Abstract
Background
Fertility-sparing surgery is indicated for patients with stage I epithelial ovarian cancers. We sought to evaluate the clinical outcomes and oncofertility in a cohort of patients of reproductive age with stage I epithelial ovarian cancer (EOC).
Methods
Overall, 108 patients of reproductive age (≤ 40 years) diagnosed with stage I EOC who were treated at Peking Union Medical College Hospital between 1999 and 2013 were included in the study. The Kaplan-Meier model and Cox regression analyses were used for the survival analysis.
Results
The type of surgery included fertility-sparing surgery (FSS) (48.1%) and radical surgery (RS) (51.9%). After a median follow-up of 83 months, we observed that grade 3 or clear-cell carcinoma was the only independent risk factor for disease-free survival and tumor-specific survival in the multivariate analysis. Patients with grade 3 or clear-cell carcinoma tended to be older than 30 years, have endometriosis, and undergo RS (p < 0.05). Fertility-sparing surgery did not affect disease-free survival or tumor-specific survival among patients of reproductive age with stage I EOC and among high-risk patients with stage IC2-3, grade 3, or clear-cell carcinoma. Thirty-four out of 52 (65.4%) FSS patients attempted to get pregnant. Twenty-eight (82.4%) achieved a successful pregnancy with a full-term delivery.
Conclusions
Grade 3 or clear-cell carcinoma was the only independent risk factor for survival of patients of reproductive age with stage I EOC. FSS can be safely performed on patients of reproductive age with grade 1-2, stage I EOC. The safety of FSS for grade 3 and clear-cell carcinoma warrants further investigation.
Similar content being viewed by others
Background
Epithelial ovarian cancer (EOC) is the most lethal of malignant ovarian tumors [1]. Clinicians have been reluctant to perform fertility-sparing surgery in certain groups of patients with stage I epithelial ovarian cancers, including those with poorly differentiated tumors or clear-cell carcinomas. Based on current evidence-based medicine, fertility-sparing surgery is indicated for patients with stage I epithelial ovarian cancers [2, 3]. Fertility-sparing surgery is only meaningful in patients with type I epithelial ovarian cancer, including endometrioid, mucinous, low-grade serous, and clear-cell carcinomas [4]. Patients with high-grade serous ovarian cancers can relapse within a short period of time and are not advised to keep the contralateral ovary [5].
Preserving the high survival rate for stage I EOC is of utmost importance. There has been increasing interest in fertility-sparing surgery that does not negatively affect survival for these patients. The reason for keeping the contralateral ovary and uterus is that most of these patients can survive for a long time and could potentially die from other conditions instead of tumor recurrence. Most studies have suggested that oncologic and reproductive outcomes of patients who undergo FSS are favorable [6, 7].
Approximately, 30% of patients with epithelial ovarian cancers are diagnosed with stage I and 13% are younger than 40 years [8]. As the child-bearing age is becoming increasingly delayed, some patients may be diagnosed with malignant ovarian tumors before child-bearing. Radical surgery can be unacceptable in these patients, because they still wish to conceive.
In this study, we compared the survival of women of reproductive age with newly diagnosed stage I EOC who underwent FSS, with those who underwent RS. We also performed a subgroup analysis of patients with high-risk disease, including grade 3, clear-cell, or stage IC2-3 tumors, and assessed the pattern of recurrence between groups, as well as subsequent reproductive outcomes of women undergoing FSS.
Methods
The study protocol was approved by the ethics committees of Peking Union Medical College Hospital. We retrospectively identified patients with stage I EOC aged ≤ 40 years at diagnosis, who underwent primary staging surgery at the Department of Obstetrics and Gynecology of Peking Union Medical College Hospital (PUMCH) between 1999 and 2013. The diagnoses and staging were reassessed based on the fourth edition of the World Health Organization Classification of Tumors of Female Reproductive Organs and International Federation of Gynecology and Obstetrics (FIGO) 2014 staging system. According to FIGO 2014, stages IC1, IC2, and IC3 were defined as intraoperative spillage, preoperative capsule rupture or surface invasion, and positive cytology results, respectively.
