Abstract
Background
The aim of this study was to evaluate a series of blood count inflammation indexes in predicting anastomotic leakage (AL) in elective colorectal surgery.
Methods
Demographic, pathologic, and clinical data of 1432 consecutive patients submitted to colorectal surgery in eight surgical centers were retrospectively evaluated. The neutrophil to lymphocyte (NLR), derived neutrophil to lymphocyte (dNLR), lymphocyte to monocyte (LMR), and platelet to lymphocyte (PLR) ratios were calculated before surgery and on the 1st and 4th postoperative days, in patients with or without AL.
Results
There were 106 patients with AL (65 males, mean age 67.4 years). The NLR, dNLR, and PLR were significantly higher in patients with AL in comparison to those without, on both the 1st and 4th postoperative days, but significance was greater on the 4th postoperative day. An NLR cutoff value of 7.1 on this day showed the best area under the curve (AUC 0.744; 95% CI 0.719–0.768) in predicting AL.
Conclusions
Among the blood cell indexes of inflammation evaluated, NLR on the 4th postoperative day showed the best ability to predict AL. NLR is a low cost, easy to perform, and widely available index, which might be potentially used in clinical practice as a predictor of AL in patients undergoing elective colorectal surgery.
Similar content being viewed by others
Background
Anastomotic leakage (AL) is one of the most severe complications in modern colorectal surgery. It has been reported to occur in 3 to 27% of patients in relation to specific risk factors, despite recent improvements in the preoperative selection and preparation of the patients, the evolution of minimally invasive surgical techniques, the stapling devices used to fashion the anastomoses, and the postoperative management of the patients [1, 2]. Anastomotic leakage represents the most common cause of unplanned reoperation in large colorectal surgery cohorts [3]. Furthermore, AL is associated with greater short-term mortality, poorer oncological outcomes and overall survival, as well as higher costs for healthcare systems [4,5,6].
Anastomotic leakage can manifest clinically in several ways, in relation to the grade of the anastomotic breakdown, the anatomical site (colon or rectum) and the type of the surgical procedure, the general condition of the patient, and the presence of a protective stoma. Generally, early AL is caused by technical errors or defects, has a major clinical impact, and reoperation is often needed to treat it. Most commonly, AL occurs between the 5th and 8th postoperative days, and has a variable clinical presentation; minor leaks can be treated conservatively using drains to evacuate possible infectious collections, while major defects require re-intervention to clean the abdomen and restore the intestinal integrity or exteriorize the bowel [7]. In all patients, a prompt diagnosis is crucial because a delay in antibiotic administration from the onset of septic shock has been associated with a decrease in survival of 7.6% per hour [8]. The discovery of biomarkers able to predict AL early after colorectal surgery would bring consistent advantages in the management and outcomes of this complication.
To this regard, several biomarkers have been evaluated so far, most of them related to the inflammatory response to surgical manipulation, and the consequent reparative events in resected tissues. Factors like interleukins, C-reactive protein (CRP), procalcitonin (PCT), Na+, tissue plasminogen activator, and soluble fibrin have been evaluated in blood samples, as well as indexes including the cells participating in the inflammatory process, like the neutrophil to lymphocyte ratio (NLR) [9, 10]. The latter has been demonstrated to be a prognostic factor in numerous diseases, including primary and metastatic colorectal cancer [11,12,13,14]. Furthermore, it has been associated with the outcomes of several types of surgical procedures, and with the onset of postoperative complications [15,16,17,18,19]. However, poor data are currently available regarding its role in predicting AL in colorectal surgery. In the present study, we investigated the role of NLR and derived neutrophil to lymphocyte ratio (dNLR) as predictive markers of AL, along with the role of the lymphocyte to monocyte (LMR) and the platelet to lymphocyte (PLR) ratios, in 1432 patients with colorectal cancer who underwent elective surgical resection in eight different centers.
Methods
Data of consecutive patients with histologically proven colorectal cancer, and undergoing elective surgery at the surgical units involved in the study from January 1, 2013 through December 31, 2017 were collected in an electronic database. Demographic and clinical data, including sex, age, body mass index (BMI), American Society of Anesthesiologists (ASA) score, Charlson comorbidity index, localization and histology of the disease, as well as the stage of the disease according to the American Joint Committee on Cancer (AJCC) staging system (7th edition), were registered. Tumor distance from the anocutaneous line was included for patients with rectal cancer. Furthermore, details regarding the surgical procedure, postoperative course, morbidity, and 30-day mortality were collected.
