Abstract
Background
E-cigarettes (electronic nicotine delivery system, ENDS) have been presented as a harm reduction strategy for people who smoke tobacco cigarettes but who cannot achieve abstinence, or for those who wish to continue to enjoy nicotine and the habit of smoking. What are the health effects of the substitution of ENDS for tobacco cigarettes? This systematic review evaluates the evidence of human clinical tests on the respiratory effects of ENDS use in participants who smoke tobacco cigarettes.
Methods
A registered and published protocol was developed conforming to PRISMA 2020 and AMSTAR2 standards. The literature search was conducted in PubMed, Scopus, and the CENTRAL Cochrane Library and updated to May 2022. Three supplementary searches and a grey literature search were performed. Studies were evaluated with the JBI quality tools and the Oxford Catalogue of Bias. Due to the heterogeneity (diversity) of the studies, a narrative data synthesis was performed on the test findings plus three sub-group analyses.
Results
The review consists of sixteen studies and twenty publications. Spirometry tests comprised the majority of the data. In total, 66 respiratory test measurements were reported, out of which 43 (65%) were not significant. Statistically significant findings were mixed, with 9 tests showing improvements and 14 measuring declines, none of which was clinically relevant. Ten studies were rated at a high risk of bias, and six had some concerns primarily due to inadequate research designs and the conduct of the studies. Reporting bias was documented in thirteen studies.
Conclusions
Most of the studies showed no difference in respiratory parameters. This indicates that ENDS substitution for smoking likely does not result in additional harm to respiratory health. Due to the low quality of the studies, confidence in the conclusions is rated as low. Robust studies with a longer duration and sufficient power are required to validate any potential benefits or possible harms of ENDS substitution.
Registration PROSPERO #CRD42021239094, International Registered Report Identifier (IRRID): DERR1-10.2196/29084.
Similar content being viewed by others
Introduction
Tobacco use annually causes over 8 million deaths and a loss of 150 million disability-adjusted life-years [1]. Smoking is the attributable mortality risk factor for many respiratory diseases [2]. Some researchers claim that e-cigarettes are potentially safer than smoking [3,4,5,6] and, therefore, could be a harm-reduction tool. E-cigarettes are called electronic nicotine delivery systems (ENDS); they are also called vapes, vape pens, tanks, mods, pod-mods, and JUUL. A review by the US National Academies of Sciences Engineering and Medicine states, “There is substantial evidence that except for nicotine, under typical conditions of use, exposure to potentially toxic substances from e-cigarettes is significantly lower compared with combustible tobacco cigarettes” [7]. The Royal College of Physicians (UK) and a number of researchers encourage people who smoke to switch from cigarettes or other combustible tobacco products to what they have evaluated as the less toxic and potentially safer ENDS [8,9,10]. While much research has focused on ENDS as a cessation tool [11], for people who do not wish to quit consuming nicotine, the substitution of ENDS may be a tobacco harm reduction option [12, 13].
To weigh the potential benefits and risks of ENDS substitution for tobacco smoking, we conducted a systematic review to answer the question, “What are the respiratory health effects, both acute and longer-term, resulting from the substitution of ENDS for tobacco cigarettes?” Our systematic review aims to critically assess and synthesize the available human clinical studies on the respiratory health effects of ENDS substitution by people who smoke.
Methods
Our research question is structured with PICOS framing (Population, Intervention, Comparator, Outcome, Studies) as follows:
-
Population: adults who smoke tobacco cigarettes.
-
Intervention: substitution of ENDS for cigarettes.
-
Comparator: either within-subject changes or comparison to participants who continue to smoke.
-
Outcomes: changes in baseline to post-intervention test measurements from spirometry tests (FEV1, FVC, FEF25–75, PEF, FEV1/FVC%), impulse oscillometry, and lung function tests (total lung capacity, residual volume, and expiratory reserve volume).
-
Studies: randomized controlled trials, quasi-experimental clinical trials, and longitudinal cohort studies.
