Abstract
Background
Neurofibromatosis type 1 (NF1) is an autosomally dominant inherited disorder characterized by multiple pigmented skin spots (café-au-lait spots) and neurofibroma. NF1 is associated with a wide variety of benign or malignant tumors. We report a NF1 patient who received surgical treatment for rectal carcinoma and multifocal small intestinal gastrointestinal stromal tumors (GISTs).
Case presentation
A 70-year-old female patient with NF1 was referred to our hospital after a positive fecal occult blood test. Locally advanced rectal carcinoma was detected in the upper rectum using colonoscopy. A submucosal tumor 20 mm in diameter was detected in the duodenal bulb during the upper gastrointestinal endoscopy. The biopsy specimen from the duodenum was GIST with positive immunostaining of KIT and CD34 microscopically. Laparoscopic low anterior resection for rectal carcinoma and local excision of the duodenal GIST were performed successfully. During the operation, five white small nodules were found on the serosa of the jejunum. One nodule was excised for histological examination. The resected rectal tumor was a well-differentiated adenocarcinoma with multiple lymph nodes metastases according to the histology. The duodenal tumor was found to be low-risk GIST. Moreover, the nodule from the jejunum was very low risk GIST. An excised skin wart was neurofibroma according to the histology.
Conclusions
GIST or carcinomas have been reported to occasionally occur in the digestive tract of the patients with NF1. We present a rare case of a NF1 patient with GISTs and colorectal carcinoma.
Similar content being viewed by others
Background
Neurofibromatosis type 1 (NF1), also called Von Recklinghausen disease, is an autosomal dominant disorder characterized by multiple pigmented skin spots and neurofibromas. NF1 patients are predisposed to a wide variety of benign and malignant tumors. NF1 patients are also at increased risk of getting gastrointestinal stromal tumors (GISTs).
Case presentation
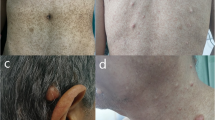
A 70-year-old female was referred to our hospital after a positive fecal occult blood test. The patient had hundreds of skin warts and pigmented spots on her body. None of her family had such skin lesions.
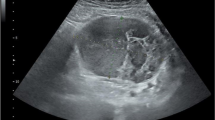
Rectal carcinoma was detected in the upper rectum during colonoscopy (Fig. 1). Histological examination of a mucosal biopsy revealed well-differentiated adenocarcinoma. A submucosal tumor 20 mm in diameter was detected in the duodenal bulb during the preoperative screening upper gastrointestinal endoscopy (Fig. 2).
Histological examination of duodenal biopsy specimen showed spindle-shaped cells with positive immunohistochemical staining for KIT and CD34. S100 was negative for staining. The duodenal tumor was diagnosed as GIST.
Abdominal-contrast CT showed the rectum wall and swelling of the lymph nodes in the mesorectum (Fig. 3). A duodenal tumor was found to protrude into the duodenal bulb (Fig. 4). Carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19-9) levels were within normal ranges.
Surgical exploration was undertaken and a diagnosis of rectal carcinoma with duodenal GIST was made. Laparoscopic low anterior resection with lymph nodes resection for the rectal carcinoma was performed followed by local resection of the duodenum with mini-laparotomy. During the operation, five white small nodules 3–6 mm in diameter were observed on the serosal surface of the jejunum approximately 20 cm from the Treiz ligament; one 6-mm nodule was excised for histological examination. A covering ileostomy was created, and one abdominal skin wart was excised for histological examination. The patient progressed favorably post operation and was discharged.
Histologically, the resected rectal carcinoma was a well-differentiated adenocarcinoma with (four) lymph node metastasis (Fig. 5)—T2N2aM0 stage IIIB according to the TNM classification of UICC, seventh edition [1] (Fig. 7). The resected duodenal tumor was 22 mm in diameter and showed 1 mitosis per 50 high-power fields (HPF) microscopically (Fig. 6). Risk of tumor progression was low according to the modified-Fletcher classification [2]. A resected small nodule from the jejunum was also positive for KIT and CD34 immunohistochemically (Fig. 7a, b) but showed no mitosis per 50 HPF and the risk was very low. The resected skin wart was confirmed as neurofibroma after nodules showed proliferated spindle-shaped cells with unclear boundaries microscopically (Fig. 8).
The patient took adjuvant chemotherapy with capecitabine plus oxaliplatin [3]. However, it was discontinued after three courses due to severe diarrhea.
Discussion
NF1 is an autosomal dominant disorder characterized by multiple pigmented skin spots and neurofibroma. Among the general population, its estimated incidence is 1 in 3000 births [4]. An increased incidence of nervous system and non-nervous system tumors such as neurofibromas, malignant peripheral nerve sheath tumors, and gliomas has been reported in the general population [5, 6]. The frequency of malignancy in NF1 patients was reported to be higher than that expected in the general population. The development of NF1-associated tumors is largely explained by the underlying defect of the NF1 gene [7]. The NF1 protein (neurofibromin 1) negatively regulates RAS proteins through GTPase activity [8, 9].
Agaimy described gastrointestinal manifestations of NF1 [10]. They are true neurogenic neoplasms, interstitial cell of Cajal lesions such as GIST, neuroendocrine tumors, and adenocarcinomas. Neuroendocrine tumors arising in the ampullary or periampullary lesions are the most common and characteristic gastrointestinal manifestations of NF-1.