Patients were eligible for inclusion if they were surgically and pathologically diagnosed with stage I (i.e., IA-B, IC1, IC2, IC3) EOC (i.e., mucinous, serous, endometrioid, and clear-cell carcinoma (CCC)) and aged ≤ 40 years at diagnosis. Patients with rare or special histological type of epithelial carcinoma (malignant Brenner tumors, squamous cell carcinomas, undifferentiated carcinomas, and epithelial carcinomas complicated with sarcoma components), carcinoma in situ, or borderline ovarian tumor were excluded from the study. Patients with incomplete clinical and pathological or follow-up information and those with disease extending beyond stage I and undergoing FSS were also excluded.
FSS was recommended to young patients with FIGO stage IA or IC1, grade 1-2 tumor, and non-clear-cell histology, with a strong desire to stay fertile and who could be monitored during followed-ups at a gynecologic oncology outpatient clinic. RS was suggested for those patients with high-risk disease, including grade 3, clear-cell histology, or stage IC2-3 tumors, based on intraoperative or final paraffin pathological diagnoses.
Comprehensive staging surgery included omentectomy, retroperitoneal, and/or para-aortic lymphadenectomy, appendectomy, excision of all suspicious nodes, peritoneal washings, and multiple-site random peritoneal biopsies. Fertility-sparing surgery included ipsilateral adnexectomy and biopsy or wedge excision of contralateral ovary. Radical surgery included hysterectomy and bilateral adnexectomy. Two independent pathologists with extensive experience in gynecological pathology reviewed all of the pathological slides and were blinded to patient outcomes.
Within the study period, adjuvant chemotherapy was administered to patients considered at increased risk for recurrence (FIGO stage IC1 or more, grade 2-3 tumor, or clear-cell histology) after the primary surgery. Chemotherapy regimens consisted of TC (paclitaxel and carboplatin), TP (paclitaxel and cisplatin), PC (cyclophosphamide and cisplatin), CC (cyclophosphamide and carboplatin), or PAC (cisplatin, epirubicin, and cyclophosphamide). The majority of patients received TC and PC chemotherapy. The number of cycles ranged from three to nine after tailoring therapy to patients.
After completion of the initial treatment, patients were followed-up monthly for the first 6 months, every 2 months after 6 months, every 3 months after 1 year, every 6 months after 2 years, and every year after 5 years. Clinical examinations performed at each visit included pelvic examination, ultrasonography scan, evaluation of carbohydrate antigen 125 (CA 125), and other previously elevated serum tumor markers. Patients were contacted by telephone or letter to obtain regular follow-up information when it was not available.
Recurrence was documented using histologic evidence of disease via tumor biopsy, fine-needle biopsy, or the appearance of new lesions on imaging examination. Disease-free survival (DFS) was defined as the time interval from the date of primary surgery to the date of disease recurrence or censoring during the last follow-up. Tumor-specific survival (TSS) was defined as the time interval from the date of the primary surgery to the date of death or censoring during the last follow-up. Endometriosis-associated ovarian cancer (EAOC) was defined as the coexistence of cancer and endometriosis in the same or contralateral ovary or extra-ovarian endometriosis [9]. All EAOCs were confirmed pathologically. We evaluated four categories of pretreatment serum tumor markers: CA 125, CA 19-9, carcinoembryonic antigen (CEA), and alpha fetoprotein (AFP). The normal upper limits of serum tumor markers CA 125, CA 19-9, CEA, and AFP were 35 and 37 U/mL and 5 and 20 ng/mL, respectively. Patients were considered to have an elevated serum tumor marker when any of these serum tumor markers were elevated.
Statistical analyses
Comparisons between the FSS and RS groups were performed using an independent t test, the Mann-Whitney U test, or the chi-square test as appropriate. DFS and TSS times were estimated using the Kaplan-Meier model and compared between the groups using the log-rank test. The Cox regression model was used for multivariate analysis. Variables with p < 0.1 in the univariate analyses were included in the multivariate analyses. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for the significant variable in the multivariate analyses. All p values reported were two tailed; p values < 0.05 were considered statistically significant. We performed statistical analyses using IBM SPSS 22.0 software for Macintosh and Graph Pad Prism 5.0.
