Abstract
Background
Due to an improving prognosis, and increased knowledge of intervention effects over time, long-term well-being among prostate cancer (PC) survivors has gained increasing attention. Yet, despite a variety of available PC interventions, experts currently disagree on optimal intervention course based on survival rates.
Methods
In January 2017, we searched multiple databases to identify relevant articles. Studies were required to assess at least two different dimensions of health-related quality of life (HRQoL) in PC survivors ≥5 years past diagnosis with validated measures.
Results
Identified studies (n = 13) were mainly observational cohort studies (n = 10), conducted in developed countries with a sample size below 100 per study arm (n = 6). External-beam radiation therapy was the most common intervention (n = 12), whereas only three studies included patients on active surveillance or on watchful waiting.
Studies were largely heterogeneous in cancer stage at diagnosis, intervention groups and instruments. All identified studies either used the EORTC QLQ-C30 (n = 5) or the SF-36 (n = 7) to assess generic HRQoL, yet 11 different instruments were employed to assess PC specific urinary, bowel and sexual symptoms. Overall, no consistent pattern between intervention and HRQoL was observed. Results from two randomized-controlled-trials (RCTs) and one observational study, comparing HRQoL by primary intervention in localized PC survivors suggest that long-term HRQoL does not differ by intervention. However, observational studies that included a combination of localized and locally advanced stage PC survivors identified HRQoL differences for various scales including physical well-being, social and role function, vitality, and role emotional.
Conclusion
This review reveals the number of publications comparing HRQoL by primary intervention in long-term PC survivors is currently limited. Robust data from two RCTs and one observational study suggest that HRQoL does not seem to differ by intervention. However, the heterogeneity of studies’ methodologies and results hindered our ability to draw a clear conclusion. Therefore, in order to answer the question of which primary intervention is superior with respect to long-term HRQoL in PC patients, more high-quality, large-scale prospective cohort studies, or RCTs with repeated HRQoL assessments, are urgently needed.
Similar content being viewed by others
Background
In economically developed countries, prostate cancer (PC) continues to be the most frequent cancer in men [1]. In Europe, for example, approximately 400,000 men are diagnosed with PC annually [2]. Patient prognosis has substantially improved due to earlier diagnosis and advancements in therapy, leading to five-year relative survival rates of 99.1% (2008) in the US [3] and 93% in Europe [4]. Consequently, the number of PC survivors is on the rise [5]. In particular, the number of long-term survivors (i.e. those still alive 5 years after initial diagnosis [6]) is substantially increasing.
A variety of intervention options, including radical prostatectomy (RP), radiotherapy (external beam (EBRT) or brachytherapy (BT)), chemotherapy (CT), cyberknife (CK), cryotherapy (CRYO), androgen deprivation therapy (ADT), active surveillance (AS) and watchful waiting (WW)) are now available. [7,8,9,10] However, there is currently no agreement on the optimal intervention, based on survival rates, especially for men with localized stage PC [8, 9, 11].
Despite increased awareness regarding long-term outcomes and patient-reported outcomes (including health-related quality of life (HRQoL)), a gold-standard definition of HRQoL does not currently exist. However, researchers agree that HRQoL is a multidimensional concept that encompasses all aspects of survivors’ well-being including physical, psychological, social and spiritual health [12, 13]. Additionally, global HRQoL (or overall health perceptions) must be added to this multidimensional concept, as it has proven to be an important predictor of individuals’ health [14].
Although HRQoL outcomes are useful to define the harmful and beneficial effects of interventions from the patient’s perspective, differences in HRQoL outcomes of long-term PC survivors (≥ 5 years since diagnosis) [15] between interventions have rarely been documented [16, 17]. Due to high PC survival rates and low PC-specific mortality rates (which do not differ between interventions [8, 18]), information on long-term HRQoL should be analyzed and subsequently considered as an additional factor in intervention decisions. HRQoL is especially relevant because other measurements (e.g. survival/mortality rates) do not currently indicate superiority of one intervention over the others [11, 19,20,21].
This systematic review aims to identify all studies assessing HRQoL among long-term PC survivors by primary intervention. Findings will be synthesized and critically discussed with respect to study design and methodology.
Method
We followed the standard systematic review methodology outlined by the Centre for Reviews and Dissemination (York, UK) [22] and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) group [23].
Study eligibility criteria
This systematic review includes all quantitative comparative studies on PC survivors diagnosed a minimum of 5 years prior to HRQoL assessment. When studies also included short- or medium-term survivors, it was critical the researchers of these studies examined results specifically pertaining to long-term PC survivors.