GISTs reportedly occur occasionally in patients with NF1 [4, 11]. NF1-associated GISTs were reported as multiple generally low-grade tumors localized at the jejunum, ileum, duodenum, and stomach [12,13,14]. In our case, the risk of GISTs was low grade in the duodenum and very low grade in the jejunum. The co-existence of a duodenal somatostatinoma and GISTs in a patient with NF1 represents a typical and almost pathognomonic feature of NF-1 [15]. In our case, neuroendocrine neoplasms were not associated.
There have been a few reports of colorectal carcinomas arising in NF1 patients. Zoller reported that how 17 of 70 NF1 patients had developed a total of 19 malignant tumors in Sweden; 4 cases of colorectal carcinoma were included [16]. Kim also reported a case of colon carcinoma in 125 Korean NF1 patients [17]. A case of synchronous multiple colon adenocarcinomas in a patient with NF1 was also reported [18].
In our case, the NF1 patient had multiple GISTs and rectal carcinoma. Apart from a few Japanese reports, no other reports in the worldwide literature have mentioned a NF1 patient with both GIST and colorectal carcinoma. It is unclear whether this colorectal carcinoma is related to the NF1 mutation or whether it is a sporadic carcinoma. The association between NF1 and adenocarcinoma of the gastrointestinal tract is thought to be casual. Negative regulation of RAS protein by neurofibromin 1 may affect the carcinogenesis of the rectal carcinoma [19]. Li reported that the germline mutations in NF1 that cause NF1 can also occur in somatic cells and contribute to the cancer development [20].
In this case, the tumor progression risks of GISTs were low in the duodenum and very low in the jejunum. Unresected small nodules of the jejunum will not affect the prognosis of the patient. The resected rectal carcinoma was locally advanced (stage IIIB). The prognosis of this patient is dependent on the relapse of the rectal carcinoma and a scheduled examination to evaluate any recurrence is required.
Conclusions
In summary we presented a rare case of NF1 patient associated with multiple GISTs and rectal carcinoma.
References
Sobin LHGM, Wittekind C. TNM classification of malignant tumours (ed 7). West Sussex, UK: Wiley-Blackwell; 2009.
Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90–6.
Schmoll HJ, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K, Haller DG. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33:3733–40.
Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10:508–15.
McGaughran JM, Harris DI, Donnai D, Teare D, MacLeod R, Westerbeek R, Kingston H, Super M, Harris R, Evans DG. A clinical study of type 1 neurofibromatosis in north west England. J Med Genet. 1999;36:197–203.
Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–4.
Rosenbaum T, Wimmer K. Neurofibromatosis type 1 (NF1) and associated tumors. Klin Padiatr. 2014;226:309–15.
Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Investig. 2017;97:146–57.
Hirata Y, Brems H, Suzuki M, Kanamori M, Okada M, Morita R, Llano-Rivas I, Ose T, Messiaen L, Legius E, Yoshimura A. Interaction between a domain of the negative regulator of the Ras-ERK pathway, SPRED1 protein, and the GTPase-activating protein-related domain of neurofibromin is implicated in Legius syndrome and neurofibromatosis Type 1. J Biol Chem. 2016;291:3124–34.
Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen's disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012;5:852–62.
Brasfield RD, Das Gupta TK. Von Recklinghausen’s disease: a clinicopathological study. Ann Surg. 1972;175:86–104.
Salvi PF, Lorenzon L, Caterino S, Antolino L, Antonelli MS, Balducci G. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int J Surg Oncol. 2013;2013:398570.
Andersson J, Sihto H, Meis-Kindblom JM, Joensuu H, Nupponen N, Kindblom LG. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol. 2005;29:1170–6.
Nishida T, Tsujimoto M, Takahashi T, Hirota S, Blay JY, Wataya-Kaneda M. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J Gastroenterol. 2016;51:571–8.
Serio G, Zampatti C, Ballabio A, Ricci R, Martini M, Zurleni F. Neurofibromatosis 1 presenting with multiple duodenal GISTS associated with a somatostatin-producing D cell neoplasm. Endocr Pathol. 2013;24:100–5.
Zoller ME, Rembeck B, Oden A, Samuelsson M, Angervall L. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer. 1997;79:2125–31.
Kim ET, Namgung H, Shin HD, Lee SI, Kwon JE, Chang MC, Park DG. Oncologic manifestations of neurofibromatosis type 1 in Korea. J Korean Surg Soc. 2012;82:205–10.
Kim IY, Cho MY, Kim YW. Synchronous multiple colonic adenocarcinomas arising in patient with neurofibromatosis type 1. Ann Surg Treat Res. 2014;87:156–60.
Rad E, Tee AR. Neurofibromatosis type 1: Fundamental insights into cell signalling and cancer. Semin Cell Dev Biol. 2016;52:39–46.
Li Y, Bollag G, Clark R, Stevens J, Conroy L, Fults D, Ward K, Friedman E, Samowitz W, Robertson M, et al. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell. 1992;69:275–81.
Acknowledgements
Not applicable.
Funding
The authors declare that they do not have funding support from any funders or grants.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
SS participated in the design of the study, the surgical procedures and the chemotherapy. YH, TT, TO, YY, TN, and MO participated in the surgical procedures. AF and YU participated in the pathological diagnosis. CS and KK participated in the dermatologic diagnosis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee of Dokkyo Medical University Koshigaya Hospital approved this study for case report (#1712).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hakozaki, Y., Sameshima, S., Tatsuoka, T. et al. Rectal carcinoma and multiple gastrointestinal stromal tumors (GIST) of the small intestine in a patient with neurofibromatosis type 1: a case report. World J Surg Onc 15, 160 (2017). https://doi.org/10.1186/s12957-017-1231-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-017-1231-3