Results
Study population
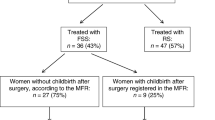
A review of the database revealed 144 patients with stage I EOC aged ≤ 40 years at diagnosis during the study period (1999–2013). Overall, 108 patients met the inclusion criteria and were included in the analysis. The selection process and reasons for patient exclusion are summarized in Fig. 1. Eleven patients with high-risk disease wanted to preserve their fertility and underwent FSS. Altogether, 52 (48.1%) patients underwent FSS and 56 (51.9%), RS.
Comparison of clinical and pathological features
Clinical and pathological variables are shown in Table 1. The median age of patients at diagnosis who underwent FSS was significantly younger (by 10 years), compared with those who underwent RS (p < 0.001). Significantly more patients who underwent FSS (94.2%) were nulliparous, compared with those underwent RS (32.1%; p < 0.001). Median tumor size in the FSS group was significantly larger than that in the RS group (p = 0.002); this could be due to the significantly higher proportion of mucinous histology (p < 0.001) in the FSS group. Most of the tumors were well-differentiated (66.7%). Eight patients had mixed tumor types. For easy statistical analysis, the epithelial component which took the larger proportion of the tumor was considered as the predominant histology. Because the RS group included more high-grade and clear-cell tumors (p < 0.001) than the FSS group, the proportion of patients receiving adjuvant chemotherapy (p = 0.006) were higher in the RS group. Patients in the RS group were more likely to have coexisting endometriosis (p = 0.006), which could be attributed to the higher proportion of clear-cell histology.
Comparison of oncologic outcomes
After a median follow-up of 83 months (range, 9–216 months), 14 (13.0%) patients relapsed, 8 (7.4%) died of progressive disease, and 100 (92.6%) were censored in the entire study cohort. The 5-year TSS and DFS rates were 92.6 and 86.6%, respectively. RS and FSS patients had a 5-year TSS rate of 89.3 and 97.3%, respectively, and a 5-year DFS rate of 83.0 and 91.0%, respectively.
Tables 2 and 3 show the results of the univariate and multivariate survival analyses of DFS and TSS, respectively. Surgery type, age, tumor size, histology, tumor grade, FIGO sub-stage, pretreatment tumor markers, and EAOC were included in the univariate analysis. Patients with grade 1-2 tumor tended to have higher 5-year DFS (p = 0.006) and TSS (p < 0.001) rates than those with a grade 3 or CCC; grade 3 and CCC were considered together (Fig. 2b–d). FSS patients tended to have better TSS than RS patients (p = 0.048). However, this was not observed with DFS (p = 0.423) (Fig. 2a–c). Women aged ≤ 30 years also had better TSS (p = 0.030) but not DFS (p = 0.106), than those aged > 30 years. Patients with EAOC had a worse DFS (p = 0.002) and TSS (p = 0.024) than those without EAOC. No adverse effects of FIGO stage IC1 or IC2-3 versus IA-B on prognosis were observed (DFS, p = 0.053; TSS, p = 0.314).
Comparison of survival in women of reproductive age with stage I epithelial ovarian cancer. Kaplan-Meier survival curves showing the effect of FSS or RS on disease-free survival (a) (p = 0.423) and tumor-specific survival (c) (p = 0.048); the effects of grade 1-2 or grade 3/clear-cell carcinoma on disease-free survival (b) (p = 0.006) and tumor-specific survival (d) (p = 0.0001)
We included tumor grade (p = 0.006), FIGO stage (p = 0.053), EAOC (p = 0.002), and pretreatment tumor markers (p = 0.071) in the multivariate analysis of DFS. Multivariate analysis confirmed the high risk for tumor grade (grade 3/CCC vs grade 1-2; HR 3.41, 95% CI 1.01–11.49, p = 0.048) for DFS. Moreover, the type of surgery (p = 0.048), age (p = 0.030), histology (p = 0.074), tumor grade (p < 0.001), and EAOC (p = 0.024) were included in the multivariate analysis of TSS. Multivariate analysis also confirmed the high risk for tumor grade (grade 3/CCC vs grade 1-2; HR 38.92, 95% CI 2.68-565.45, p = 0.007) in TSS.