This review adheres to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 [14]. The protocol for this systematic review has been registered with PROSPERO #CRD42021239094 and has been published in a peer-reviewed journal [15]. The PRISMA 2020 checklist is in Additional file 1: Table S1. Below is an overview of how the review was conducted. A more detailed description of the search and study selection processes is available in the published protocol [15].
Search strategy
The database search was performed on January 31, 2021, with updates on April 29, 2021, and May 18, 2022. The publication date delimiter for the database search was 2010, and the databases used were Scopus, PUBMED, and the CENTRAL Cochrane Library. The search terms “electronic nicotine” AND “e-cigarette” were combined with OR for the respiratory keywords “respiratory,” “lungs” and “pulmonary.” The search syntaxes are displayed in Additional file 1: Figure S1. Common terms for ENDS (“Juul” “vaping”) were tested as keywords but did not retrieve any additional studies for inclusion. Keyword “vap*” was not used because it yielded thousands of false retrievals of chemistry studies.
Three secondary searches were conducted in February 2021. First, the reference lists of systematic and narrative reviews published since 2018 were examined for additional studies. Next, a secondary literature search was conducted in Google Scholar. Two experts in the field of ENDS research reviewed the list of included studies. Finally, a grey literature search was conducted at the websites of 53 respiratory medical organizations (listed in Additional file 1: Table S2).
Inclusion, exclusion, and study eligibility criteria
Study designs included in the review were human subject research with randomized and non-randomized controlled trials, clinical trials, prospective and retrospective cohort studies, and case–control studies. The first exclusion of articles was conducted on titles, and where a title was not sufficient for a determination, the abstract was reviewed. In vitro (cell), animal, and cross-sectional studies were excluded.
The second process of inclusion was a full paper review. Three inclusion criteria were applied. One, a study had to be one of the research designs listed above. Two, a study was required to have either a comparator group who smoked tobacco (cigarettes) or within-subject testing of participants who had substituted ENDS for smoking. Third, the study had to report an outcome of a respiratory test. All three criteria had to be satisfied for a study to be included.
The inclusion and exclusion of studies were conducted independently by two reviewers after training, and initial discrepancies were resolved by discussion. Where agreement could not be reached, the Project Leader made the final decision. Inter-rater reliability was 98% for title sorting and 95% for full paper review.
Data extraction
The data extraction process was conducted independently by two reviewers after training using a pre-specified data extraction form drawn from the JBI Manual [16] and the Cochrane Collaboration Handbook [17]. Any discrepancies in data extraction were resolved by discussion.
Quality assessment and risk of bias
Two independent reviewers assessed the study quality using the JBI quality assessment tools [18] and a report list of biases drawn from the Oxford Centre for Evidence-Based Medicine Catalogue of Bias [19] further supplemented with our teams’ prompt questions. In the case where multiple articles were published on one study, each article was assessed separately. Interim publications of longer duration studies were not assessed for quality, but were referenced for data as necessary. Discrepancies were resolved by discussion.
The overall rating of study quality consisted of a combination of the JBI score and the biases report. Studies were rated in three classifications from the Cochrane guidelines [17]: low risk of bias, some concerns of bias, and high risk of bias. The rater (RO) was blinded to study outcomes and funders. The final rating was endorsed by the team members who conducted the JBI and bias assessments. The rating rubric is in Additional file 1: Table S3.
Data analysis and synthesis
As per protocol, we conducted a narrative synthesis by study design, population, test measurements, and biases.
A meta-analysis was not conducted due to the heterogeneity between the studies. These differences across studies included the ENDS nicotine strength, the ENDS models, wide disparities in study populations, and differing tests.
Three sub-group analyses of testing measurements were conducted for (1) concurrent use of ENDS and cigarettes (dual use), (2) populations with prior disease conditions, and (3) ENDS use of a duration of 1 year or longer.
Sensitivity analyses were conducted. One excluded all studies at high risk of bias. The second was on the effect modifications on findings. Finally, the certainty of the evidence was evaluated with Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [20, 21].