The inclusion criteria were as follows: (a) patients with histologically proven colorectal cancer; (b) patients undergoing elective surgical procedure with an open or laparoscopic approach; (c) patients with available clinical, surgical, and pathological data; (d) patients with available blood cell counts before surgery, and at the 1st and 4th postoperative days; and (e) patients who signed an informed consent for each procedure performed. The exclusion criteria were the following: (a) patients younger than 18, (b) those operated on an emergency setting, and (c) those who did not have an anastomosis. Patients who had a clinically manifested anastomotic leakage (Extended Clavien-Dindo classification stage III through V) [20] within 30 days from surgery were included in the AL group; when necessary, AL was confirmed by imaging or endoscopic techniques. All the operations were performed by senior surgeons, and the anastomoses were made up hand-sewn or with stapling devices; the choice of the technique or the stapling device was made by the surgeon based on the localization of the disease, the anatomical conditions of the patients, and his/her experience. The study was carried out in accordance with the principles of the Declaration of Helsinki, and was approved by the ethics committee of University Hospital (A.O.U.) of Cagliari (Italy).
Regarding laboratory tests, fasting blood samples were obtained with standard procedures and methodologies dictated by the current international and national guidelines, adopted by the institutions involved in the study; the samples were processed and analyzed in certified laboratories. Complete blood counts before the operation, on the 1st postoperative day, and on the 4th postoperative day were retrieved, and the NLR, dNLR (neutrophils/white blood cells—neutrophils), LMR, and PLR were calculated.
All results were expressed as mean (mean ± SD) or median values (median and IQR). Variable distribution was assessed by the Shapiro-Wilcoxon test. Statistical differences between groups were compared using unpaired Student’s t test or Mann-Whitney rank sum test, as appropriate. Correlations between variables were assessed by Pearson’s correlation or Spearman’s correlation, as appropriate. Multiple comparisons were performed by one-way ANOVA, student-Newman-Keuls test or Kruskal-Wallis test, as appropriate. Levene’s test for equality of error variances was employed. Logistic regression analysis was employed to investigate the association of NLR and other risk factors with AL.
The ability of the studied parameters to predict AL was analyzed using receiver operating characteristic (ROC) curve analysis. Optimal cutoff maximizing sensitivity and specificity was selected. Sensitivity and specificity were reported using the optimal ROC curve value according to the Youden index. The results of the area under the curve (AUC) represented the global accuracy of the tests performed, 0.91–1.00 (excellent), 0.81–0.90 (good), 0.71–0.80 (fair), 0.61–0.70 (poor), and 0.51–0.60 (fail). Statistical analyses were performed using MedCalc for Windows, version 15.4 64 bit (MedCalc Software, Ostend, Belgium) and SPSS for Windows, version 14.0 32 bit (IBM Corporation; Armonk, NY, USA).
Results
The global number of patients enrolled in the study was 1432; among them, 817 (57%) were male, and the mean age was 65.8 (± 13.7). Globally, 106 (7.4%) patients with AL fulfilling the selection criteria were registered; among them, 59 (55.7%) were affected by rectal or sigmoid cancer. The demographic, anthropometric, and clinical data of patients with and without AL are summarized in Table 1. Patients with AL had a significantly lower mean BMI value (23.5 ± 4.2 vs 25.2 ± 4.1, p = 0.0002) and a significantly higher mean Charlson comorbidity index (6.5 ± 3.0 vs 5.5 ± 2.3, p = 0.0017). Furthermore, there was a significantly higher percentage of TNM stage III patients (51.9% vs 35.5%, p = 0.0368) in the group of patients with AL in comparison to those without; the latter had significantly more patients of TNM stage II (33.9% vs 19.8%, p = 0.0353) and significantly less pathological grade 3 tumors (13.7% vs 27.4%, p = 0.0027). AL was treated with a surgical operation in most cases (72.6%) and without surgery in the remaining cases. AL patients had significantly higher percentage of concomitant complications (50% vs 17.1%, p = 0.0042), a greater length of stay (24.1 ± 17.3 vs 10.6 ± 4.4, p < 0,0001), and significantly worse 30-day postoperative mortality (10.4% vs 0.5%, p < 0,0001).