At minimum, study outcomes had to report on overall/general HRQoL plus one HRQoL domain, or at least two HRQoL domains. Domains were defined as physical, psychological, social and spiritual well-being [12, 24]. Only validated assessment instruments were included, such as the European Organization for Research and Treatment of Cancer Core Questionnaire (EORTC QLQ-C30) [25], the 36-item Short Form Health Survey (SF-36) [26] or the Functional Assessment of Cancer Therapy - General (FACT-G) [27]. Further, we required HRQoL results to be explicitly reported by type of primary intervention. Interventions could be either RP, EBRT, BT, ADT, CT, CK, CRYO, AS or WW, as well as, combinations of these interventions. It was necessary each study compared the HRQoL of different interventions, or one intervention to the HRQoL of a reference group (e.g. general population). Without an available gold-standard classification of intervention options (e.g. active surveillance), all intervention options are classified as “intervention,” for our purposes [28,29,30]. Moreover, researchers had to report on information regarding age and date of diagnosis and time post diagnosis. All included articles were published in English, German, French or Italian.
Search strategy and study selection
The literature search was completed in January 2017 using the following electronic databases: Pubmed, Medline, Embase, PsychInfo, Cinahl, Web of Science and Cochrane Central Register of Controlled Trials. Additionally, we hand-searched the bibliographies of reviews, conference proceedings, and supplements to identify further relevant studies. Authors of these publications were contacted for further details.
The following combinations were used: “quality of life, HRQoL, patient satisfaction, well-being, general health status assessment, qlq c30, pr 25, sf 36” AND “cancer survivor, long-term, year after” AND “prostate cancer, prostate adenocarcinoma, prostate neoplasm, prostate neoplasia, prostate carcinoma” (Additional file 1: Appendix A).
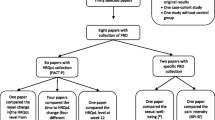
One author (SaA) assessed eligibility and selected the articles by screening records based on title/abstract review. Further, two reviewers (SaA and AF) assessed the full-texts according to predefined, hierarchically ordered inclusion and exclusion criteria. In the case of doubt, a third reviewer (VA) made the final decision. The flow diagram of the search and selection process is outlined in Fig. 1.
Data extraction and quality assessment
Data were independently extracted by two reviewers (SaA, AF) using a systematic scheme containing the following study characteristics: title, first author, year, country, study design, age range, cancer stage, intervention(s), comorbidities, response rate, time since diagnosis/randomization, HRQoL instrument(s), statistical methods and results. Only data pertaining to long-term survivors was extracted. Reviewers described study results and indicated whether they were statistically significant and/or clinically meaningful [31,32,33]. The same reviewers assessed the methodological quality of each article, following the risk of bias (RoB) criteria based on the GRADE approach [34], with the following additional criteria: adjustment for attrition error, sample size power, control for confounding, reporting of results appropriate (plots/diagrams/tables printed sufficiently, lack of selective reporting of results), statistical significance test(s) performed and baseline data available.
Results
Literature search results
Two thousand sixty articles were identified through the literature search. After removing duplicates, 1236 articles remained. Screening of titles and abstracts identified 94 potentially eligible articles (Figure 1). Full-text analyses identified 13 articles, which were included in data extraction [35,36,37,38,39,40,41,42,43,44,45,46,47].
Study characteristics
Studies were exclusively conducted in developed countries: seven in Europe [35, 37,38,39, 41, 43, 47], three in Japan [44,45,46], two in the US [40, 42] and one completed in the US and Europe [36] (Table 1). The majority were observational prospective cohort studies (n = 7) [35, 38, 40, 42, 44,45,46], three were observational retrospective cohort studies [39, 43, 47] and three were randomized controlled trials (RCTs) [36, 37, 41] (Tables 1 and 2).
Recruitment of survivors
Recruitment was monocentric hospital-based in nine studies [35, 38,39,40,41,42, 44,45,46], multicentric hospital-based in one study [36] and population-based in three studies [37, 43, 47]. In ten studies, survivors were diagnosed with PC, on average (mean, median), five to 10 years before the time of HRQoL assessment. [36,37,38,39,40, 43,44,45,46,47] In three studies, survivors were diagnosed more than 10 years before the time of HRQoL assessment. [35, 41, 42] Most studies included long-term PC survivors with localized (TNM stage: T1 & T2) and locally advanced (TNM stage: T3 & T4) PC [35, 38, 39, 42, 43, 45, 46] (categorization based on [48, 49]). Whereas two studies specifically recruited survivors after locally advanced PC [36, 44], four studies recruited survivors after only localized PC. [37, 39, 41, 47]. Ten studies [35,36,37,38,39,40,41, 45,46,47] provided no information on how they analyzed recurrent PC cancer survivors and whether recurrent PC cases were included in their dataset. Two studies [42, 43] included recurrent cancer patients and one excluded them, as they died during the follow-up time [44].