Deviations from protocol
There were a few deviations from the protocol. Due to journal word count limits and reporting needs, we excluded the narrative summary of individual studies. A sensitivity analysis for conflict of interest for industry-funded studies was not conducted because all industry studies were at high risk of bias (independently of their industry funding). An analysis of effect modifications was added to conform to PRISMA 2020 requirements. Because no meta-analysis was conducted, a formal assessment of publication bias could not be performed. The planned data repository was changed.
As per protocol, we have transitioned the review from a living systematic review (ongoing searches and updates) to a completed systematic review with the final search date of May 2022 because of the insufficient number of new studies published. Only one new article for inclusion was published in the 18 months after the baseline search, so the living component is not justified at this time.
Results
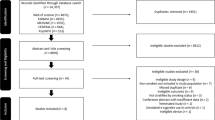
The search results are reported in the PRISMA 2020 flow diagram, Additional file 1: Figure S1. Publications excluded at full paper review, including “near misses” [14], are listed in Additional file 1: Table S4 with their reason for exclusion.
Our systematic review retrieved sixteen studies [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] with twenty publications [38,39,40,41]. One of the studies had two publications with different analyses [34, 38] so both were referenced together. Three studies [39,40,41] were interim publications of longer-term studies [32, 33]; only the final results were included in the analysis. Basic information on the studies and publications are reported in Table 1 Characteristics of studies. The studies were conducted in Greece (5), United Kingdom (4), USA (3), Italy (2), and one each from Belgium and Hungary. The participants ranged in age from 18 to 73, comprising 1,357 participants who smoked. Six studies included participants with asthma or chronic obstructive pulmonary disease (COPD). Seven studies had acute testing data; nine studies presented follow-up data ranging from 5 days to 5 years. Ten studies were rated at high risk of bias, six were rated as some concerns, and zero studies were rated as at low risk of bias, see Table 2.
Narrative synthesis: test findings
The test findings are for within-subject changes from baseline to final test measurement after ENDS substitution. Subgroup results are indicated in parentheses. Test measurements reaching statistical significance are reported in Additional file 1: Table S5 Statistically significant test measurements pre/post-test—acute studies and Additional file 1: Table S6 for follow-up studies.
Forced expiratory volume in 1 s (FEV1)
The FEV1 test measures the volume of air that is forced out of the lungs in 1 s; it can assess the severity and development of COPD [42, 43]. Twelve studies tested FEV1, five acute (one data not reported), and seven follow-up. Four acute tests were nonsignificant [27, 29, 30 (cigarettes), 30 (asthma)] and one acute study reported a significant decrease [23]. Three follow-up studies found no significant changes [25, 36, 38], four found statistically significant improvement [24, 26, 32, 33] and one reported a significant decrease [37].
Forced vital capacity (FVC)
FVC is the total volume of air that can be exhaled during a maximally forced expiration effort [44]. Twelve studies tested FVC within-subject. All five acute tests were nonsignificant [23, 27, 29, 30] (cigarettes), 30 (asthma)]. In the follow-up studies, three reported no significant effects [25, 36, 38], four found a significant increase [24, 26, 32, 33], and one [37] had a statistically significant decrease.
FEF25–75
The FEF25–75 is the average flow starting from the point at which 25% of the FVC has been exhaled to the point at which 75% has been exhaled [44]. It is potentially a sensitive marker of obstructive peripheral airflow [45]. Six studies conducted this test. One acute test was nonsignificant [27]. In the follow-up studies, three tests were nonsignificant [25, 36, 38], one indicated benefit [33] and one had a statistically significant decline [37].
Peak expiratory flow (PEF)
Peak expiratory flow (PEF) is the maximum airflow generated during a forceful exhalation, starting from full lung inflation [46]. Ten studies conducted this test. Four acute studies measured PEF; three tests had non-significant results [23, 27, 30] cigarettes] and a significant decline in two studies [29, 30] asthma]. Five follow-up studies showed no significant impact of ENDS use on PEF [24, 25, 28, 36, 38] and one had a significant decline [37].
