Abstract
Background
This study aimed to develop and validate a novel nomogram to predict the cancer-specific survival (CSS) of patients with ascending colon adenocarcinoma after surgery.
Methods
Patients with ascending colon adenocarcinoma were enrolled from the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2015 and randomly divided into a training set (5930) and a validation set (2540). The cut-off values for age, tumour size and lymph node ratio (LNR) were calculated via X-tile software. In the training set, independent prognostic factors were identified using univariate and multivariate Cox analyses, and a nomogram incorporating these factors was subsequently built. Data from the validation set were used to assess the reliability and accuracy of the nomogram and then compared with the 8th edition of the American Joint Committee on Cancer (AJCC) tumour-node-metastasis (TNM) staging system. Furthermore, external validation was performed from a single institution in China.
Results
A total of 8470 patients were enrolled from the SEER database, 5930 patients were allocated to the training set, 2540 were allocated to the internal validation set and a separate set of 473 patients was allocated to the external validation set. The optimal cut-off values of age, tumour size and lymph node ratio were 73 and 85, 33 and 75 and 4.9 and 32.8, respectively. Univariate and multivariate Cox multivariate regression revealed that age, AJCC 8th edition T, N and M stage, carcinoembryonic antigen (CEA), tumour differentiation, chemotherapy, perineural invasion and LNR were independent risk factors for patient CSS. The nomogram showed good predictive ability, as indicated by discriminative ability and calibration, with C statistics of 0.835 (95% CI, 0.823–0.847) and 0.848 (95% CI, 0.830–0.866) in the training and validation sets and 0.732 (95% CI, 0.664–0.799) in the external validation set. The nomogram showed favourable discrimination and calibration abilities and performed better than the AJCC TNM staging system.
Conclusions
A novel validated nomogram could effectively predict patients with ascending colon adenocarcinoma after surgery, and this predictive power may guide clinicians in accurate prognostic judgement.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer-related death worldwide, with the predominant type being adenocarcinoma [1]. In the USA, there were an estimated 104,270 new cases and 52,980 deaths due to colon cancer in 2021 [2]. The colon can be divided into the left and right colon according to the localization of the primary tumour, with the splenic flexure as the boundary. The right side of the colon is historically derived from the embryological midgut, and the left side originates in the embryological hindgut. Epidemiological, clinical and molecular biological differences between left-sided and right-sided colon cancer have been elucidated and studied in many studies [3, 4]. It has been suggested that left and right-sided colon cancer may be two different entities [5]. Right-sided colon tumours usually exhibit larger size and higher tumour grade, leading to worse prognosis compared to left-sided cancer [5, 6]. The caecum and ascending colon are considered the most common tumour sites in right-sided colon [7]. Unlike the caecum, which develops from the caecal diverticulum in the 5-week-old embryo, the ascending colon arises from the caudal limb of the midgut loop. There may be differences between caecum and ascending colon carcinomas due to different locations and developmental processes. The colonic subsite should be taken into consideration when cancer is classified [7]. Further detailed analysis may be necessary to investigate the epidemiology and prognosis of tumours in different areas of the colon to facilitate a more precise and individualized treatment plan and the prediction of survival.
Surgical resection and adjuvant chemotherapy are the mainstay treatments for patients with colon cancer. The pathological data and lymph node status guide prognosis and adjuvant therapy recommendations [8], defined as the proportion of positive regional nodes among the number of examined regional nodes, and have been demonstrated to have a significant prognostic role in many cancers [9,10,11,12], even superior to pN stage in many studies [13].
The American Joint Committee on Cancer (AJCC) tumour-node-metastasis (TNM) staging system is recognized and widely used in predicting the prognosis of tumours. However, there are limitations concerning TNM classification because all colorectal tumours share the same stage standards even when they do not fit into either subdivision. The TNM staging system cannot serve as the best prognostic tool since it only includes the anatomical information of the tumour and ignores the clinical features and laboratory tests of the patients.