Subgroup analysis based on high-risk and low-risk disease
Overall, 38 (35.2%) patients had high-risk disease (grade 3 or stage IC2-3 or clear-cell carcinoma). Among them, 11 (28.9%) patients underwent FSS and 27 (71.1%) patients, RS.
Among the low-risk patients, those who underwent RS and FSS had a 5-year DFS rate of 88.7 and 91.3%, a 5-year TSS rate of 96.6 and 96.6%, with no statistical significance (DFS, p = 0.580; TSS, p = 0.883) (Fig. 3a–b). The high-risk RS and FSS patients had a 5-year DFS rate of 77.0 and 68.2, a 5-year TSS rate of 77.0 and 100%, and also with no statistical significance (DFS, p = 0.776; TSS, p = 0.111) (Fig. 3c, d).
Comparison of survival in patients with low- and high-risk stage I epithelial ovarian cancer. Kaplan-Meier survival curves showing the effects of FSS or RS on disease-free survival (a) (p = 0.580) and tumor-specific survival (b) (p = 0.883) among low-risk patients; the effect of FSS or RS on disease-free survival (c) (p = 0.776) and tumor-specific survival (d) (p = 0.111) among high-risk patients
The distribution of grade 3 and clear-cell carcinoma in stage I EOC cohort of reproductive age
Since grade 3 and clear-cell carcinoma was the only independently significant predictor of DFS and TSS, we evaluated the correlation of grade 3 and clear-cell carcinoma with age, endometriosis, and surgery type. As can be seen from Table 4, 87.5% of patients with grade 3/CCC versus 38.1% of patients with grade 1-2 aged more than 30 years (p < 0.001); 41.7% of patients with grade 3/CCC versus 13.1% of patients with grade 1-2 were coexistent with endometriosis (p = 0.002); and 87.5% of patients with grade 3/CCC versus 41.7% of patients with grade 1-2 underwent radical surgery (p < 0.001).
Comparison of pattern of recurrence
As can be seen from Table 5, 22.2% (2/9) in the RS group versus 80.0% (4/5) in FSS had localized relapses. In the FSS group, 80.0% of recurrences were confined to the contralateral ovary. Furthermore, 71.4% (5/7) in grade 1-2 versus 14.3% (1/7) in grade 3/CCC had localized relapses.
Four in five FSS patients had a localized relapse in the contralateral ovary; the remaining patient had a disseminated relapse in the lung. Most patients in the RS group had multiple relapse sites and lost the opportunity to undergo follow-up surgery.
Taking a closer look at outcomes, seven (77.8%) of nine patients in the RS group had relapses and one (20.0%) of five patients in the FSS group who relapsed died of progressive disease; the remaining two (22.2%) of nine patients in the RS group and one (20.0%) of five patients in the FSS group were alive with the disease; another three (60.0%) patients in the FSS group were salvaged with repeated surgery and long-term survival without tumor was achieved.
Reproductive outcomes for patients in the FSS group
Of 52 patients in the FSS group, 34 (65.4%) attempted to become pregnant. Five (14.7%) patients were unable to conceive and diagnosed with infertility. Among the remaining 29 patients, 32 pregnancies were recorded, including 28 live births (82.4%, 28/34), 1 induced abortion, 2 miscarriages, and 1 intrauterine death. None of the patients underwent radical surgery after child-bearing.
Two patients failed to become pregnant and had recurrent disease of the retained ovary, concurrent with unexpected endometrial malignancy. One patient in the FSS group had an endometriosis relapse rather than malignant tumor on the contralateral ovary before she successfully became pregnant; the contralateral ovary was then resected. In another patient, relapse occurred in the contralateral ovary at 32 weeks of gestation. A cesarean section and restaging surgery were performed; the child was delivered and the woman survived without tumor for a long time.
