in OS and DFS [20]. In a report by Duraes et al., higher CD grades were associated with worse OS and RFS after colorectal cancer resection [21]. Cienfuegos et al. reported worse OS and DFS in patients with major complications beyond in CD grade IIIb than in those without major complications [22]. In contrast, Mark et al. reported similar oncological results after resection for rectal cancer among patients who had major complications, those who had minor complications and those without complications [19]. Oh et al. reported similar oncological results in patients with major (CD grade III or IV) or minor (grade I or II) complications after colorectal cancer surgery [24]. In the present study, RFS did not differ between patients who developed major SSI and those who developed minor SSI. The inclusion of only patients after laparoscopic surgery and stages I–III with negative resection margins might have influenced these results. In addition, the small number of patients with SSI might have been another reason for the negative result.
The mechanisms by which SSI results in worse RFS remain speculative. A possible explanation is that delayed or difficult postoperative adjuvant chemotherapy may be responsible for the inferior RFS in patients who had SSI [30]. Actually, the proportion of stage III patients in the present study who received adjuvant chemotherapy was marginally lower among patients who had SSI than among those who did not. However, this small difference in adjuvant chemotherapy may not sufficiently explain the significant difference in RFS between patients who did and did not show SSI.
Another issue for speculation is the negative influence of systemic or local inflammatory response caused by SSI on RFS. A number of studies have explored the influence of preoperative nutritional state and inflammation on oncological outcomes of various cancers [38,39,40,41,42,43,44,45,46,47,48]. Although we did not examine nutritional or inflammatory biomarkers in the present study, changes in such biomarkers caused by SSI may play a role in the long-term oncological outcomes. The relationship of perioperative nutritional or inflammatory biomarkers with long-term oncological results may be an interesting subject for future studies.
Although the present study found significant differences for some sites of recurrence (liver, lymph nodes, and peritoneum) between patients with and without SSI, the relationship between the site of recurrence and SSI is difficult to speculate. Inflammatory cells produce tumor necrosis factor α, transforming growth factor β, interleukin-6, and other cytokines, and these inflammatory agents regulate nuclear factor (NF)-κβ and the STAT3 pathway and encourage metastasis in tumor cells [49, 50]. Moreover, Helbig et al. reported that NFκβ induces the chemokine receptor CXCR4 which regulates organ-specific metastasis in several solid cancers and promotes cancer cell migration and metastasis [51]. These mechanisms should be investigated in future molecular studies.
The present study has some limitations that merit consideration when interpreting the results. First, this study included an inherent selection bias owing to its retrospective design. In particular, the selection bias was present with regard to selecting the surgical approach. In addition, co-morbidities other than DM might have affected the incidence of SSI and resulted in confounding bias. Second, the small number of patients makes it difficult to support the results conclusively. Third, this study was restricted to patients from a single institution. Details of the surgical technique for laparoscopic colorectal resection might differ between institutions, and different surgical techniques are likely to exert different influences on RFS, even among patients who had SSI. A multi-institutional survey of infectious complications including SSI and RFS using a unified protocol for the diagnosis of infectious complications and adjuvant chemotherapy may further clarify the influence of SSI on RFS.
In conclusion, the present study revealed that RFS after potentially curative laparoscopic resection of stage I–III colorectal cancer may be compromised by postoperative SSI. Further improvements in surgical technique and refinement of perioperative care to reduce SSI may contribute not only to the safety of colorectal surgery, but also to the improvement of long-term oncological results.
Availability of data and materials
The datasets supporting the conclusion of this article are included within the article. The underlying datasets are available from the corresponding author on reasonable request.
References
Kiran RP, El-Gazzaz GH, Vogel JD, Remzi FH. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: data from national surgical quality improvement program. J Am Coll Surg. 2010;211:232–8.
Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, et al. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23–30.
Zheng H, Wu Z, Wu Y, Mo S, Dai W, Liu F, et al. Laparoscopic surgery may decrease the risk of clinical anastomotic leakage and a nomogram to predict anastomotic leakage after anterior resection for rectal cancer. Int J Color Dis. 2019;34:319–28.