FEV1/FVC%
The FEV1/FVC% is the percentage of the FVC expired in 1 s [44]. It is an indicator of obstructive defects, restrictive or mixed patterns of deteriorating lung function [47, 48]. Eight studies calculated this measurement. One acute study found a significant decrease [30] asthma] and three tests were not significant [27, 29, 30] cigarettes] and five follow-up studies had results that were not significant [24, 32, 33, 36, 38].
Impulse oscillometry (IOS)
The IOS test measures resistance to airflow and is more sensitive than spirometry for measuring peripheral airway disease [49]. Only the acute studies conducted this testing. Three studies found increased resistance with acute ENDS use [30, 31, 35], and in one study, test measurements were not significant after 30 min [22]. Three studies tested IOS on participants with asthma; two showed significant declines [30, 31] and one had no significant changes [22]. One study testing participants with COPD had nonsignificant test results [31].
Other lung function tests
Other tests were conducted in the acute studies. One test was total lung capacity, the volume of air in the lungs at maximal inflation [44]; one acute study [23] observed no significant changes in this test. Three tests conducted in two acute studies [23, 30] found no significant effects on Residual Volume, the volume of gas in the airways after maximal exhalation. One acute study [30] testing of Expiratory Reserve Volume, the volume of gas maximally exhaled after end-inspiratory tidal breathing [50] observed no significant changes for participants who smoked or for those with asthma.
Tabulation of testing findings
In summary, 66 test measurement findings were reported in the studies, out of which 43 (65%) were not significant. Significant findings were mixed, with 14 measuring declines and 9 showing improvement in lung function. A sensitivity analysis excluded the studies at high risk of bias, and the percentages were very similar for studies rated at some concerns (none industry-funded). None of the statistically significant test measurements was clinically relevant. See Additional file 1: Tables S5 and S6.
Narrative synthesis: sub-group analyses
Dual use
Six studies evaluated differences in respiratory function between participants who exclusively used ENDS and those who used both ENDS and cigarettes (dual use). Four studies found no significant differences or improvements in those with dual use [25, 26, 34, 36]. Studies by Polosa et al. on asthma [33] and on COPD [32] observed that those who used ENDS exclusively had significant improvements in lung function tests FEV1, FVC and FEF25–75. While participants with dual use also showed improvements in these studies, the improvements were not as great compared to participants with exclusive ENDS.
Populations with underlying disease
Studies included participants with asthma, COPD, and serious mental illness.
Four follow-up studies were conducted with participants with various severities of asthma, with mixed findings. In these studies, participants with asthma with dual use showed improvement in all lung function tests, except the FVC test with exclusive ENDS use.
In one study [33], patients with mild to moderate asthma using ENDS on at least two consecutive follow-up visits over 24 months showed significant improvements in lung function tests FEV1, FVC, FEF27–75 for both exclusive and dual ENDS use, both groups of participants experiencing fewer exacerbations of asthma. Additional evidence from this study supported that ENDS substitution resulted in improvements: two patients who relapsed to smoking after ENDS use experienced worsening of their asthma outcomes. The study’s small sample size of 16 participants limits the confidence in these findings.
Three of four acute studies measuring IOS in participants with moderate asthma (N = 63) showed increased airway resistance with ENDS use [30, 31, 35], and one found no significant change [22]. These findings suggest possible airway irritation from ENDS use, but the test measurements in all three studies were not clinically relevant.
Only two studies were conducted with participants with COPD. A 5-year study of 39 patients with COPD [32] demonstrated significant improvements in participants with exclusive ENDS use aged 66.9 (± 5.8) that demonstrates that in older age, switching to ENDS may result in improvements in lung function over a longer period of time. In the other study [31], airway resistance in 16 patients with COPD after 10 min of ENDS use did not produce significant changes.
A cessation study [28] of patients with a serious mental illness found no clinically significant changes in their respiratory tests with ENDS substitution.
ENDS usage > 1 year
Three studies [32, 33, 37] with a longer duration of ENDS use indicated improved lung function in healthy participants and for those with an underlying health condition of COPD or asthma.
