Abstract
Background
The aims of this study were to evaluate the associations between the maximum standardized uptake value (SUVmax) and prognostic factors in invasive lobular carcinoma (ILC) and to compare these results with those in invasive ductal carcinoma (IDC).
Methods
The study included pathologically confirmed ILCs (n = 32) and IDCs (n = 73). We retrospectively evaluated the preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and measured the SUVmax. The pathologic results were reviewed regarding the size, histological type, histological grade, estrogen receptor (ER) and progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), and Ki-67 of the primary tumor. We also compared the associations between the SUVmax and prognostic factors.
Results
The mean SUVmax of the ILCs was significantly lower compared with that of the IDCs (P = 0.032). The SUVmax increased with tumor grade (P < 0.001) and was higher with ER negativity compared with ER positivity (P = 0.007) in IDC. The SUVmax was higher with EGFR positivity compared with EGFR negativity (P = 0.013) in IDC and higher with Ki-67 positivity compared with Ki-67 negativity in IDC and ILC (P < 0.001 and P = 0.002, respectively). The SUVmax was not significantly different regarding PR or HER2 for both tumor groups. The correlation between the tumor size and the SUVmax was demonstrated for IDCs (r = 0.57), but not for ILCs (r = 0.25).
Conclusions
The SUVmax was significantly different according to the tumor grade, ER, EGFR, and Ki-67 for IDCs. The SUVmax exhibited a positive association with Ki-67 in ILC; however, it was not significantly different with other factors, which suggests that the role of 18F-FDG PET/CT may be limited in ILC.
Similar content being viewed by others
Background
Breast cancer is a heterogeneous disease with a broad range of therapeutic responses, recurrence risk, and overall prognosis [1]. Predicting the prognosis of breast cancer is very important to determine the direction of treatment. The conventional prognostic factors include the tumor nuclear grade, tumor size, and presence of lymph node metastasis. The immunohistochemical prognostic factors include hormone receptors, such as estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), and the Ki-67 proliferation index [2].
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has become an increasingly important tool in the evaluation of patients with invasive breast cancer by revealing the functional properties of breast tumors [1]. 18F-FDG uptake in tumors is affected by various mechanisms, such as glucose transporter-1 (GLUT1) expression, hexokinase activity in tumor cells, tumor vascularity, tumor necrosis, the density of tumor cells, and the mitotic activity index [3]. The maximum standardized uptake value (SUVmax) measured with FDG PET is a sensitive indicator for metabolic activity in breast cancer [4,5], which can be used to assess tumor aggressiveness and is associated with prognostic factors, such as the histological type, histological grade, immunohistochemical factors, and proliferation index [2-4,6-10]. These studies have primarily included invasive ductal carcinoma (IDC), which comprises 72% to 80% of all invasive breast cancers [11]. Invasive lobular carcinoma (ILC) is the second most common breast cancer after IDC and accounts for 5% to 15% of all breast cancers [11,12], but there is a lack of data associating the SUVmax and prognostic factors in ILC in the existing literature. ILCs have exhibited low FDG uptake in previous studies [3,4,7-9,13-15].
To our knowledge, no study has associated FDG uptake with prognostic factors in this specific type of breast cancer. Thus, the aims of this study were to associate the SUVmax with histopathological and immunohistochemical prognostic factors in ILC and to compare these findings with those in IDC.
Methods
Patients
Institutional review board approval was obtained for this retrospective study. The informed consent was waived. We reviewed medical records and identified 32 patients with ILC between January 2004 and June 2012 and 73 patients with IDC in 2009. All patients underwent preoperative 18F-FDG PET/CT, in addition to breast magnetic resonance imaging and subsequent surgical diagnosis and staging.
18F-FDG PET/CT imaging
All patients underwent preoperative 18F-FDG PET/CT examinations using a dedicated PET/CT scanner with two-slice CT (Siemens Biograph Classic; Siemens Medical Solutions, Knoxville, TN, USA) (n = 60) or with a PET/CT scanner with 40-slice CT (Siemens Biograph TruePoint; Siemens Medical Solutions, Knoxville, TN, USA) (n = 45). The patients were asked to fast for a minimum of 6 h prior to the examination. Serum glucose levels were measured to ensure euglycemia (blood glucose level <130 mg/dl). Approximately 370 to 550 MBq of 18F-FDG were then injected with saline infusion. Following 60 min of bed rest after the injection, the PET scans were obtained. Seven to eight bed positions were acquired, with an acquisition time of 2 min each. All patients were in a supine position with their arms raised during the PET/CT scanning. Noncontrast CT scanning was initiated at the orbitomeatal line and progressed to the upper thigh (30 mAs; 130 kV; slice thickness 5 mm); the corresponding PET imaging immediately followed over the same body region. The CT data were used for attenuation correction, and the images were reconstructed using the standard ordered subset expectation maximization (OSEM; two iterations, eight subsets) algorithm.