Discussion
In this study, we evaluated women of reproductive age with stage I EOC who underwent FSS or RS. Grade 3/CCC was the only significant independent risk factor for DFS and TSS. Tumor-specific survival in FSS was better than that in the RS group in univariate analysis, because up to 71.1% of the high-risk patients underwent RS. In addition, up to 87.5% of patients with grade 3/CCC underwent RS. In the cohort, we defined the tumor with stage IC2-3 or grade 3 or clear-cell histology as high-risk disease. Several studies have already confirmed that no significant difference in patient survival exists between stage IC1 and stage IA tumors [10, 11]. Based on these data, we did not classify the tumor stage IC1 as high-risk disease, which is different from previous studies [12, 13]. The survival advantage of FSS compared with RS was not observed among high-risk patients. Similar to our study, studies published to date comparing FSS and RS have found no significant influence of FSS on prognosis, even among high-risk patients, including those with stage IC1-3, grade 3, or clear-cell carcinoma [12, 13]. Grade 3 and CCC were considered together in our cohort, because there were only four grade 3 tumors. This was understandable because malignant ovarian tumors confined to stage I were usually well-differentiated [13].
In an Italian study, researchers evaluated 240 patients with early-staged EOC (eEOC) treated with FSS [14]. Similar to our study, they found that grade 3 tumors were the only factor that negatively affected the prognosis of patients [14]. They subsequently evaluated 1031 patients with eEOC treated with FSS or RS [13] and found that grade 3 tumor was associated with shorter DFS and overall survival. However, in both studies, the classification of the tumor differentiation of CCC was not specified [13]. Although we did not observe the effect of sub-staging on DFS in our study, the p value (0.053) indicated a little significance. Most studies reported adverse effect of stage IC2/3 on DFS [10, 12, 13].
Our preliminary study has found that patients with EAOC might own an improved survival, but endometriosis per se was not an independently significant predictor in the multivariate analysis [9, 15, 16]. However, focusing on young (aged ≤ 40 years) patients with stage I EOC, EAOC patients had significantly poorer DFS and TSS than those with non-EAOC in the univariate analysis. It is possible that this trend is due to a higher proportion of grade 3/CCC in patients with EAOC than in those with non-EAOC (47.6 vs. 16.1%, p = 0.002).
Similarly, patients aged ≤ 30 years had a better TSS than those aged > 30 years in the univariate analysis. The incidence of grade 3/CCC was significantly greater in patients after they were aged 30 years. Patients aged > 30 years had significantly more grade 3/CCC than those aged ≤ 30 years (39.6 vs. 5.5%, p < 0.001). The literature suggests that younger age is more correlated with low-grade tumors [13], and increased age, with worse overall survival [12]. Patients with stage I EOC at a reproductive age tended to have a mucinous histology, low-grade, sub-staged early, and had better intrinsic biological behavior, compared with those at a non-reproductive age [13]. Therefore, the high-risk of age > 30, endometriosis, and radical surgery on survival in univariate analysis was attributed to the higher proportion of grade 3/CCC.
It should also be noted that the preservation of the uterus and contralateral ovary does not seem to affect patient survival [17]. In a Japanese study, researchers evaluated 16 patients with stage I CCC who underwent FSS, 205 patients who underwent RS, and 64 patients with stage I non-CCC who underwent FSS [18]. They found that patients with stage I CCC who underwent FSS did not have a poorer prognosis than those receiving RS and those with non-CCC who underwent FSS [18]. Researchers suggested that FSS was adequate for patients with stage I EOC, regardless of the stage, grade, and histological subtype [13].