As shown by Table 2, no significant differences between patients with and without AL were found in the median values of white blood cells, neutrophils, monocytes, lymphocytes, and platelets before surgery; similarly, the red cell distribution width (RDW), as well as cell ratios were not statistically different, with the only exception of PLR (200 vs 178, p = 0.038) which showed a limited but statistically significant difference. On the first postoperative day, some further differences in blood cell populations and indexes were observed, but the greatest statistical differences were registered on the 4th postoperative day. On that day, patients with AL had significantly greater mean WBC (10 vs 7.9, p < 0.0001) and neutrophil (8 vs 5.7, p < 0.0001) values, but lower mean lymphocyte (0.9 vs 1.10, p < 0.0001) values. In addition, the mean NLR (9.6 vs 5.3, p < 0.0001), dNLR (4.7 vs 2.9, p < 0.0001), and PLR (254 vs 218, p < 0.0001) values were consistently greater in patients who developed an AL (Table 2). In a multiple regression analysis model including the most impacting risk factors on AL and the indexes with statistically significant differences between patients with and without AL in the 4th postoperative day, the BMI (OR 0.881, 95% CI 0.832–0.948, p < 0.001), Charlson comorbidity index (OR 1.278, 95% CI 1.152–1.417, p < 0.001), and 4th postoperative day NLR (OR 1.068, 95% CI 1.021–1.117, p = 0,004) were shown to be independent factors associated with AL.
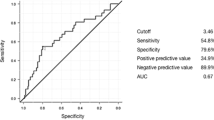
We performed ROC curve analysis for the indexes which showed statistically significant differences between the two groups of patients on the 4th postoperative day (Table 3, Fig. 1). NLR at a cutoff point of 7.1 showed the best AUC (0.744, 95% CI 0.719–0.768) with a sensitivity and specificity of 72.7% and 73.4%, respectively, followed by the dNLR (0.732, 95% CI 0.707–0.757) at a cutoff point of 3.8. PLR showed a poor result in ROC analysis.
Discussion
Anastomotic leakage is one of the most serious postoperative adverse events in colorectal surgery [1,2,3,4,5,6]. This was confirmed also in the present study considering the rate of postoperative concomitant complications, length of stay, and 30-day mortality, which were significantly higher in patients with AL than in those with an uncomplicated postoperative course. The rate of AL in our series (7.4%) was similar to that reported in other recent articles [10, 15]. AL patients in our study had lower mean BMI values in comparison to those without AL, and BMI was found to be an independent factor influencing AL in multivariate analysis; this result is somewhat unexpected, considering that obesity is traditionally considered one of the main risk factors of AL [21]. On the other hand, these patients had a worse Charlson comorbidity index, as well as higher tumor TNM stage and histological grade. This may be associated with a greater level of systemic inflammation, which in one hand determines the increased AL rates, and on the other alters several hematological biomarkers, like those investigated herein. Charlson comorbidity index and NLR in the 4th postoperative day (other than BMI) were shown to be independent factors associated with AL in a multivariate analysis model including also age, sex, ASA score, TNM stage of the tumors, and 4th postoperative day RDW, dNLR, and PLR. No significant correlation between the type of surgery performed (open or laparoscopy) and the occurrence of AL was observed. AL required surgical re-operation in most cases (72.6%); in the remaining cases, it was treated conservatively, mainly through a “wait and see” approach in patients with drain tubes and clinical conditions which permitted the resolution of the leakage without surgery. No statistically significant correlations with NLR values in the 4th postoperative day were found between patients with and without resurgery for AL.
To our knowledge, this is the first study to evaluate the role of the dNLR, LMR, and PLR in predicting AL. These simple blood count indexes, together with the NLR and RDW, have been demonstrated to have a prognostic potential in several chronic pathological conditions, including colorectal cancer and colorectal liver metastases [22,23,24,25,26], and a potential role in predicting outcomes in surgical procedures [15,16,17,18,19, 27,28,29]. NLR is the most studied index to this purpose. Josse et al. retrospectively investigated its role in predicting complications in 583 patients who underwent surgical resection for suspected or confirmed colorectal cancer [29]. The authors found that a preoperative NLR greater or equal to 2.3 was significantly associated with a major perioperative complication rate; on multivariate analysis, a high NLR and Charlson comorbidity index ≥ 3 were significantly related to major morbidity. Nevertheless, they did not detect any relationship between an elevated preoperative NLR and specific complication types, although there was a trend towards higher NLR values in patients with AL [29].