The average age of PC survivors at HRQoL assessment was around 75 years, ranging from 53 to 90 years of age. The RCTs, and some prospective cohort studies, only reported age at study enrollment (baseline). Thus, for these studies, the study population age at different HRQoL assessment time points can only be estimated.
One study excluded survivors with comorbid conditions [35], whereas four [43,44,45, 47] studies explicitly considered long-term PC survivors with comorbidities. These studies showed that >60% of long-term PC survivors were diagnosed with at least one comorbid condition.
Participation rate and number of participants
Sample size was defined at time of HRQoL assessment. Half of the studies had a sample size below 100 participants [35, 38, 39, 42, 45, 46], five had a sample size between 101 and 200 participants [36, 40, 41, 44, 47], one had 780 participants [43] and one study cohort consisted of 1463 participants 5 years post-randomization, with 1413 participants remaining for analysis 6 years post-randomization [37]. Participation rate (defined as the number of participants divided by the number of eligible patients at the time of long-term HRQoL assessment) was over 90% in one study [46], between 70 and 90% in ten studies [35,36,37, 39,40,41,42,43,44,45] and below 60% in one study [47].
Intervention comparisons and stage at diagnosis
Interventions were generally classified as RP, EBRT (referring to the external delivery of any type of radiation), ADT, BT, WW or AS. Studies either compared HRQoL by primary intervention in long-term survivors with:
Unfortunately, one study did not reveal information about the cancer stage. This study was categorized as stage X [40] (Tables 4 and 5).
Additionally, alternative comparison methods for HRQoL among primary intervention groups were identified. Studies either compared:
-
T1)
HRQoL of PC survivors undergoing a specific primary intervention with controls from the general population at certain points over time [35, 38, 42, 45, 47],
-
T2)
HRQoL of PC survivors undergoing different interventions to each other at certain time points [36,37,38,39,40,41, 43, 46, 47] or.
-
T3)
HRQoL of PC survivors undergoing different interventions over a certain time period [36, 37, 44] (Tables 1 and 3).
Overall, EBRT was the most commonly evaluated intervention, followed by RP. The most common control group was the general population (n = 10) [35, 38, 42,43,44, 47] (Table 2).
Assessment of health-related quality of life and prostate cancer specific symptoms
Included studies employed generic, as well as, disease-specific HRQoL instruments.(Table 3) Seven studies employed the SF-36 questionnaire as a generic HRQoL assessment instrument [40, 42,43,44,45,46,47], and five studies used the EORTC QLQ-C30 (Version 1.0 and 3.0) [35, 36, 38, 39, 41].
One study [37] used both the abbreviated form of the SF-36, the SF-12, and the EORTC QLQ C30. Additionally, two studies [43, 47] made use of the Dutch version of the Quality of Life-Cancer Survivors (QoL-CS) questionnaire [50]. The EORTC QLQ-C30 consists of five functional scales, nine symptom specific subscales and a global health status scale [25]. In contrast, both the SF-36 and the SF-12 consist of eight scales. The scales include general health perception, which encompasses two general domains: physical and mental well-being [26, 51]. Scales in both instruments are linearly transformed to values from 0 to 100 [52]. In the EORTC QLQ-C30, a high score for a functional scale represents a high/healthy level of functioning, a high score for the global health status/QoL represents a high QoL. Generally, a high score for a symptom scale/item represents a high level of symptomatology [52]. Most studies reported statistically significant differences [36,37,38,39,40,41, 43,44,45,46,47]. Five studies completed an additional analysis if the results were clinically meaningful [35,36,37, 39, 43].
PC specific symptoms were assessed with 11 different instruments. [53,54,55,56,57,58,59,60,61,62,63] (Table 3). Additionally, the Hospital Anxiety and Depression Scale (HADS) [64] was used in two studies. [37, 46] Six studies [37, 38, 40, 41, 45, 46] combined different instruments, six [35, 36, 39, 42, 44, 47] used one instrument, and one study did not assess PC specific symptoms [43]. Scales of disease-specific HRQoL instruments were mainly related to urinary, bowel and sexual functions/problems.
Study findings
Overall, studies were heterogeneous and most had potential limitations. Therefore, we decided to systematically report but not pool (e.g. in a meta-analysis), the main results. Further, we divided the results between RCTs and observational studies and grouped them by disease stage. (Tables 4 and 5, Additional file 1: Appendix Tables B and C).