Huang MJ, Liang JL, Wang H, Kang L, Deng YH, Wang JP. Laparoscopic-assisted versus open surgery for rectal cancer: a meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Color Dis. 2011;26:415–21.
Kitano S, Inomata M, Mizusawa J, Katayama H, Watanabe M, Yamamoto S, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2017;2:261–8.
Nienhuser H, Heger P, Schmitz R, Kulu Y, Diener MK, Klose J, et al. Short- and long-term oncological outcome after rectal cancer surgery: a systematic review and meta-analysis comparing open versus laparoscopic rectal cancer surgery. J Gastrointest Surg. 2018;22:1418–33.
Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, et al. Impact of postoperative infection on long-term survival after potentially curative resection for gastric cancer. Ann Surg Oncol. 2009;16:311–8.
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83.
Yuan P, Wu Z, Li Z, Bu Z, Wu A, Wu X, et al. Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer. 2019;19:833.
Hirai T, Yamashita Y, Mukaida H, Kuwahara M, Inoue H, Toge T. Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today. 1998;28:576–9.
Murthy BL, Thomson CS, Dodwell D, Shenoy H, Mikeljevic JS, Forman D, et al. Postoperative wound complications and systemic recurrence in breast cancer. Br J Cancer. 2007;97:1211–7.
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–9.
Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum. 2016;59:236–44.
Goto S, Hasegawa S, Hida K, Uozumi R, Kanemitsu Y, Watanabe T, et al. Multicenter analysis of impact of anastomotic leakage on long-term oncologic outcomes after curative resection of colon cancer. Surgery. 2017;162:317–24.
Kim IY, Kim BR, Kim YW. The impact of anastomotic leakage on oncologic outcomes and the receipt and timing of adjuvant chemotherapy after colorectal cancer surgery. Int J Surg. 2015;22:3–9.
Tsujimoto H, Ueno H, Hashiguchi Y, Ono S, Ichikura T, Hase K. Postoperative infections are associated with adverse outcome after resection with curative intent for colorectal cancer. Oncol Lett. 2010;1:119–25.
Richards CH, Platt JJ, Anderson JH, Mckee RF, Horgan PG, Mcmillan DC. The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Ann Surg. 2011;254:83–9.
Smith JD, Paty PB, Guillem JG, Temple LK, Weiser MR, Nash GM. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg. 2012;256:1034–8.
Mrak K, Eberi T, Jagoditch M, Fritz J, Tschmelitsch J. Impact of postoperative complications on long-term survival after resection for rectal cancer. Dis Colon Rectum. 2013;56:20–8.
Odermatt M, Miskovic D, Flashman K, Khan J, Senapati A, O'Leary D. Major postoperative complications following elective resection for colorectal cancer decrease long-term survival but not the time to recurrence. Color Dis. 2015;17:141–9.
Duraes LC, Stocchi L, Steele SR, Kalady MF, Church JM, Gorgun E. The relationship between Clavien-Dindo morbidity classification and oncologic outcomes after colorectal cancer resection. Ann Surg Oncol. 2018;25:188–96.
Cienfuegos JA, Baixauli J, Beorlegui C, Ortega PM, Granero L, Zozaya G. The impact of major postoperative complications on long-term outcomes following curative resection of colon cancer. Int J Surg. 2018;52:303–8.
Huh JW, Lee WY, Park YA, Cho YB, Kim HC, Yun SH, et al. Oncological outcome of surgical site infection after colorectal cancer surgery. Int J Color Dis. 2019;34:277–83.
Oh CK, Huh JW, Lee YJ, VChoi MS, Pyo DH, Lee SC, et al. Long-term oncologic outcome of postoperative complications after colorectal cancer surgery. Ann Coloproctol. 2020;36:273–80.
Park EJ, Baik SH, Kang J, Hur H, Min BS, Lee KY, et al. The Impact of Postoperative complications on long-term oncologic outcomes after laparoscopic low anterior resection for rectal cancer. Medicine (Baltimore). 2016;95:e3271.
Japanese Society for Cancer of The Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d English edition [Secondary Publication]. J Anus Rectum Colon. 2019;3:175–95.
Tsilidis KK, Kasimis JC, Lopez DS, Ntzani E, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of metaanalysis of observational studies. BMJ. 2015;350:g7607.