Imaging interpretation
The 18F-FDG PET/CT imaging data were interpreted retrospectively by a nuclear medicine physician. All PET/CT images were reviewed at a workstation with fusion software (Syngo; Siemens Medical Solutions, Knoxville, TN, USA), which provided multiplanar reformatted images and displayed PET images, CT images, and PET/CT fusion images. A nuclear medicine physician reviewed the PET/CT images, and the interpretation was visually performed. For semi-quantitative analysis, SUVmax of 18F-FDG was measured by visually placing the regions of interest (ROIs) around the primary cancer mass that had perceptible 18F-FDG uptake. For compensation of two different PET/CT scanner types, the mean liver SUV values were obtained for all patients. The tumor/liver SUV ratio was calculated and compared between two groups using the different PET/CT scanner types. The tumor/liver SUV ratio was not statistically different according to the PET/CT scanner types (2.11 ± 1.89 for biograph classic with two-slice CT versus 1.75 ± 1.26 for biography true point with 40-slice CT, P = 0.299).
Histopathological analysis
We obtained the histopathological findings, including the size of invasive cancer, histological type, histological grade, ER and PR status, HER2, epidermal growth factor receptor (EGFR), and Ki-67 of the primary tumor by reviewing the pathology reports. Histopathological grading was performed using the Elson-Elis method, in which tubule formation, pleomorphism, and mitotic counts are scored 1 to 3 points. The cases scored within the 3 to 5 range were designated as grade 1, within 6 to 7 as grade 2, and within 8 to 9 as grade 3. Immunohistochemistry was used to assess the expression of the following molecular markers: ER, PR, HER2, EGFR, and Ki-67. ER and PR positivity was defined as the presence of 10% or more positively stained nuclei in ten high-power fields. The intensity of HER2 immunohistochemical (IHC) staining was scored as 0, 1+, 2+, or 3+. The tumors with 3+ were classified as positive, whereas 0 and 1+ were negative. For an HER2 score of 2+, fluorescence in situ hybridization (FISH) was used to determine HER2 positivity. EGFR was considered positive if membrane staining was observed. A Ki-67 of ≥15% was considered positive expression, which is comparable to 2011 Galen Consensus [16] and previous other studies of our institution [2,17].
Statistical analysis
Continuous variables are shown as the mean ± standard deviation (SD) with or without median (interquartile range), and the categorical variables are presented as the frequency and percentage. The differences between patient age and histopathological findings in ILCs and IDCs were compared using the Wilcoxon rank sum and Kruskal-Wallis tests. The association between the histopathological variables (for example, histological type, histological grade, ER and PR status, HER2, EGFR, and Ki-67 of the primary tumor) and the SUVmax were compared in each group of total carcinomas, ILCs, and IDCs using the Wilcoxon rank sum and Kruskal-Wallis tests. The correlations between the tumor size and the SUVmax were determined by the Spearman correlation coefficient and P value.
Significance was established at P < 0.05. The evaluation of the results was performed using the SAS system for Windows V 9.1 (SAS Institute, Cary, NC, USA).
Results
Patients and tumor characteristics
The clinical and histopathological data are summarized in Table 1. The tumor size was significantly larger in the ILC group compared with the IDC group (2.64 ± 1.13 vs. 2.08 ± 1.69 cm; P = 0.002). Compared with IDCs, ILCs were more likely to be positive for ER (93.6% vs. 75.3%; P = 0.031) and PR (83.9% vs. 61.1%; P = 0.023) and less likely to overexpress HER2 (6.7% vs. 24.6%; P = 0.039) and Ki-67 (18.5% vs. 51.4%; P = 0.003).
The patient’s age, tumor grade, and EGFR of the primary tumor were not different between the ILCs and IDCs.
SUVmax and histopathological variables
The association between the SUVmax and the histopathological variables are summarized in Table 2. The mean SUVmax of the ILCs (1.99 ± 1.72) was significantly lower compared with the IDCs (3.91 ± 3.99) (P = 0.032). The SUVmax increased with tumor grade (P < 0.001) and was higher with ER negativity compared with ER positivity (6.39 ± 5.06 vs. 3.09 ± 3.23; P = 0.007) in the IDC group (Figure 1). The SUVmax was higher with EGFR positivity compared with EGFR negativity (6.92 ± 5.55 vs. 3.26 ± 3.22; P = 0.013) in the IDC group (Figure 1) and higher with Ki-67 positivity compared with Ki-67 negativity (5.58 ± 4.66 vs. 2.19 ± 2.15, IDC, P < 0.001; 4.18 ± 0.88 vs. 1.45 ± 1.22, ILC, P = 0.002) in both the IDC and ILC groups (Figures 1 and 2). The SUVmax was not significantly different according to PR or HER2 for both tumor groups.