HRQoL by primary intervention in long-term survivors with localized PC
Three studies assessed HRQoL in long-term survivors with localized stage PC [37, 41, 47]. Comparisons were drawn from two RCTs, comparing either AS vs. RP vs. EBRT, or RP vs. EBRT [37, 41] and one observational study comparing AS vs. EBRT. Both interventions used controls from the general population [47].
These three studies showed that long-term survivors with localized stage PC have comparable HRQoL independent from the chosen intervention. (Tables 4 and 5) Moreover, one study revealed that PC survivors do not experience any reduction in their HRQoL, except for deficits in physical function, when compared with controls from the general population. [47] However, in two studies [37, 47] EBRT had an effect on bowel function. Additionally, one RCT reported that RP had the greatest negative effect on urinary and sexual function, compared to survivors on AS or survivors treated with EBRT [37] (Additional file 1: Appendix Tables B and C).
HRQoL by primary intervention in long-term survivors with locally advanced PC
Two studies (one RCT, one observational study) assessed HRQoL in long-term survivors with locally advanced PC [36, 44]. The RCT compared PC survivors treated with ADT vs. ADT + EBRT [36] and the observational study RP vs. EBRT [44]. Only the RCT reported results for intervention comparisons at specific time points. In this RCT, no difference in HRQoL or PC symptoms could be identified. After 5 years, the observational study shows both interventions have good outcomes, whereas PC patients treated with RP reported better well-being [36].
HRQoL by primary intervention in long-term survivors with localized or locally advanced PC
Seven observational studies compared HRQoL in survivors with localized and locally advanced stage PC [35, 38, 39, 42, 43, 45, 46]. In four studies [35, 38, 42, 43], PC survivors treated with EBRT were compared with controls from the general population, whereas in three [35, 42, 43] of these four studies, PC survivors were additionally treated with ADT. In these four studies, no uniform pattern in HRQoL differences could be identified. Three [35, 38, 42] studies reported significant, or even clinically relevant, functioning in different HRQoL domains (social, role and emotional functioning) and a higher burden of diarrhea, appetite loss, nausea, pain and insomnia. Conversely, the fourth study [43] revealed that patients reported comparable HRQoL, and less bodily pain, in comparison to a control group from the general population. However, for PC specific symptoms, authors could identify more detriments in sexual function domains (n = 2) [35, 42] and more urinary bowel problems (n = 2) [38, 42] when compared to controls from the general population (Tables 4 and 5, Additional file 1: Appendix Tables B and C).
When PC survivors treated with EBRT were compared to either PC survivors treated with RP or WW, no significant results in HRQoL could be identified [39, 46]. The same result applies for the comparison of PC survivors treated with RP vs. controls from the general population [45].
The one study comparing PC survivors treated with RP vs. EBRT vs. ADT vs. WW showed significant differences were observed in physical functioning and physical well-being, whereas survivors treated with RP had the best scores in these domains. Further, survivors treated with ADT had the lowest scores. In a separate analysis comparing all the intervention groups with controls from the general population, no intervention group reported worse HRQoL [43].
Discussion
Five and 10 year PC-specific survival rates are nearing 100%, seemingly independent from type of primary intervention [18]. Consequently, experts continue to disagree on a preferred intervention course, particularly in the disease’s early stages.
This review identified 13 studies (three RCTs and 10 observational studies), which evaluated HRQoL and PC specific symptoms in long-term PC survivors at different cancer stages. Studies varied in terms of intervention comparison groups, instruments used, and whether/how studies reported results on primary interventions for localized PC, locally advanced PC, or on both together without distinction.
The main tested intervention group was EBRT (plus ADT), and only limited information was available on PC survivors treated with ADT only, and on PC survivors on AS or WW. AS and WW are only recently considered standard care. Thus, the lack of studies in this review focusing on long-term PC survivors (and two earlier reviews including short-term survivors) undergoing AS or WW, is not surprising [65, 66]. The limited number of studies assessing HRQoL in PC survivors treated with ADT is also logical, as ADT is mainly indicated in patients with advanced stage PC, which has a shorter survival time [67].
To assess generic HRQoL, studies either used the SF-36, or EORTC QLQ-C30, thus allowing for comparisons to be drawn across at least some domains. However, our review reveals a diverse number of instruments employed in assessing PC specific symptoms. UCLA-PCI (n = 4) was the most commonly employed instrument, followed by the EPIC (n = 2) and IPSS (n = 2). The first two questionnaires (UCLA-PCI and EPIC) focus on urinary, sexual and bowel symptoms, whereas the latter (IPSS) evaluates only urinary symptoms. The studies in this review: (1) focused on only one questionnaire, (2) used different combinations of the questionnaires, or (3) did not evaluate PC specific symptoms at all, making it impossible to pool results across studies.