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930–8.
Zimmermann MS, Wellner U, Laubert T, Ellebrecht DB, Bruch HP, Keck T, et al. Influence of anastomotic leak after elective colorectal cancer resection on survival and local recurrence: a propensity score analysis. Dis Colon Rectum. 2019;62:286–93.
Sueda T, Tei M, Yoshikawa Y, Furukawa H, Matsumura T, Koga C, et al. Prognostic impact of postoperative intra-abdominal infections after elective colorectal cancer resection on survival and local recurrence: a propensity score-matched analysis. Int J Color Dis. 2020;35:413–22.
Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, et al. Incidence of and risk factors for anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Am J Surg. 2011;202:259–64.
Konishi T, Watanabe T, Kishimoto J, Nagawa H. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg. 2006;244:758–63.
Goto S, Hasegawa S, Hata H, Yamaguchi T, Hida K, Nishitai R, et al. Differences in surgical site infection between laparoscopic colon and rectal surgeries: sub-analysis of a multicenter randomized controlled trial (Japan-Multinational Trial Organization PREV 07-01). Int J Color Dis. 2016;31:1775–84.
Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–12.
Amri R, Bordeianou LG, Sylla P, Berger DL. Association of radial margin positivity with colon cancer. JAMA Surg. 2015;150:890–8.
Roxburgh CS, Crozier JE, Maxwell F, Foulis AK, Brown J, Mckee RF, et al. Comparison of tumour-based (Petersen Index) and inflammation-based (Glasgow Prognostic Score) scoring systems in patients undergoing curative resection for colon cancer. Br J Cancer. 2009;100:701–6.
Proctor MJ, Talwar D, Balmar SM, O'reilly DS, Foulis AK, Horgan PG, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870–6.
Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14.
Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'reilly DS, Foulis AK, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–34.
Mehta RS, Chong DQ, Song M, Meyerhardt JA, Ng K, Nishihara R, et al. Association between plasma levels of macrophage inhibitory cytokine-1 before diagnosis of colorectal cncer and mortality. Gastroenterology. 2015;149:614–22.
Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265:539–46.
Iseki Y, Shibutani M, Maeda K, Nagahara H, Tamura T, Ohira G, et al. The impact of the preoperative peripheral lymphocyte count and lymphocyte percentage in patients with colorectal cancer. Surg Today. 2017;47:743–54.
Olsen RS, Nijm J, Andersson RE, Dimberg J, Wagsater D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol. 2017;23:6212–9.
Peng J, Zhang R, Zhao Y, Wu X, Chen G, Wan D, et al. Prognostic value of preoperative prognostic nutritional index and its associations with systemic inflammatory response markers in patients with stage III colon cancer. Chin J Cancer. 2017;36:96.
Rossi S, Basso M, Strippoli A, Schinzari G, D'argento E, Larocca M, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16:264–74.
Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y, et al. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer. 2017;17:744.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epitherial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13.
Helbig G, Christopherson KW, Bhat-Naksshatri P, Kumar S, Kishimoto H, Miller KD, et al. NF-kappaB promotes breast cancer cell migration and metastasis by including the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–8.
Acknowledgements
None.
Funding
This research received no funding support.
Author information
Authors and Affiliations
Contributions
NS, TO, and MO designed the study. NS, TO, ET, HO, YH, SM, MT, and MO participated in the surgical procedures. NS, TO, TM, TN, HY, and MO reviewed the clinical records. NS, TO, and MO analyzed the data. All authors participated in the study design, data interpretation, and critical discussion. NS, TO, and MO wrote the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Before the operation, written informed consent for the present study was obtained from all patients. This study was conformed to the 1964 Helsinki Declaration its later amendments or with comparable ethical standards and was approved by the institutional review board at Saitama Medical Center, Dokkyo Medical University (No. 1582).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sugamata, N., Okuyama, T., Takeshita, E. et al. Surgical site infection after laparoscopic resection of colorectal cancer is associated with compromised long-term oncological outcome. World J Surg Onc 20, 111 (2022). https://doi.org/10.1186/s12957-022-02578-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02578-2