Miyakita et al. published a study on 260 patients with rectal cancer who underwent radical surgery to examine the relations between complications and 5 types of risk scores, including the preoperative NLR [15]. Complications developed in 56 patients (21.5%), and 18 patients with AL were encountered. The authors evidenced that the levels of NLR calculated in blood samples obtained at initial presentation and before chemo-radiotherapy were significantly associated with surgical complications in general, and especially to AL. In particular, they established that a preoperative NLR cutoff point at 2.21 was an independent predictor of AL (p = 0.0089, odds ratio = 8.24) and that the sensitivity and specificity of the test at this cutoff point was 83% and 47%, respectively [15]. This cutoff value seems very low to invest a clinical role in detecting AL, and was lower from the median values observed in both patients with and without AL in our cohort; in addition, we did not detect any statistically significant difference in the preoperative values of NLR between the two groups of patients.
Another study recently published by Mik et al. included 724 patients who underwent elective open colorectal surgery, and (among them) the rate of AL was 4.6% [10]. In this study, blood samples were obtained also on the 1st and 4th postoperative days, and both CRP and NLR were evaluated. The authors found a statistically significant difference in the mean value of NLR on the 4th postoperative day, between patients with (9.03 ± 4.13) and without (4.45 ± 2.25) AL (p = 0.0012). The ROC analysis showed a sensitivity of 69% (95% CI, 65–73), and a specificity of 78% (95% CI, 74–82) at a cutoff point of 6.5, with an AUC of 0.68 [10]. In our cohort, the AUC was greater (0.744), the sensitivity was slightly higher (73%), and the specificity lower (73%) at an NLR cutoff point of 7.1 on postoperative day 4. This value was relatively close to the NLR cutoff value in patients with AL found in the study of Mik et al.; in addition, the median NLR value found in our series (9.60) was close to the median value found by Mik et al. (9.03) in this subset of patients.
More recently, Walker et al. published their results on the predictive roles of CRP, PCT, and NLR on 136 patients retrospectively enrolled (eleven AL patients, 8.1%) [30]. Median CRP values were found to be significantly higher in patients with AL in comparison to those with an uncomplicated course on postoperative days 2 through 5. PCT median value differences never reached statistical significance within the same time frame, while those of NLR were significantly higher in AL patients on postoperative days 3 and 4. ROC analysis evidenced that the cutoff for CRP (105 mg/L) with the highest sensitivity (100%) and specificity (56.5%) was on postoperative day 5; this figure is consistently lower than those reported by Mik et al. The NLR showed the best predictive ability at a cutoff value of 6.15 in day 4 (sensitivity of 100% and specificity of 61.8%), as observed also in our cohort. Another recent study, performed in 44 case-matched patients, reported a poor AUC (0.697) in predicting AL at an NLR cutoff value of 8.7, with sensitivity and specificity of 52% and 88%, respectively [31].
The RDW and the LMR did not show any predictive ability for the early detection of AL in our study. To this regard, a retrospective pilot study by Paliogiannis et al. investigated the role of the preoperative RDW and mean platelet volume (MPV) in 42 case-matched patients who underwent oncological colorectal surgery [32]. The authors found higher mean values for both indexes in patients with than without AL, but in multiple regression analysis only, the RDW remained significantly associated to the AL, and the AUC reported was poor (0.673, cutoff 11%, sensitivity 90.2%, and specificity 38.1%). In the present study, the RDW did not show any predictive ability neither before nor after surgery. As opposed, the PLR was significantly higher in AL patients in all the evaluations performed, with the statistical significance increasing from the preoperative to the 4th postoperative day. Also in this case, the AUC on the 4th postoperative day was poor, but the finding deserves further evaluation in future studies.
Our study has some limitations, mainly the retrospective design, the lack of information about treatments that may alter the indexes under evaluation (like steroids), as well as potential variability in the perioperative management of the patients and laboratory testing. On the other hand, it includes the largest cohort employed so far for the study of the role of specific inflammatory indexes in predicting AL, some of them tested for the first time. Further prospectively designed trials are necessary to investigate the prognostic effect and the clinical applicability of NLR in patients who develop AL following colorectal resection.