A nomogram is a graphical predictive tool with a calculated score that is used to predict tumour prognosis, and it can provide individualized, evidence-based, accurate risk estimation [14]. Furthermore, nomograms improve the decision-making processes and are relatively easy to use. A number of nomograms have been developed to predict outcomes in various types of malignancies. Nevertheless, no data were available in nomograms including LNR to predict the prognosis in ascending colon adenocarcinoma after surgery. Cancer-specific survival (CSS), which directly reflects cancer prognosis, is calculated from diagnosis to death from the tumour. In this study, we aimed to assess the predictive ability of a nomogram incorporating the LNR in patients with ascending colon adenocarcinoma after surgery. In addition, we also compared the predictive accuracy and discriminability of the nomogram for predicting CSS with the current TNM staging system.
Materials and methods
Patients
Data from patients with ascending colon adenocarcinoma were extracted from the SEER database using SEER*stat software (version 8.3.6, NCI, Bethesda, USA). The SEER database collects patient demographics and publishes cancer incidence and survival data, covering approximately 34% of the US population. Patient data was collected for those diagnosed with ascending colon adenocarcinoma or included an adenocarcinoma as a component of their primary malignancy (C18.2) between 2004 and 2015, and histological classification was based on the International Classification of Diseases Codes for Oncology (ICD-O) proposed in 2000 (8140/3,8144/3,8210/3,8211/3,8213/3,8220/3,8244/3,8255/3,8260/3,8261/3,8262/3,8263/3,8323/3,8480/3,8481/3,8560/3,8574/3). The inclusion criteria were as follows: only one primary tumour after surgery; definite age, race, tumour size and pathology data; complete information of AJCC TNM stage, follow-up, CSS time and status; and detailed information about regional nodes, level of CEA and perineural invasion. An external validation set complying with the above criteria was collected from The Central Hospital of Xuzhou, Affiliated Hospital of Nanjing Medical University (from 2011 to 2020). Patients were reclassified according to the 8th edition of the TNM classification based on the 7th edition data provided by the SEER database. All methods were performed in accordance with the relevant guidelines and regulations of the SEER database. Written informed consent for publication was obtained from all participants, and ethics committee approval was obtained from a local ethics committee.
Statistical analysis
Statistical analysis was carried out using SPSS statistics software, version 25.0 (SPSS, Chicago, IL, USA), and R version 3.6.2 software (The R Foundation for Statistical Computing, Vienna, Austria.http://www.r-project.org). The optimum cut-off values for LNR, age and tumour size were generated by X-tile version 3.6.1 software (Yale University School of Medicine, New Haven, Conn). Variables with a p value <0.05 in the univariate analysis were entered into the multivariate analysis via the Cox proportional hazards model in the training set. A nomogram for 1-, 3- and 5-year CSS was constructed according to the results of multivariate survival analysis. The discrimination accuracy was measured by Harrell’s concordance index (C-index) and receiver operating characteristic (ROC) curves, and the corresponding areas under the curves (AUCs) were computed. Time-dependent ROC analysis was also performed. Calibration curves were generated to assess the consistency of the nomogram by using the bootstrap method (resampling = 1000) in the training and validation sets. Decision curve analysis (DCA) [15], a novel method to evaluate prediction models by calculating the clinical net benefit, was conducted for decision making in both the internal and external validation cohorts. The performance of the nomogram was compared with the traditional AJCC 8th TNM staging system. P values <0.05 were considered statistically significant.