In this study, the pattern of recurrence was more advantageous in FSS than that in the RS group. According to literature, relapse on the retained ovary has a good possibility of rescue with surgery and chemotherapy and did not affect the long-term survival of FSS patients [7, 19, 20]. Whereas patients who relapse in the lymph nodes and widely spread in the peritoneum, which is typical of clear cell histology, had a poor prognosis [4]. Compared with grade 1-2 tumors, grade 3 tumors give rise to a higher rate of extra-ovarian recurrences [14, 20]. Thus, the higher proportion of an isolated ovarian recurrence after FSS in our study could also be due to the lower proportion of grade 3/CCC. In our study, we found no evidence of fertility damage among patients in the FSS group. Previously published data suggest that there is an 80% rate of successful pregnancy after FSS [7, 14]. Besides, more fertility-preservation techniques, such as ovarian tissue cryopreservation and pharmacological protection against gonadotoxic agents, are developed to prevent the loss of reproductive fitness in these women [21, 22].
Considering the unbalanced distribution of grade 3/clear-cell carcinoma between groups, only 12.5% (3/24) of patients with grade 3 or clear-cell carcinomas underwent FSS, and the safety of FSS for patients with grade 3 or clear-cell carcinomas was uncertain. Patients aged ≤ 30 had only three (5.5%, 3/55) grade 3/clear-cell carcinomas, reflecting that young women tended to have more well-differentiated tumors. In addition, 86.5% (45/52) of patients in the FSS group were aged ≤ 30, indicating that FSS was safe in this patient population.
Conclusion
Grade 3 or clear-cell carcinoma was the only independent risk factor for survival of patients of reproductive age with stage I EOC. FSS can be safely performed on patients of reproductive age with grade 1-2, stage I EOC. The safety of FSS for grade 3 and clear-cell carcinoma warrants further investigation.
Abbreviations
- CCC:
-
Clear-cell carcinoma
- DFS:
-
Disease-free survival
- EAOC:
-
Endometriosis-associated ovarian cancer
- EOC:
-
Epithelial ovarian cancer
- FSS:
-
Fertility-sparing surgery
- RS:
-
Radical surgery
- TSS:
-
Tumor-specific survival
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Chan JL, Wang ET. Oncofertility for women with gynecologic malignancies. Gynecol Oncol. 2017;144:631–6.
Morice P, Denschlag D, Rodolakis A, Reed N, Schneider A, Kesic V, Colombo N, Fertility Task Force of the European Society of Gynecologic O. Recommendations of the Fertility Task Force of the European Society of Gynecologic Oncology about the conservative management of ovarian malignant tumors. Int J Gynecol Cancer 2011; 21: 951-963.
Groen RS, Gershenson DM, Fader AN. Updates and emerging therapies for rare epithelial ovarian cancers: one size no longer fits all. Gynecol Oncol. 2015;136:373–83.
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–88.
Nam JH, Park JY. Fertility-sparing surgery for young women with early-stage epithelial ovarian cancer. Gynecol Obstet Investig. 2013;76:14–24.
Zapardiel I, Diestro MD, Aletti G. Conservative treatment of early stage ovarian cancer: oncological and fertility outcomes. Eur J Surg Oncol. 2014;40:387–93.
Heintz APM, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HYS, Pecorelli S, Beller U. Carcinoma of the ovary. Int J Gynecol Obstet. 2006;95:S161–S92.
Ye S, Yang J, You Y, Cao D, Bai H, Lang J, Chen J, Shen K. Comparative study of ovarian clear cell carcinoma with and without endometriosis in People’s Republic of China. Fertil Steril. 2014;102:1656–62.
Kajiyama H, Mizuno M, Shibata K, Yamamoto E, Kawai M, Nagasaka T, Kikkawa F. Recurrence-predicting prognostic factors for patients with early-stage epithelial ovarian cancer undergoing fertility-sparing surgery: a multi-institutional study. Eur J Obstet Gynecol Reprod Biol. 2014;175:97–102.
Kajiyama H, Mizuno M, Shibata K, Umezu T, Suzuki S, Yamamoto E, Mitsui H, Sekiya R, Niimi K, Kawai M, Nagasaka T, Kikkawa F. A recurrence-predicting prognostic factor for patients with ovarian clear-cell adenocarcinoma at reproductive age. Int J Clin Oncol. 2014;19:921–7.