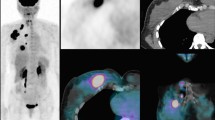
A 57-year-old woman with invasive ductal carcinoma in the right breast. (a) 18F-FDG PET/CT shows focal FDG uptake in the right upper outer breast with a maximum standardized uptake value (SUVmax) measured at 13.6. The tumor grade was III. On immunohistochemical study, ER and PR were negative and Ki-67 and EGFR were positive. (b) 18F-FDG PET/CT at axillary level shows focal FDG uptake in the right axillary lymph node with a SUVmax measured at 5.8. On histologic examination, right axillary lymph node metastases were noted in 7 of 16 dissected lymph nodes. EGFR, epidermal growth factor receptor; ER, estrogen receptor; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; PR, progesterone receptor.
A 76-year-old woman with invasive lobular carcinoma in the left breast. 18F-FDG PET/CT shows focal FDG uptake in the left mid outer breast with a maximum standardized uptake value (SUVmax) measured at 5.3. On immunohistochemical study, Ki-67 was positive. 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
A correlation between the tumor size and the SUVmax was identified in IDCs (r = 0.57), but not ILCs (r = 0.25) (Table 3).
Discussion
The objectives of this study were to associate the SUVmax with prognostic factors in ILC and to compare these results with the associations in IDC.
In the present study, the tumor size was significantly larger in the ILC group compared with the IDC group. Compared with IDCs, ILCs were more likely to be positive for ER and PR and less likely to overexpress HER2 and Ki-67. The results of the present study are consistent with previous studies [12,18,19].
In the present study, because the distributions of SUVmax values were skewed, the association between clinical/histopathological variables and SUVmax values was performed by the Wilcoxon rank sum test. Our study revealed that the mean SUVmax of ILCs was significantly lower compared with that of IDCs, which was consistent with previous results [3,4,7-9,13-15]. The lower FDG uptake of ILC was explained by diffuse infiltrative growth patterns, a low tumor cell density, a low level of GLUT1 expression, and a decreased proliferation rate [3,13,15]. The SUVmax was significantly different according to the tumor grade, ER, EGFR, and Ki-67 for IDCs; however, only Ki-67 was significantly different for ILCs in the present study. The SUVmax was not significantly different according to PR or HER2 for both tumor groups. These results for IDCs were consistent with previous studies regarding breast cancers in which the SUVmax was significantly higher in patients with tumors with negative ER and grade III [2,4,6-10,14,20]. There have been some controversies regarding the association between the SUVmax and the negativity of PR [2,4,6,8-10,14,20] and the positivity of EGFR [2,9]. There was no association between the SUVmax and HER2 status in IDC or ILC in the present study, and these findings are nearly identical to previous results [2,4,6,8-10,14,20]; however, the current findings are in disagreement with previous results that indicated a positive association between the SUV max and HER2 positivity [10,14].
In contrast with IDC, the SUVmax showed a positive association only with Ki-67 in ILC in the present study. The association between the SUVmax and the Ki-67 proliferation index in breast carcinoma has been reported in previous studies [2,4,6,7]; however, the majority (84% to 100%) of cases in these studies had IDCs. To the best of our knowledge, no previous report has evaluated the association between the SUVmax and prognostic factors in ILC. The role of 18F-FDG PET/CT as an imaging biomarker to predict the prognosis in breast cancers has been demonstrated in many papers [2-4,6-10,14]. We have also demonstrated the role of 18F-FDG PET/CT as an imaging biomarker for the prognostic prediction of breast cancers in IDC. However, the role of 18F-FDG PET/CT may be assumed to be limited in ILC.
The correlation between the tumor size and the SUVmax was demonstrated in IDCs, but not in ILCs in the present study. The IDC results in the present study were similar to previous studies that reported a positive relationship between SUVmax and tumor size in breast cancer [2,6,7,9,10,14], but not consistent with previous result that indicated there was no correlation between the SUVmax and T stage [8].