Results
Clinicopathologic and follow-up data
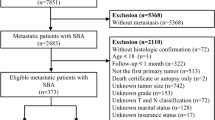
A total of 8470 patients from the SEER database and 473 patients from a single centre in China were eventually enrolled in this study. Patients from SEER were randomly divided into a training set (n=5930) and an internal validation set (n=2540) at a ratio of 7t3. The mean age of the patients was 68.6 years, with males accounting for 45.7%. In terms of race, 6575 (77.6%) patients were white, 1170 (13.8%) were black and 725 (8.5%) were of other races. A minority of the patients exhibited increased CEA levels (3447, 40.6%) and perineural invasion (965, 14.7%). Of all 8470 patients, 594 (7.0%) were well differentiated, 5842 (69.0%) were moderately differentiated, 1681 (19.8%) were poorly differentiated and 353 (4.2%) were undifferentiated. The distribution of the 8th TNM stage among the patients was as follows: stage 0 and I, 1605 (18.9%); stage II, 3077 (36.3%); stage III, 2645 (31.2%); and stage IV, 1143 (13.5%). Postoperative pathological tumour staging suggested that 719 (8.4%) patients in the Tis and T1 stage, 1188 (14.0%) patients in the T2 stage, 5213 (61.5%) in the T3 stage and 1350 (15.9%) were in the T4 stage, while 4825 (57.0%) patients were in the N0 stage, 2172 (25.6%) in the N1 stage and 1473 (17.4%) in the N2 stage. In total, 1143 patients (13.4%) showed metastasis on pathology, and 5555 (65.5%) received adjuvant chemotherapy. According to the X-tile programme from the training set, the tumour size distribution was classified as ≤3.3 cm, 3.3–7.5 cm and >7.5 cm; the age distribution was classified as ≤73, 73–85 and >85; and the LNR distribution was classified as ≤4.9%, 4.9–32.8% and >32.8% (Fig. 1). Patient demographics and clinical and pathological characteristics of the training, internal and external validation sets are presented in Table 1.
CSS was defined as the survival time from diagnosis to cancer-associated death (CSD) and death from other causes, or those still alive were censored on the date of last follow-up. Based on the follow-up data, CSD was observed in 1745 patients from SEER and 64 in external validation (Fig. 1).
Nomogram construction and validation
Univariate and multivariate Cox proportional hazard analyses revealed that age, serum CEA level, AJCC 8th edition T stage, N stage, M stage, chemotherapy, tumour differentiation and LNR were independent predictors of CSS (Table 2). The corresponding nomogram was constructed on the basis of the results of Cox regression analyses (Fig. 2). The C-index of the nomogram for CSS prediction was 0.835 (95% CI, 0.823–0.847), 0.848 (95% CI, 0.830–0.866) and 0.732 (95% CI, 0.664–0.799) in the training, internal validation and external validation sets, respectively. The C-index of the AJCC TNM 8th edition staging system was 0.787 (95% CI, 0.767–0.807) and 0.701 (95% CI, 0.648–0.754) in the internal and external validation sets, respectively. The AUCs of 1-, 3- and 5-year CSS prediction were 0.859, 0.876 and 0.874 in the training set; 0.889, 0.876 and 0.869 in the internal validation set; and 0.824, 0.752 and 0.700 in the external validation set, respectively (Fig. 3). Furthermore, the two time-dependent ROC curves of the nomogram were higher than those of the TNM staging system (Fig. 4), indicating comparative stability and adequate discriminability in both the internal and external validation sets.
The calibration curves showed good agreement between the nomogram predictions and the actual proportion in both the training set and the validation set (Fig. 5). The DCA curve, which evaluates models from the perspective of clinical consequence, confirmed the clinical validity of our nomogram for CSS, and our nomogram yielded superior clinical net benefit compared with the AJCC 8th edition TNM staging system (Fig. 6).
Decision curve analysis of 1-, 3- and 5-year CSS predicting the nomogram and TNM stage system in the internal validation (A, B and C) and external validation sets (D, E and F). The x-axis shows the threshold probabilities. The y-axis represents net benefit. The grey line indicates the assumption that all patients died from ascending colon adenocarcinoma. The black line indicates the assumption that all patients survived
Discussion
Nomograms are more accurate than traditional staging systems in predicting the prognosis of various tumours [16,17,18]. In this study, a novel nomogram that incorporates haematological biomarkers, clinical features and clinicopathological factors was well developed in predicting CSS for patients with ascending colon adenocarcinoma undergoing surgery and was further verified in the validation set.