GRADE
The certainty of the evidence was moderate to low in the acute studies and moderate to very low in the follow-up studies. Overall, the confidence in the evidence was rated as low. Ten RCTs and clinical trials were reduced to low confidence due to multiple risks of bias. Four RCTs and clinical trials were rated at some concerns, lowering their assessment to moderate. The two cohort studies were assessed as some concerns of bias, lowering their certainty to very low confidence. See Additional file 1: Table S7 for GRADE rating.
Discussion
Summary of main results
The 16 studies in this review conducted a total of 66 respiratory test measurements. No significant differences were reported in 43 tests (65%) between ENDS use and cigarette use. Nine follow-up studies found improvements in lung function tests. Declines in lung function tests were reported in 14 tests, 10 of which were from the acute studies, and all negative test results were from studies rated at high risk of bias. None of the statistically significant results indicated clinically relevant changes in lung functioning.
Findings on the respiratory health effects of ENDS substitution for smoking varied by health status and by the duration of ENDS use. For participants without respiratory disease, the acute studies did not show a clinically meaningful worsening of pulmonary function with ENDS use. Four acute and five short-term studies recorded no changes in healthy participants using ENDS. Also, one short-term study showed a decline in respiratory functioning in participants after they reverted from ENDS to cigarettes.
However, for participants with respiratory illnesses, the findings were mixed. For participants with asthma, two acute studies found a worsening of pulmonary function [30, 31], and one reported no significant change [22]. Yet these findings were not confirmed by a 24-month follow-up study [33] that observed no decline in respiratory functioning in participants with diagnosed asthma using ENDS and instead reported statistically significant improvement. Two studies were conducted with participants with COPD but the studies’ durations were diametrically different. A 5-year study showed significant test score improvements in patients with COPD who switched to ENDS [32] while an acute study reported no significant effects of ENDS use on COPD [31].
Effect modification
A major problem with the findings is that the studies were not of sufficient duration. The beneficial effects of quitting cigarette use on lung function are not immediate and may take up to 2 years to manifest [51]. After stopping cigarette use, it takes 3 months for a reduction in the presence and severity of respiratory symptoms, 1 year for improvements in airway inflammation, and 8 years for improvements in lung diffusion capacity [52]. It is worth noting that improvements in spirometry testing can occur due to participants’ familiarity with the testing process rather than a clinically relevant improvement [53].
The duration of cessation is critical to accurately interpreting the results of the FEV1 test because improvements or lower rates of decline do not occur until after 1 year of cigarette abstinence [51, 52]. Nine studies conducted FEV1 tests, but with a duration of less than a year. Three studies conducted this test after at least 1 year of ENDS use and two reported statistically significant improvement in the test results [32, 33] and one found a significant decrease [37].
Improvement in symptoms after quitting cigarettes takes even longer for patients with respiratory diseases [54]. Lung function for COPD patients who stop smoking never improves; the loss of function is irreversible, and cessation can only help prevent further progression of the disease [51]. Evidence of the effects of ENDS substitution cannot be obtained from short-term studies if the duration does not account for recovery periods [55].
Another concern with the results of FEV1 tests is the age of the participants. The FEV1 test can measure improvements in those who stop cigarette use before age 30, but those who stop smoking after age 40 will show declines in FEV1 measurements that are not significantly different from those who continue to smoke [51]. Three studies had large age ranges in the participants. Seven studies included participants aged 30 (± 15), and two studies had participants aged 40 and above. Seven studies were conducted with participants 30 years old and younger. None of the authors accounted for the age of their participants as an effect modification of their findings for this spirometry test.
Quality and bias assessments
One of the key observations of this review is the poor quality of much of the research literature, with ten of sixteen studies rated at high risk of bias and no studies rated at low risk of bias. Without discussing every item, we report below on the major areas of concern for biases in the research design, the conduct of the study, and reporting.