The limitations of the current study included the small number of cases, especially for ILC, and the retrospective design. Furthermore, the reviewers were aware that all cases had breast cancer, although they did not know whether the diagnosis was IDC or ILC, which may have resulted in selection bias. Because the review of histopathological report was also retrospective, the data file included a certain amount of missing values. The high rate of missing values for HER2 was due to lack of FISH results for cases showing HER2 score of 2+ on IHC staining, because the FISH was not a routine exam but an ancillary test based on request by clinicians. The missing values had less than 15%, so the list-wise deletion was used for missing data.
Conclusions
In conclusion, the mean SUVmax of ILCs was significantly lower compared with that of IDCs. The SUVmax was significantly different according to the tumor grade, ER, EGFR, and Ki-67 in IDCs, but only for Ki-67 in ILCs. Therefore, the role of preoperative 18F-FDG PET/CT in terms of prognosis prediction may be more difficult in ILCs than IDCs.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- ER:
-
Estrogen receptor
- 18F-FDG PET/CT:
-
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- GLUT1:
-
Glucose transporter-1
- HER2:
-
Human epidermal growth factor receptor 2
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular carcinoma
- PR:
-
Progesterone receptor
- SUVmax:
-
Maximum standardized uptake value
References
Bolouri MS, Elias SG, Wisner DJ, Behr SC, Hawkins RA, Suzuki SA, et al. Triple-negative and non-triple-negative invasive breast cancer: association between MR and fluorine 18 fluorodeoxyglucose PET imaging. Radiology. 2013;269:354–61.
Choi BB, Kim SH, Kang BJ, Lee JH, Song BJ, Jeong SH, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012;10:126.
Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–87.
Koolen BB, Vrancken Peeters MJ, Wesseling J, Lips EH, Vogel WV, Aukema TS, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39:1830–8.
Flanagan FL, Dehdashti F, Siegel BA. PET in breast cancer. Semin Nucl Med. 1998;28:290–302.
Ekmekcioglu O, Aliyev A, Yilmaz S, Arslan E, Kaya R, Kocael P, et al. Correlation of 18F-fluorodeoxyglucose uptake with histopathological prognostic factors in breast carcinoma. Nucl Med Commun. 2013;34:1055–67.
Gil-Rendo A, Martinez-Regueira F, Zornoza G, Garcia-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96:166–70.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espie M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15:588–93.
Sanli Y, Kuyumcu S, Ozkan ZG, Isik G, Karanlik H, Guzelbey B, et al. Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med. 2012;26:345–50.
Arps DP, Healy P, Zhao L, Kleer CG, Pang JC. Invasive ductal carcinoma with lobular features: a comparison study to invasive ductal and invasive lobular carcinomas of the breast. Breast Cancer Res Treat. 2013;138:719–26.
Lim ST, Yu JH, Park HK, Moon BI, Ko BK, Suh YJ. A comparison of the clinical outcomes of patients with invasive lobular carcinoma and invasive ductal carcinoma of the breast according to molecular subtype in a Korean population. World J Surg Oncol. 2014;12:56.
Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495–502.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8.
Buck AK, Schirrmeister H, Mattfeldt T, Reske SN. Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging. 2004;31 Suppl 1:S80–7.
Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: summary of the consensus discussion. Breast Care (Basel). 2011;6:136–41.
Kim JY, Kim SH, Kim YJ, Kang BJ, An YY, Lee AW, et al. Enhancement parameters on dynamic contrast enhanced breast MRI: do they correlate with prognostic factors and subtypes of breast cancers? Magn Reson Imaging. 2015;33:72–80.
Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, Rotmensz N, et al. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat. 2012;133:713–23.
Gonzalez-Angulo AM, Sahin A, Krishnamurthy S, Yang Y, Kau SW, Hortobagyi GN, et al. Biologic markers in axillary node-negative breast cancer: differential expression in invasive ductal carcinoma versus invasive lobular carcinoma. Clin Breast Cancer. 2006;7:396–400.
Soussan M, Orlhac F, Boubaya M, Zelek L, Ziol M, Eder V, et al. Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS One. 2014;9:e94017.
Acknowledgements
The statistical consultation was supported by the Catholic Research Coordinating Center of the Korea Health 21 R&D Project (A070001), the Ministry of Health & Welfare, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NYJ and SHK participated in conception of the study, data collection and analysis, and drafted the manuscript. NYJ, SHK, BBC, and SHK participated in the data collection and analysis. MSS participated in manuscript editing and proof. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Jung, N.Y., Kim, S.H., Choi, B.B. et al. Associations between the standardized uptake value of 18F-FDG PET/CT and the prognostic factors of invasive lobular carcinoma: in comparison with invasive ductal carcinoma. World J Surg Onc 13, 113 (2015). https://doi.org/10.1186/s12957-015-0522-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-015-0522-9