Ditto A, Martinelli F, Bogani G, Lorusso D, Carcangiu M, Chiappa V, Reato C, Donfrancesco C, De Carrillo KJ, Raspagliesi F. Long-term safety of fertility sparing surgery in early stage ovarian cancer: comparison to standard radical surgical procedures. Gynecol Oncol. 2015;138:78–82.
Fruscio R, Ceppi L, Corso S, Galli F, Dell'Anna T, Dell'Orto F, Giuliani D, Garbi A, Chiari S, Mangioni C, Milani R, Floriani I, Colombo N, et al. Long-term results of fertility-sparing treatment compared with standard radical surgery for early-stage epithelial ovarian cancer. Br J Cancer. 2016;115:641–8.
Fruscio R, Corso S, Ceppi L, Garavaglia D, Garbi A, Floriani I, Franchi D, Cantu MG, Bonazzi CM, Milani R, Mangioni C, Colombo N. Conservative management of early-stage epithelial ovarian cancer: results of a large retrospective series. Ann Oncol. 2013;24:138–44.
Bai H, Cao D, Yuan F, Sha G, Yang J, Chen J, Wang Y, Zhang Z, Shen K. Prognostic value of endometriosis in patients with stage I ovarian clear cell carcinoma: experiences at three academic institutions. Gynecol Oncol. 2016;143:526–31.
Wang S, Qiu L, Lang JH, Shen K, Huang HF, Pan LY, Wu M, Yang JX, Guo LN. Prognostic analysis of endometrioid epithelial ovarian cancer with or without endometriosis: a 12-year cohort study of Chinese patients. Am J Obstet Gynecol. 2013, 209:241. e1-9
Wright JD, Shah M, Mathew L, Burke WM, Culhane J, Goldman N, Schiff PB, Herzog TJ. Fertility preservation in young women with epithelial ovarian cancer. Cancer. 2009;115:4118–26.
Kajiyama H, Shibata K, Mizuno M, Hosono S, Kawai M, Nagasaka T, Kikkawa F. Fertility-sparing surgery in patients with clear-cell carcinoma of the ovary: is it possible? Hum Reprod. 2011;26:3297–302.
Marpeau O, Schilder J, Zafrani Y, Uzan C, Gouy S, Lhomme C, Morice P. Prognosis of patients who relapse after fertility-sparing surgery in epithelial ovarian cancer. Ann Surg Oncol. 2008;15:478–83.
Bentivegna E, Fruscio R, Roussin S, Ceppi L, Satoh T, Kajiyama H, Uzan C, Colombo N, Gouy S, Morice P. Long-term follow-up of patients with an isolated ovarian recurrence after conservative treatment of epithelial ovarian cancer: review of the results of an international multicenter study comprising 545 patients. Fertil Steril. 2015;104:1319–24.
De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–10.
Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11.
Acknowledgements
We thank the members of the Department of Pathology of PUMCH for their help with the pathological reviews in this study.
Funding
This study was supported by the National High Technology Research Development Program of China (863 program, grant no. 2012AA02A507), the National Natural Science Foundation of China (grant nos. 81172482 and 81372780), and the National Science and Technology Support Program of China (grant no. 2008BAI57B02).
Availability of data and materials
Please contact the corresponding author for data requests.
Author information
Authors and Affiliations
Contributions
XJ and JXY are the main authors of the manuscript and have made substantial contributions to the conception and design of the study. MY, WMX, and DYC have been involved in the requisition of the data. MW, LYP, HFH, and YY have been involved in the provision of the study materials or patients and in the analysis and interpretation of the data. KS has been involved in the analysis of the data and gave the final approval and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of PUMCH approved the study. Written informed consent was obtained from the patients for their participation.
Consent for publication
The written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jiang, X., Yang, J., Yu, M. et al. Oncofertility in patients with stage I epithelial ovarian cancer: fertility-sparing surgery in young women of reproductive age. World J Surg Onc 15, 154 (2017). https://doi.org/10.1186/s12957-017-1222-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-017-1222-4