The training and internal validation sets were completely randomized from the same respective population (SEER), so the C-index of the two sets did not differ much in this study (0.835 vs. 0.848). Owing to ethnic and geographical differences, the C-index of the external set (0.732) was inferior to the internal validation set but still higher than TNM stage (0.701). Both internal validation and external validation presented higher discrimination and satisfactory calibration and achieved a better and more robust predictive performance than that of the 8th edition of the AJCC TNM staging system.
Patients with right-sided colon cancer exhibit more advanced tumour stages and poorly differentiated tumours and, more commonly, abdominal implantation compared with left-sided colon and rectal cancer [19]. Many earlier studies reported that right-sided colon patients were more likely to be female [19,20,21,22], and 4813 female patients were enrolled in our study, accounting for 53.6% of all the patients included in this study. The results of our study support the conclusions described above. The literature has reported that patients older than 60 years make up 65% of the cases of carcinoma of the ascending colon [23]. There was a linear relationship between age and location of colon carcinoma: an older age was more common and more proximal to the tumour [7]. Likewise, our study also demonstrated that older age, with cut-off values of 73 and 85, could act as an independent factor influencing the prognoses of patients with ascending colon cancer after surgery.
Perineural invasion (PNI) is regarded as a reasonable risk factor when incorporating all possible ways of tumour spread, and the prognostic impact of PNI has been demonstrated in many studies, including other cancers [24,25,26]. The College of American Pathologists has highlighted the importance of PNI and recommended reporting PNI in patients with carcinoma of the colon and rectum since 2009 [27]. In the current study, PNI was also incorporated into the novel nomogram as an independent prognostic factor.
It has been widely recognized that T stage, N stage, M stage and chemotherapy are prognostic factors in patients with colon tumours [28,29,30]. The level of serum CEA is an important prognostic marker for cancer treatment, recurrence and metastasis [31]. Serum CEA has shown great value for the differential diagnosis of malignant tumours and postoperative prediction of CRC. Konishi et al. [32] showed that elevated CEA is an important predictor of recurrence in colon cancer, which could further affect the CSS of patients. These independent prognostic factors were also included as components of our novel nomogram.
Studies indicate that right-sided tumours exhibit poorer differentiation than left-sided tumours [33, 34]. The degree of differentiation of the tumour is an important index for assessing malignancy risk and disease prognosis. The poorer the tumour differentiation degree is, the more malignant the degree will be, which could reduce the survival rate of patients. Tumour differentiation was regarded as an independent factor in our nomogram. The LNR has been investigated as an important parameter, and the LNR can be used to estimate prognosis and identify high-risk patients [35]. The LNR was an independent value for discriminating survival outcomes and was even more precise than the classic N stage [36]. Our study showed that 4.9% and 52.8% were the cut-off values of LNR, and the range of LNR varied according to the type and site of tumour [37,38,39], suggesting that the value should be individualized.
Data from various studies are conflicting and contradictory regarding the prognostic significance of tumour size. Some studies have demonstrated that tumour diameter, particularly larger size, was not an influencing factor of prognosis [40, 41], while others identified it as an important influencing factor [42, 43]. The common denominator of these studies above was that subanalysis according to each site was not performed, which might account for the contradiction. A previous study [44] showed that a smaller tumour size (<40 mm) was an independent risk factor for CSS in patients with RCC. Right colon tumours, especially ascending colon tumours, often exhibit an exophytic growth pattern and larger and more advanced stages [45]. Our data derived from SEER indicated that the average tumour size of all patients was 50 mm. Multivariate Cox regression analysis revealed that tumour size was not an independent prognostic factor. As a result, tumour size was not included in the development of the current nomogram for ascending colon tumours exclusively. This conclusion suggested that further subgroup analyses based on the primary tumour location are warranted.