Study design
Blinding is a basic component of clinical research, where participants, clinicians, and researchers are prevented from knowing the intervention (or treatment); the participant receives in order to avoid the introduction of bias. With some studies, the blinding of participants is not possible because the difference appearances of ENDS and cigarettes is obvious, plus the lack of vapor with sham vaping is easy to identify. Yet it is possible to blind participants and clinicians to nicotine strengths or no nicotine, as was done by Veldheer et al. [36]. Researchers performing the statistical analyses can easily be blinded from identifying the intervention group of the individual participants. In five of the seven RCTs, blinding was not performed. See Table 2 JBI assessment and study biases.
Follow-up duration was a major limitation in study design. The seven acute studies per force and three follow-up studies [24, 26, 38] had a duration of less than 3 months. These studies have limited relevance for observing the potential effects of ENDS substitution because improvements in respiratory function take a minimum of 3 months to show benefit from cessation, and 2 years or more of cessation are needed for improvements in respiratory function (see Effect modification above).
Study conduct
A red flag in clinical research is unreported deviations from the study protocol (plan) because it may be an indication of the potential “cooking” of data to obtain desired or favorable results [56,57,58]. Two research teams did not indicate if they had a protocol [27, 29], and one had an unpublished protocol [24]. Four studies with protocols had discrepancies from the research design [25, 26, 28, 36], three of which we considered serious.
Another source of error in the findings is compliance bias—differences in subjects’ adherence to the planned treatment regimen or intervention [59]. Compliance bias was detected in six out of sixteen studies. Many participants continued to smoke even when they were instructed or “encouraged” not to [25, 37]. Four follow-up studies [24, 32, 33, 36] failed to report if the participants experienced adverse effects with ENDS use therefore it is not possible to determine if any of their participants curtailed ENDS use.
Reporting bias
Reporting bias is scientific misconduct. It happens from the selective reporting of results and excluding or concealing data [60,61,62,63]. Reporting bias also occurs when the study authors manoeuvre their discussion only to sources that conform to their desired conclusions [64, 65]. Reporting bias was detected in thirteen studies.
Most egregious, several studies published selective test results or did not provide actual pulmonary test measurements. Several authors characterized test results as “not significant” without reporting any data. Some figures in the articles included only p values (a probability statistic) or average differences between ENDS and cigarette user groups, but not the actual test measurement data.
In several studies, the authors manipulated their discussions or conclusions. In four studies [25, 27, 28, 32], the authors’ discussion presented the assumption that all other studies were in accord with their findings, that only one position exists (all’s well literature bias [66, 67]). In six studies [22,23,24,25, 30, 31], the authors offered only studies in support of their findings (one-sided reference bias [68]). Some authors unevenly highlighted one side of their study with the framing effect of language focused on the loss of health or risks [69,70,71].
As for conclusions, in eight studies, the conclusion was based on secondary endpoints (i.e. not the primary outcome) having no clinical significance. In the conclusions of five studies, there was an over-reliance on the statistical significance of p values [72] although the test results were not clinically relevant. See Table 2 JBI assessments and study biases.
Comparison to other systematic reviews
Six systematic reviews published since 2020 have covered studies on the respiratory effects of ENDS, but their analysis does not match up with ours because of the very different types of studies they included. This makes comparing their conclusions with our conclusions untenable. The one partially comparable systematic review is the Larue et al. [73] meta-analysis based on 17 studies of acute respiratory outcomes from good-quality-rated cross-over studies and randomized parallel group studies. In accord with our findings, their meta-analysis did not find significant changes in spirometry tests with ENDS use.
Two systematic reviews conducted meta-analyses of cross-sectional studies (population surveys). Goniewicz et al. [74] examined two cross-sectional studies and one longitudinal population study on the respiratory effects of ENDS substitution. Their meta-analysis calculated ENDS substitution as producing a ~ 40% lower odds of negative respiratory outcomes of COPD, asthma, chronic bronchitis, emphysema, and wheezing. The meta-analysis of Chand and Hosseinzadeh [75] was comprised of 13 cross-sectional studies and calculated a significant association of between current e-cigarette use and asthma. Our review based on respiratory testing did not validate either the substantial benefit or the negative association with ENDS use. As is well established, cross-sectional studies are evidence of a possible association, but not causation.