Conclusions
Among the blood cell indexes of systemic inflammation investigated in this study, NLR evaluated on the 4th postoperative day showed the better results in predicting AL. Therefore, NLR which is a simple, inexpensive, and widely available index, might be a useful tool in clinical practice in predicting the occurrence of AL in patients undergoing elective colorectal surgery. Nevertheless, its potential usefulness in daily practice needs to be further evaluated in future prospective studies.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- AL:
-
Anastomotic leakage
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- LMR:
-
Lymphocyte to monocyte
- NLR:
-
Neutrophil to lymphocyte
- PCT:
-
Procalcitonin
- PLR:
-
Platelet to lymphocyte
- RDW:
-
Red blood cell distribution width
References
Matthiessen P, Henriksson M, Hallbook O, Grunditz E, Noren B, Arbman G. Increase of serum C-reactive protein is an early indicator of subsequent symptomatic anastomotic leakage after anterior resection. Colorectal Dis. 2008;10:75–80.
Iancu C, Mocan LC, Todea-Iancu D, Mocan T, Acalovschi I, Ionescu D, Zaharie FV, Osian G, Puia CI, Muntean V. Host-related predictive factors for anastomotic leakage following large bowel resections for colorectal cancer. J Gastrointest Liver Dis. 2008;17:299–303.
Michaels AL, Mullen MG, Guidry CA, Krebs ED, Turrentine FE, Hedrick TL, Friel CM. Unplanned reoperation following colorectal surgery: indications and operations. J Gastrointest Surg. 2017;21:1480–5.
Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, Carmichael JC, Mills S, Stamos MJ. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148:65–71.
Ashraf SQ, Burns EM, Jani A, Altman S, Young JD, Cunningham C, Faiz O, Mortensen NJ. The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: are we adequately remunerating them? Colorectal Dis. 2013;15:190–8.
Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W. Adverse effects of anastomotic leakage on local recurrence and survival after curative anterior resection for rectal cancer: a systematic review and meta-analysis. World J Surg. 2017;41:277–84.
Paliogiannis P, Attene F, Scognamillo F, Trignano E, Torre C, Pulighe F, Trignano M. Conservative management of minor anastomotic leakage after open elective colorectal surgery. Ann Ital Chir. 2012;83:25–8.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving sepsis campaign guidelines committee including the pediatric subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2013;39:165–228.
Su’a BU, Mikaere HL, Rahiri JL, Bissett IB, Hill AG. Systematic review of the role of biomarkers in diagnosing anastomotic leakage following colorectal surgery. Br J Surg. 2017;104:503–12.
Mik M, Dziki L, Berut M, Trzcinski R, Dziki A. Neutrophil to lymphocyte ratio and C-reactive protein as two predictive tools of anastomotic leak in colorectal cancer open surgery? Dig Surg. 2018;35:77–84.
Tang H, Li B, Zhang A, Lu W, Xiang C, Dong J. Prognostic significance of neutrophil-to-lymphocyte ratio in colorectal liver metastasis: a systematic review and meta-analysis. PloS One. 2016;11:e0159447.
Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403–13.
Madonia M, Paliogiannis P, Solinas T, Mangoni AA, Carru C, Zinellu A. Neutrophil to lymphocyte ratio and muscular invasion in early-stage bladder cancer: a meta-analysis. Eur J Oncol. 2018;23:65–71.
Paliogiannis P, Scognamillo F, Bellomo M, Pittalis ML, Pisano IP, Karligkiotis A, Bozzo C, Sotgiu G, Attene F. Neutrophil to lymphocyte ratio as a predictor of thyroid papillary carcinoma. Acta Med Mediterr. 2015;31:371–5.
Miyakita H, Sadahiro S, Saito G, Okada K, Tanaka A, Suzuki T. Risk scores as useful predictors of perioperative complications in patients with rectal cancer who received radical surgery. Int J Clin Oncol. 2017;22:324–31.
Vaughan-Shaw PG, Rees JR, King AT. Neutrophil lymphocyte ratio in outcome prediction after emergency abdominal surgery in the elderly. Int J Surg. 2012;10:157–62.
Giakoumidakis K, Fotos NV, Patelarou A, Theologou S, Argiriou M, Chatziefstratiou AA, Katzilieri C, Brokalaki H. Perioperative neutrophil to lymphocyte ratio as a predictor of poor cardiac surgery patient outcomes. Pragmat Obs Res. 2017;8:9–14.