Although our nomogram achieves good prediction, there are several limitations worth noting. First, this is a retrospective study of a large population, so unavoidable confounding factors might limit the validity of this study, and further prospective studies are needed to confirm these findings. Second, the detailed information for chemotherapy and specific values of CEA within the SEER database remain unclear. Third, this model was developed and validated from the SEER database and externally validated in a single centre in China; however, whether the model is applicable to other ethnic or racial groups needs further investigation, and it still requires external validation from other centres before it can be widely utilized in the clinical setting.
Conclusion
Our study indicates that the novel nomogram could effectively predict the prognosis of patients with ascending colon adenocarcinoma after surgery. The predictive ability of our nomogram is relatively promising, and after more extensive evaluation and broadened analysis from different populations, it may improve the predictive power and assist clinicians in a more precise prediction of prognosis.
Availability of data and materials
The raw data of this manuscript are available upon reasonable request from the corresponding author.
Abbreviations
- CSS:
-
Cancer-specific survival
- SEER:
-
Surveillance, Epidemiology, and End Results
- LNR:
-
Lymph node ratio
- AJCC:
-
American Joint Committee on Cancer
- TNM:
-
Tumour-node-metastasis
- CEA:
-
Carcinoembryonic antigen
- CRC:
-
Colorectal cancer
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- DCA:
-
Decision curve analysis
- CSD:
-
Cancer-associated death
- PNI:
-
Perineural invasion
References
Udayasiri DK, Skandarajah A, Hayes IP. Laparoscopic compared with open resection for colorectal cancer and long-term incidence of adhesional intestinal obstruction and incisional hernia: a systematic review and meta-analysis. Dis Colon Rectum. 2020;63(1):101–12.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Shen H, Yang J, Huang Q, Jiang MJ, Tan YN, Fu JF, et al. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol. 2015;21(21):6470–8.
Yang SY, Cho MS, Kim NK. Difference between right-sided and left-sided colorectal cancers: from embryology to molecular subtype. Expert Rev Anticancer Ther. 2018;18(4):351–8.
Yang L, Xiong Z, Xie Q, He W, Liu S, Kong P, et al. Prognostic value of total number of lymph nodes retrieved differs between left-sided colon cancer and right-sided colon cancer in stage III patients with colon cancer. BMC Cancer. 2018;18(1):558.
Wang CB, Shahjehan F, Merchea A, Li Z, Bekaii-Saab TS, Grothey A, et al. Impact of tumor location and variables associated with overall survival in patients with colorectal cancer: a Mayo Clinic Colon and Rectal Cancer Registry study. Front Oncol. 2019;9:76.
Benedix F, Schmidt U, Mroczkowski P, Gastinger I, Lippert H, Kube R. Colon carcinoma--classification into right and left sided cancer or according to colonic subsite?--Analysis of 29,568 patients. Eur J Surg Oncol. 2011;37(2):134–9.
Fortea-Sanchis C, Martínez-Ramos D, Escrig-Sos J. The lymph node status as a prognostic factor in colon cancer: comparative population study of classifications using the logarithm of the ratio between metastatic and nonmetastatic nodes (LODDS) versus the pN-TNM classification and ganglion ratio systems. BMC Cancer. 2018;18(1):1208.
Guo S, Shang M, Dong Z, Zhang J, Wang Y, Zhao Y. The assessment of the optimal number of examined lymph nodes and prognostic models based on lymph nodes for predicting survival outcome in patients with stage N3b gastric cancer. Asia Pac J Clin Oncol. 2021;17(2):e117–e124.
Spoerl S, Gerken M, Mamilos A, Fischer R, Wolf S, Nieberle F, et al. Lymph node ratio as a predictor for outcome in oral squamous cell carcinoma: a multicenter population-based set study. Clin Oral Investig. 2021;25(4):1705–13.