Interestingly, the RCTs evaluated in this systematic review included either PC survivors with localized PC [37, 41] or locally advanced PC [36], whereas only two observational studies [44, 47] made this distinction. Therefore, the results of these observational studies should be interpreted carefully, because the choice of intervention is dependent on stage at diagnosis [10].
In addition to the use of diverse instruments, the majority of reviewed studies had potential limitations. These limitations prevented our ability to draw firm conclusions on HRQoL’s dependency on primary intervention in long-term PC survivors. First, only three studies [37, 43, 47] had sufficient power to detect predetermined differences in scores between groups. For example, to detect a difference of ten points with a power of 80% and alpha = 0.05, a sample size of 100 per group in the EORTC QLQ-C30, and of around 70 in the SF-36 questionnaire, is needed. [68, 69] Second, ten studies [31, 32, 34, 35, 38,39,40,41,42,43] were prone to confounding, as they were observational studies. In these observational studies, control for potential confounding was performed to varying degrees by only half of the studies [31, 34, 39, 42, 43]. Age, stage, comorbidity and other factors are strongly associated with HRQoL and with intervention decision. Thus, observational studies should carefully account for potential confounding by these factors. Third, most studies did not assess the results’ clinical significance [34, 36,37,38, 40,41,42,43], which limits clinical relevance. Finally, selection bias may occur if patients experiencing PC recurrence are excluded from sample analysis. Only two studies explicitly stated whether survivors with recurrent disease were included in the analysis, or not.
The strong heterogeneity across studies, and their potential limitations, reveals an urgent need for more high-quality, large-scale, prospective cohort studies, or RCTs with repeated follow-up HRQoL assessments.
However, some robust data exist from two RCTs and one population-based observational, retrospective cohort study comparing HRQoL by primary intervention in survivors with localized stage PC. The data do not suggest HRQoL differs by intervention. However, these three studies had different comparisons and included, in total, four different interventions, whereas pooling of study findings was not possible.
No consistent results could be seen in other studies based on survivors with locally advanced PC, or on combining localized or locally advanced PC stage. Intervention detriments are seen for various scales: (1) physical well-being, (2) social and role function, (3) vitality and (4) role emotional. However, results are contractionary due to the previously discussed limitations and the heterogeneity of included studies. Therefore, the question of whether HRQoL varies by primary intervention and (if yes), which intervention options are superior with respect to HRQoL, cannot be answered based on these studies.
Further, our systematic review has some of its own limitations. As the aim was to compare the influence of primary interventions on HRQoL in long-term PC survivors, all studies that did not have a comparison group (either general population or another intervention group) were excluded from the review. Additionally, qualitative studies were not included as we only wanted to review and compare quantitative studies using validated questionnaires. Furthermore, as consensus exists that HRQoL is a multidimensional concept that encompasses all aspects of survivors’ well-being, three studies that reported or assessed HRQoL on only one domain were not included. Additionally, due to the limitations and variations of the instruments, and comparison groups of the included studies, result pooling was not possible for the observational studies, or for the RCTs.
Conclusion
Despite an increasing number of publications studying HRQoL and/or disease specific symptoms in PC survivors, only a limited number of publications is available focusing on long-term PC survivors and primary intervention. This systematic review exposes the heterogeneity of PC intervention studies in terms of (1) stage at diagnosis, (2) intervention groups and (3) instruments used. In addition, most studies are limited by low sample size, and in the case of observational studies, potential confounding by indication, or due to insufficient adjustment.
Robust data from two RCTs and one observational study, comparing HRQoL by primary intervention in localized PC survivors, suggest that HRQoL does not seem to differ by intervention. However, data from observational studies assessing HRQoL by primary intervention of PC survivors and combining localized, or locally advanced stage PC, identified differences for various scales: physical well-being, social and role function, vitality and role emotional. However, study heterogeneity and limitations prevent the identification of clear patterns.
Therefore, a review of the existing studies reveals an urgent need for more high-quality, large-scale, prospective cohorts or RCTs with repeated follow-up HRQoL assessments in order to provide clinicians and patients with sound evidence. Currently, it is unclear whether HRQoL varies by primary intervention and (if yes) which primary intervention is superior with respect to long-term HRQoL in PC patients. Additionally, studies should indicate clinical meaningfulness in addition to statistically significant differences, in order to better inform patient/caregiver decision-making.