Wills et al. [76] conducted a meta-analysis of human epidemiological studies. Eleven of the 15 asthma studies were with adolescent populations; they may not be indicative of outcomes for adults because adolescent asthma is known to go into remission in adulthood [77]. Their analysis incorporated studies of ENDS use on participants who had never smoked. Our PICO specified adults who smoke, so their conclusions are based on findings that do not match up with our study population.
Finally, the systematic reviews of Bozier et al. [78] and Bravo-Gutiérrez et al. [79] each anchored their conclusions in the evidence from in vitro (cell) and rat studies as well as including cross-sectional studies. We excluded in vitro studies because they “may not directly translate to adverse effects relevant to disease outcomes” in tobacco research [55]. As for animal tests on ENDS, almost all on rats, this study design does not reflect real life-use or human exposure levels as the rats’ exposure to ENDS is administered via intra-tracheally or nasally administered liquids or whole-body aerosol exposure [8]. Furthermore, respiratory studies on rodents have been dismissed by some researchers as not relevant to humans [80]. Because their analyses include non-human studies, these two systematic reviews are not comparable with ours.
Recommendations for future research
Like other researchers of ENDS, our call is for longer-duration studies. Improved study quality is critical, requiring that research is conducted with an adequate number of participants. For statistical precision, future longitudinal studies should assess and stratify the results by smoking behaviour and history. Given the issues with treatment fidelity, exclusive ENDS use and dual use with cigarettes should be identified as separate categories. Reporting biases must be rooted out, either by the authors or by peer reviewers.
One concerning study design that should not be used is having ENDS users revert to smoking, as was done by Barna et al. [24]. This experimental design puts participants at risk for relapse to smoking.
Limitations
Our systematic review has limitations, some derive from the quality of the studies themselves, and others from our conduct of the review. The studies have many limitations. The majority of studies were rated at high risk of bias, and no studies were at low risk. More than half of the studies, ten out of sixteen, were conducted with small sample sizes limiting their conclusions and precluding generalizability. In addition, acute effects contributed little to identifying health outcomes, nor did findings with significant p values indicate clinically relevant outcomes.
The review had limitations in its conduct. First, the quality and bias assessments were labor intensive, and the findings required more discussion than anticipated. Second, we had expected to find sufficient new studies published to continue this systematic review in the living mode (regular, ongoing updates), but this was not the case. We believe that our 100% compliance with PRISMA 2020 and AMSTAR2 requirements has served us well in conducting a rigorous and transparent systematic review with strong validity and reliability.
Conclusions
Most of the studies showed no difference in respiratory parameters. Nearly two-thirds of the respiratory function tests found no significant effects of ENDS substitution for cigarette smoking. None of the statistically significant changes in test measurements was of clinical relevance. This indicates that ENDS substitution for smoking likely does not result in additional harm to respiratory health. Due to the high risk of bias and the small sample sizes in the majority of the studies, our certainty in this conclusion is low. Unfortunately, reporting spin is rampant, further eroding our confidence in the conclusions articulated by many of the study authors. To be able to inform policy and clinical practice, well done and robust studies are sorely needed to assess if ENDS substitution is a worthwhile harm reduction option for people who smoke.
Availability of data and materials
All data in this review were extracted from the original studies and available in the article. The data extraction forms and bias assessment reports are available in the Zenodo data repository https://zenodo.org/record/4835883#.YPnMqECxUuU.
Change history
13 March 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12954-024-00968-1
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- ENDS:
-
Electronic nicotine delivery system, e-cigarette
- RCT:
-
Randomized controlled trial
References
Britton J. Death, disease, and tobacco. Lancet. 2017. https://doi.org/10.1016/S0140-6736(17)30867-X.
Institute for Health Metrics and Evaluation. Findings from the global burden of disease study 2017. Seattle WA: IHME.