Wang Y, Pang Z, Chen X, Yan T, Liu J, Du J. Development and validation of a prognostic model of resectable small-cell lung cancer: a large population-based set study and external validation. J Transl Med. 2020;18(1):237.
Guo Q, Zhu J, Wu Y, Wen H, Xia L, Ju X, et al. Validation of the prognostic value of various lymph node staging systems for cervical squamous cell carcinoma following radical surgery: a single-center analysis of 3,732 patients. Ann Transl Med. 2020;8(7):485.
Lei BW, Hu JQ, Yu PC, Wang YL, Wei WJ, Zhu J, et al. Lymph node ratio (LNR) as a complementary staging system to TNM staging in salivary gland cancer. Eur Arch Oto-Rhino-Laryngol 2019;276(12):3425-3434.
Guan X, Ma CX, Quan JC, Zhao ZX, Chen HP, Sun P, et al. A prognostic index model to individually predict clinical outcomes for colorectal cancer with synchronous bone metastasis. J Cancer. 2020;11(15):4366–72.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565-74.
Zhou T, Cai Z, Ma N, Xie W, Gao C, Huang M, et al. A novel ten-gene signature predicting prognosis in hepatocellular carcinoma. Front Cell Dev Biol. 2020;8:629.
Han D, Yang J, Xu F, Huang Q, Bai L, Wei YL, et al. Prognostic factors in patients with gallbladder adenocarcinoma identified using competing-risks analysis: a study of cases in the SEER database. Medicine (Baltimore). 2020;99(31):e21322.
Wang S, Zhang L, Yu Z, Chai K, Chen J. Identification of a glucose metabolism-related signature for prediction of clinical prognosis in clear cell renal cell carcinoma. J Cancer. 2020;11(17):4996–5006.
Shida D, Inoue M, Tanabe T, Moritani K, Tsukamoto S, Yamauchi S, et al. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol. 2020;55(10):958–68.
Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57–64.
Karim S, Brennan K, Nanji S, Berry SR, Booth CM. Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol. 2017;3(10):1386–92.
Qiu MZ, Pan WT, Lin JZ, Wang ZX, Pan ZZ, Wang FH, et al. Comparison of survival between right-sided and left-sided colon cancer in different situations. Cancer Med. 2018;7(4):1141–50.
Wang H, Lu H, Yang H, Zhang X, Thompson EW, Roberts MS, et al. Impact of age on risk of lymph node positivity in patients with colon cancer. J Cancer. 2019;10(9):2102–8.
Lian Z, Zhang H, He Z, Ma S, Wang X, Liu R. Impact of positive surgical margin location and perineural invasion on biochemical recurrence in patients undergoing radical prostatectomy. World J Surg Oncol. 2020;18(1):201.
Zhang Z, Liu R, Jin R, Fan Y, Li T, Shuai Y, et al. Integrating clinical and genetic analysis of perineural invasion in head and neck squamous cell carcinoma. Front Oncol. 2019;9:434.
Michalek J, Brychtova S, Pink R, Dvorak Z. Prognostic and predictive markers for perineural and bone invasion of oral squamous cell carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2019;163(4):302–8.
Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Laboratory Med. 2009;133(10):1539–51.
Dienstmann R, Mason MJ, Sinicrope FA, Phipps AI, Tejpar S, Nesbakken A, et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28(5):1023–31.
Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16(7):30.
Gupta P, Chiang SF, Sahoo PK, Mohapatra SK, You JF, Onthoni DD, et al. Prediction of Colon Cancer Stages and Survival Period with Machine Learning Approach. Cancers (Basel). 2019;11(12):2007.
Sato M, Kitani H, Kojima K. Development and validation of scFv-conjugated affinity silk protein for specific detection of carcinoembryonic antigen. Sci Rep. 2017;7(1):16077.