Additionally, when HRQoL is assessed, domains other than physical well-being and PC specific problems (e.g. incontinence or impotence) should be addressed, as differences occurred in various scales.
Change history
07 August 2018
The original article [1] contains errors whereby some information provided in Tables 2 and 5 in the online version is missing in the PDF version; in addition, some details regarding the study by Mols et al., Johnstone et al. and Fransson et al. (2008) in Tables 1 and 5 require correction.
Abbreviations
- ADT:
-
Androgen deprivation therapy
- AS:
-
Active surveillance
- BT:
-
Brachytherapy
- CK:
-
Cyberknife
- CRYO:
-
Cryotherapy
- CT:
-
Chemotherapy
- EBRT:
-
External beam radiation therapy
- HRQoL:
-
Health-related quality of life
- PC:
-
Prostate cancer
- QoL:
-
Quality of life
- RCT:
-
Randomized controlled trial
- RP:
-
Radical prostatectomy
- WW:
-
Watchful waiting
References
American Cancer Society. Global Cancer Facts & Figures. Am Cancer Soc. 2015;3(Edition(700)):1–57. https://doi.org/10.1002/ijc.27711.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403. https://doi.org/10.1016/j.ejca.2012.12.027.
National Cancer Institute. Cancer trends progress report. National Cancer Institute; 2012. https://progressreport.cancer.gov/after/survival#field_most_recent_estimates. Accessed 20 Feb 2017.
Sant M, Minicozzi P, Primic-Žakelj M, Otter R, Francisci S, Gatta G, Berrino F, De Angelis R. Cancer survival in Europe, 1999–2007: doing better, feeling worse? Eur J Cancer. 2015;51:2101–3.
National Coalition of Cancer Survivorship. The NCCS Definition of a “Cancer Survivor.” http://www.canceradvocacy.org/news/defining-cancer-survivorship/. Accessed 25 June 2015.
American Cancer Society. Cancer facts and figures—2000. Atlanta: American Cancer Society; 2000.
Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932–42. https://doi.org/10.1056/NEJMoa1311593.
Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377(2):132–42. https://doi.org/10.1056/NEJMoa1615869.
Sooriakumaran P, Nyberg T, Akre O, et al. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. BMJ. 2014;348:g1502.
Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. https://doi.org/10.1016/j.eururo.2010.10.039.
Gomella LG, Johannes J, Trabulsi EJ. Current prostate cancer treatments: effect on quality of life. Urology. 2009;73(5 SUPPL):S28–35.
Ferrell BR, Grant MM, Funk B, Otis-Green S, Garcia N. Quality of life in breast cancer survivors as identified by focus groups. Psychooncology. 1997;6(1):13–23. https://doi.org/10.1002/(SICI)1099-1611(199703)6:1<13::AID-PON231>3.0.CO;2-S.
Velikova G, Stark D, Selby P. Quality of life instruments in oncology. Eur J Cancer. 1999;35(11):1571–80. https://doi.org/10.1016/S0959-8049(99)00193-8.
Iran B, Wilson PDC. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65.
Deimling GT, Sterns S, Bowman KF, Kahana B. The health of older-adult, long-term cancer survivors. Cancer Nurs. 2005;28(6):415–24.
Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials. JAMA. 2013;309(8):814. https://doi.org/10.1001/jama.2013.879.
Efficace F, Osoba D, Gotay C, Sprangers M, Coens C, Bottomley A. Has the quality of health-related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Ann Oncol. 2006;18(4):775–81. https://doi.org/10.1093/annonc/mdl494.
Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–24. https://doi.org/10.1056/NEJMoa1606220.
Drummond FJ, Kinnear H, Leary EO, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. In: Results from the PiCTure (prostate cancer treatment, your experience) study; 2014. https://doi.org/10.1007/s11764-014-0419-6.
Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888–92. https://doi.org/10.1093/jnci/djp114.
Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. Br Med J. 2009;339:12. https://doi.org/10.1136/bmj.b4817.
Tobergte DR, Curtis S. Systematic reviews - CRD’s guidance for undertaking reviews in health care, vol. 53; 2013. https://doi.org/10.1017/CBO9781107415324.004.
Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data. JAMA. 2015;313(16):1657. https://doi.org/10.1001/jama.2015.3656.
Kassianos AP, Raats MM, Gage H, Peacock M. Quality of life and dietary changes among cancer patients: a systematic review. Qual Life Res. 2014;24(3):705–19. https://doi.org/10.1007/s11136-014-0802-9.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Gandek B, Sinclair SJ, Kosinski M, Ware JE. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5–25.
Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. https://doi.org/10.1200/jco.1993.11.3.570.
Postma R. Treatment of prostate cancer. Ann Oncol. 2006;17(10):207–10. https://doi.org/10.1093/annonc/mdl261.
Prostate Cancer Foundation. What is Active Surveillance? https://www.pcf.org/c/active-surveillance/. Accessed 13 Nov 2017.
Henson CC, Burden S, Davidson SE, Lal S. Nutritional interventions for reducing gastrointestinal toxicity in adults undergoing radical pelvic radiotherapy. In: Henson CC, editor. Cochrane database of systematic reviews. Chichester: Wiley; 2013. https://doi.org/10.1002/14651858.CD009896.pub2.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–44.
Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution- and anchor-based approaches in interpretation of changes in health-related quality of life. Med Care. 2001;39(10):1039–47. https://doi.org/10.1097/00005650-200110000-00002.
Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Bost New Engl Med Cent. 1993:1 v. (various pagings).
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924.
Berg A, Lilleby W, Bruland OB, Fossa SD. 10-year Sruvival and quality of life in PAtients with high-risk pN0 prostate cancer following definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(4):1074–83. https://doi.org/10.1016/j.ijrobp.2007.04.031.
Brundage M, Sydes MR, Parulekar WR, Warde P, Cowan R, Bezjak A. Impact of radiotherapy when added to androgen deprivation therapy for locally advanced prostate cancer: long-term quality-of-life outcomes from the NCIC CTG PR3/MRC PR07 randomized trial. J Clin Oncol. 2015;33(19):2151–7. https://doi.org/10.1200/JCO.2014.57.8724.
Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–37. https://doi.org/10.1056/NEJMoa1606221.
Fransson P. Patient-reported lower urinary tract symptoms, urinary incontinence, and quality of life after external beam radiotherapy for localized prostate cancer – 15 years’ follow-up. A comparison with age-matched controls. Acta Oncol (Madr). 2008;47:852–61. https://doi.org/10.1080/02841860701654325.
Fransson P, Damber J, Widmark A. Health-related quality of life 10 years after external beam radiotherapy or watchful waiting in patients with localized prostate cancer. Scand J Urol Nephrol ISSN. 2009;43:119–26. https://doi.org/10.1080/00365590802519396.
Galbraith ME, Arechiga A, Ramirez J, Pedro LW. Prostate cancer survivors’ and partners’ self-reports of health-related quality of life, treatment symptoms, and marital satisfaction 2.5-5.5 years after treatment | ONF. Oncol Nurs Forum. 2005;32(2):E30–41.
Giberti C, Chiono L, Gallo F, Schenone M, Gastaldi E. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: a prospective study. World J Urol. 2009;27(5):607–12. https://doi.org/10.1007/s00345-009-0418-9.
Johnstone PAS, Gray C, Powell CR. Quality of life in T1-3N0 prostate cancer patients treated with radiation therapy with minimum 10-year follow-up. Int J Radiat Oncol Biol Phys. 2000;46(4):833–8.
Mols F, Van De Poll-Franse LV, Vingerhoets AJJM, et al. Long-term quality of life among Dutch prostate cancer survivors: results of a population-based study. Am Cancer Soc. 2006;107(9):2186–96. https://doi.org/10.1002/cncr.22231.
Namiki S, Tochigi T, Ishidoya S, Ito A, Numata I, Arai Y. Long-term quality of life following primary treatment in men with clinical stage T3 prostate cancer. Qual Life Res. 2011;20(1):111–8. https://doi.org/10.1007/s11136-010-9721-6.
Namiki S, Kaiho Y, Mitsuzuka K, Saito H, Yamada S, Nakagawa H. Original Article : clinical investigation long-term quality of life after radical prostatectomy : 8-year longitudinal study in Japan. Int J Urol. 2014;21:1220–6. https://doi.org/10.1111/iju.12586.
Shinohara N, Maruyama S, Shimizu S, et al. Longitudinal comparison of quality of life after real-time tumor-tracking intensity-modulated radiation therapy and radical prostatectomy in patients with localized prostate cancer. J Radiat Res. 2013;54(6):1095–101. https://doi.org/10.1093/jrr/rrt049.
Thong MSY, Mols F, Kil PJM, Korfage IJ, Van De Poll-franse LV. Prostate cancer survivors who would be eligible for active surveillance but were either treated with radiotherapy or managed expectantly: comparisons on long-term quality of life and symptom burden. BJU Int. 2009;105:652–8. https://doi.org/10.1111/j.1464-410X.2009.08815.x.