Mohri Y, Tanaka K, Toiyama Y, Ohi M, Yasuda H, Inoue Y, Kusunoki M. Impact of preoperative neutrophil to lymphocyte ratio and postoperative infectious complications on survival after curative gastrectomy for gastric cancer: a single institutional cohort study. Medicine (Baltimore). 2016;95:e3125.
Lan H, Zhou L, Chi D, Zhou Q, Tang X, Zhu D, Yue J, Liu B. Preoperative platelet to lymphocyte and neutrophil to lymphocyte ratios are independent prognostic factors for patients undergoing lung cancer radical surgery: a single institutional cohort study. Oncotarget. 2017;8:35301–10.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, Sasako M. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Vasiliu EC, Zarnescu NO, Costea R, Neagu S. Review of risk factors for anastomotic leakage in colorectal surgery. Chirurgia (Bucur). 2015;110:319–26.
Huang XZ, Chen WJ, Zhang X, Wu CC, Zhang CY, Sun SS, Wu J. An elevated platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with colorectal cancer: a meta-analysis. Dis Markers. 2017;2017:1053125.
Lin ZX, Ruan DY, Li Y, Wu DH, Ma XK, Chen J, Chen ZH, Li X, Wang TT, Lin Q, Wen JY, Wu XY. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015;21:10898–906.
Facciorusso A, Del Prete V, Crucinio N, Serviddio G, Vendemiale G, Muscatiello N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J Gastroenterol. 2016;22:4211–8.
Paliogiannis P, Fois AG, Sotgia S, Mangoni AA, Zinellu E, Pirina P, Negri S, Carru C, Zinellu A. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27:147.
Paliogiannis P, Zinellu A, Mangoni AA, Capobianco G, Dessole S, Cherchi PL, Carru C. Red blood cell distribution width in pregnancy: a systematic review. Biochem Med (Zagreb). 2018;28:030502.
Silberman S, Abu-Yunis U, Tauber R, Shavit L, Grenader T, Fink D, Bitran D, Merin O. Neutrophil-lymphocyte ratio: prognostic impact in heart surgery. Early outcomes and late survival. Ann Thorac Surg. 2018;105:581–6.
Paliogiannis P, Ginesu GC, Tanda C, Feo CF, Fancellu A, Fois AG, Mangoni AA, Sotgia S, Carru C, Porcu A, Zinellu A. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J Surg. 2018;88:616–20.
Josse JM, Cleghorn MC, Ramji KM, Jiang H, Elnahas A, Jackson TD, Okrainec A, Quereshy FA. The neutrophil-to-lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Colorectal Dis. 2016;18:O236–42.
Walker PA, Kunjuraman B, Bartolo DCC. Neutrophil-to-lymphocyte ratio predicts anastomotic leakage. ANZ J Surg. 2018. https://doi.org/10.1111/ans.14369.
Paliogiannis P, Feo CF, Scognamillo F, Mulas S, Xidas A, Zinellu A, Carru C, Porcu A. Re: Neutrophil-to-lymphocyte ratio predicts anastomotic leakage. ANZ J Surg. 2018;88:939.
Paliogiannis P, Attene F, Porcu A, Cossu ML, Fancellu A, Scanu AM, Ginesu GC, Cherchi G, Niolu P, Coppola M, Carru C, Zinellu A. Red cell distribution width and mean platelet volume as predictors of anastomotic leakage in colorectal surgery a pilot multicenter case-match study. Ann Ital Chir. 2018;89:419–24.
Acknowledgements
The authors wish to thank the doctors Mohamed Yehia Elbarmelgi and Mostafa Mahran for their contribution in collecting data for the study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
PP and AZ conceived and designed the study, and they performed statistical analyses. SD, SM, TP, AF, AM, EM, DP, MM, JL, FSc, FSa, CFF, GC, AX, AR, and LZ contributed in collecting and interpreting data as well as in drafting and critically revising the manuscript. All authors approved the final version of the manuscript and are accountable for the accuracy and integrity in all aspects of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was performed in accordance with the principles of the Declaration of Helsinki, and was approved by the ethics committee of University Hospital (AOU) of Cagliari (Italy).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Paliogiannis, P., Deidda, S., Maslyankov, S. et al. Blood cell count indexes as predictors of anastomotic leakage in elective colorectal surgery: a multicenter study on 1432 patients. World J Surg Onc 18, 89 (2020). https://doi.org/10.1186/s12957-020-01856-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-020-01856-1