Konishi T, Shimada Y, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018;4(3):309–15.
Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw. 2017;15(3):411–9.
Wang SM, Jiang B, Deng Y, Huang SL, Fang MZ, Wang Y. Clinical significance of MLH1/MSH2 for stage II/III sporadic colorectal cancer. World J Gastrointestinal Oncol. 2019;11(11):1065–80.
Baqar AR, Wilkins S, Wang W, Oliva K, McMurrick P. Log odds of positive lymph nodes is prognostically equivalent to lymph node ratio in non-metastatic colon cancer. BMC Cancer. 2020;20(1):762.
Lv Y, Feng QY, Lin SB, Mao YH, Xu YQ, Zheng P, et al. Exploration of exact significance of lymph node ratio and construction of a novel stage in colon cancer with no distant metastasis. Cancer Manag Res. 2019;11:6841–54.
Polterauer S, Schwameis R, Grimm C, Hillemanns P, Juckstock J, Hilpert F, et al. Lymph node ratio in inguinal lymphadenectomy for squamous cell vulvar cancer: Results from the AGO-CaRE-1 study. Gynecol Oncol. 2019;153(2):286–91.
Lee J, Lee SG, Kim K, Yim SH, Ryu H, Lee CR, et al. Clinical value of lymph node ratio integration with the 8(th) edition of the UICC TNM classification and 2015 ATA risk stratification systems for recurrence prediction in papillary thyroid cancer. Sci Rep. 2019;9(1):13361.
Hwang JE, Kim H, Shim HJ, Bae WK, Hwang EC, Jeong O, et al. Lymph-node ratio is an important clinical determinant for selecting the appropriate adjuvant chemotherapy regimen for curative D2-resected gastric cancer. J Cancer Res Clin Oncol. 2019;145(8):2157–66.
Gangireddy VGR, Coleman T, Kanneganti P, Talla S, Annapureddy AR, Amin R, et al. Polypectomy versus surgery in early colon cancer: size and location of colon cancer affect long-term survival. Int J Colorectal Dis. 2018;33(10):1349–57.
Saha S, Shaik M, Johnston G, Saha SK, Berbiglia L, Hicks M, et al. Tumor size predicts long-term survival in colon cancer: an analysis of the National Cancer Data Base. Am J Surg. 2015;209(3):570–4.
Crozier JE, McMillan DC, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. Tumor size is associated with the systemic inflammatory response but not survival in patients with primary operable colorectal cancer. J Gastroenterol Hepatol. 2007;22(12):2288–91.
Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88(7):1739–57.
Li X, An B, Ma J, He B, Qi J, Wang W, et al. Prognostic value of the tumor size in resectable colorectal cancer with different primary locations: a retrospective study with the propensity score matching. J Cancer. 2019;10(2):313–22.
Karahan B, Argon A, Yıldırım M, Vardar E. Relationship between MLH-1, MSH-2, PMS-2,MSH-6 expression and clinicopathological features in colorectal cancer. Int J Clin Exp Pathol. 2015;8(4):4044–53.
Acknowledgements
We would like to thank ResearchHome (www.home-for-researchers.com) for the help with the English language and Shuwen Cheng for his technical assistance in R software.
Funding
None.
Author information
Authors and Affiliations
Contributions
Yifan Zhang, Cheng Ma—conception and design, data collection and statistical analyses. Yifan Zhang—drafting of the manuscript. Cheng Ma—R software usage and plotting. Xiaoping Qian—overall revision of the manuscript for important content. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of The Xuzhou School of Clinical Medicine of Nanjing Medical University and performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y.F., Ma, C. & Qian, X.P. Development and external validation of a novel nomogram for predicting cancer-specific survival in patients with ascending colon adenocarcinoma after surgery: a population-based study. World J Surg Onc 20, 126 (2022). https://doi.org/10.1186/s12957-022-02576-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02576-4