Klein, Eric A, Vogelzang, Nicholas, Lee, Robert W, Richie, Jerome P, Ross, MER. Patient education: Prostate cancer treatment; stage I to III cancer. 2017. https://www.uptodate.com/contents/prostate-cancer-treatment-stage-i-to-iii-cancer-beyond-the-basics. Accessed 12 Oct 2017.
American Cancer Society. Prostate Cancer Stages. 2016. https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/staging.html. Accessed 12 Oct 2017.
Ferrell BR, Hassey Dow K, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4(6):523–31. https://doi.org/10.1007/BF00634747.
Ware JE, Kosisnki M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston: The Health Institute, New England Medical Center, Second Edition; 1995.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JCJM, Kaasa S, Klee MC, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw KCA, Sullivan M, Takeda F. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-76.
Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA prostate cancer index: development, reliability, and validity of a health-related quality of life measure on JSTOR. Med Care. 1998;36(7):1002–12.
van Andel G, Bottomley A, Fosså SD, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44(16):2418–24. https://doi.org/10.1016/j.ejca.2008.07.030.
Fransson P, Tavelin B, Widmark A. Reliability and responsiveness of a prostate cancer questionnaire for radiotherapy-induced side effects. Support Care Cancer. 9(3):187–98. https://doi.org/10.1007/S005200000146.
Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Prostate cancer index composite (Epic) for comprehensive assessment of health-related. Urology. 2000;56(6):899–905. https://doi.org/10.1016/S0090-4295(00)00858-X.
Barry MJ, Fowler FJ, O’Leary MP, et al. The American urological association symptom index for benign prostatic hyperplasia. The measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–57. discussion 1564
Lenderking R, Ph D, Barber B, et al. * a brief male sexual function inventory for urology - inventory. p. 697–706.
Korfage IJ, Essink-Bot ML, Madalinska JB, Kirkels WJ, Litwin MS, de Koning HJ. Measuring disease specific quality of life in localized prostate cancer: the Dutch experience. Qual Life Res. 2003;12(4):459–64.
Rosen, Wagner, Riley. A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(97):822–30.
Donovan JL, Peters TJ, Abrams P, Brookes ST. De aa rosette JJ, Schäfer W. Scoring the short form ICSmaleSF questionnaire. International continence society. J Urol. 2000;164(6):1948–55.
Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322–30. https://doi.org/10.1002/nau.20041.
Moinpour CM, Hayden KA, Thompson IM, Feigl P, Metch B. Quality of life assessment in southwest oncology group trials. Oncology (Williston Park). 1990;4(5):79–84. 89; discussion 104
Costantini M, Musso M, Viterbori P, et al. Detecting psychological distress in cancer patients: validity of the Italian version of the hospital anxiety and depression scale. Support Care Cancer. 1999;7(3):121–7.
Marenghi C, Bellardita L, Rancati T, et al. Active surveillance in patients with low-risk prostate cancer: PRIAS experience at the national cancer institute (Milan). Anticancer Res. 2011;31:1944–5.
Whiting PF, Moore THM, Jameson CM, et al. Symptomatic and quality-of-life outcomes after treatment for clinically localised prostate cancer: a systematic review. BJU Int. 2016;118(2):193–204. https://doi.org/10.1111/bju.13499.
Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53(1):68–80. https://doi.org/10.1016/j.eururo.2007.09.002.
Scott N, Fayers P, Aaronson N, et al. EORTC QLQ-C30 Reference Values. 2008;(July):419.
Walters SJ. Sample size and power estimation for studies with health related quality of life outcomes : a comparison of four methods using the. 2004;17:1-17.
Acknowledgements
The work was supported by the Epidemiological, Biostatics and Prevention Institute (EBPI), the National Institute of Cancer (NICER) and Swiss Bridge. The authors would also like to thank Martina Gosteli, from UZH Library for supporting the literature search and Ali Weihofen for language editing the manuscript.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Contributions
SaA and VA developed the concept and study protocol of this systematic review. The eligibility and selection of articles were assessed by screening records based on title/abstract review by one author (SaA) and assessing the full-texts according to predefined hierarchical ordered inclusion and exclusion criteria by two reviewers (SaA and AF). SaA, AF, SR, and VA read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Appendices. (DOCX 41 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Adam, S., Feller, A., Rohrmann, S. et al. Health-related quality of life among long-term (≥5 years) prostate cancer survivors by primary intervention: a systematic review. Health Qual Life Outcomes 16, 22 (2018). https://doi.org/10.1186/s12955-017-0836-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12955-017-0836-0