Marques P, Piqueras L, Sanz M-J. An updated overview of e-cigarette impact on human health. Respir Res. 2021. https://doi.org/10.1186/s12931-021-01737-5.
Hartmann-Boyce J, McRobbie H, Lindson N, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2021. https://doi.org/10.1002/14651858.CD010216.pub4.
Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019. https://doi.org/10.1056/NEJMoa1808779.
McNeill A, Brose L Calder R, Hitchman SC. E-cigarettes: an evidence update; a report commissioned by Public Health England. Report no. 2015260, August 2015. London: Public Health England.
National Academies of Sciences Engineering, and Medicine. Public health consequences of e-cigarettes. Washington DC: National Academies Press; 2018. p. 198.
Wang G, Liu W, Song W. Toxicity assessment of electronic cigarettes. Inhal Toxicol. 2019. https://doi.org/10.1080/08958378.2019.1671558.
Harrell PT, Marquinez NS, Correa JB, Meltzer LR, Unrod M, Sutton SK, Simmons VN, Brandon TH. Expectancies for cigarettes, e-cigarettes, and nicotine replacement therapies among e-cigarette users (aka vapers). Nicotine Tob Res. 2015. https://doi.org/10.1093/ntr/ntu.
Royal College of Physicians. Nicotine without smoke: tobacco harm reduction. London: RCP; 2016.
Hartmann-Boyce J, McRobbie H, Butler AR, Lindson N, Bullen C, Beg R, Theodoulou A, Notley C, Rigotti NA, Turner T, Fanshaw TR. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2021(9).
Notley C, Ward E, Dawkins L, Holland R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduct J. 2018. https://doi.org/10.1186/s12954-018-0237-7.
O’Leary R, Polosa R. Tobacco harm reduction in the 21st century. Drugs Alcohol Today. 2020. https://doi.org/10.1108/DAT-02-2020-0007.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/bmj.n71.
O’Leary R, Qureshi MA, La Rosa GRM, Vernooij RWM, Odimegwu DC, Bertino G, Polosa R. Respiratory and cardiovascular health effects of e-cigarette substitution: protocol for two living systematic reviews. JMIR Res Protoc. 2021. https://doi.org/10.2196/29084.
JBI Global. Critical appraisal tools. Adelaide: JBI Global; 2020.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions. Version 6.3, 2022. www.training.cochrane.org/handbook. Accessed 6 Jun 2022.
Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. Adelaide: JBI Global; 2020.
Centre for Evidence-Based Medicine. Catalogue of bias. https://catalogofbias.org/. Accessed 12 Jan 2021.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, Debeer H. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011. https://doi.org/10.1016/j.jclinepi.2010.04.026.
Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011. https://doi.org/10.1016/j.clinepi.2011.06.004.
Lappas AS, Tzortzi AS, Konstantinidi EM, Teloniatis SI, Tzavara CK, Gennimata SA, Koulouris NG, Behrakis PK. Short-term respiratory effects of e-cigarettes in healthy individuals and smokers with asthma. Respirology. 2018. https://doi.org/10.1111/resp.13180.
Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, Starczewska E, Briki R, de Hemptinne Q, Zaher W, Debbas N. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol. 2019. https://doi.org/10.1152/ajplung.00492.2018.
Barna S, Rózsa D, Varga J, Fodor A, Szilasi M, Galuska L, Garai I. First comparative results about the direct effect of traditional cigarette and e-cigarette smoking on lung alveolocapillary membrane using dynamic ventilation scintigraphy. Nucl Med. 2019. https://doi.org/10.1097/MNM.0000000000000957.
Cravo AS, Bush J, Sharma G, Savioz R, Martin C, Craige S, Walele T. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol. 2016. https://doi.org/10.1016/j.yrtph.2016.10.003.
D’Ruiz CD, O’Connell G, Graff DW, Yan XS. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regul Toxicol Pharmacol. 2017. https://doi.org/10.1016/j.yrtph.2017.05.002.
Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsaki AM, Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013. https://doi.org/10.3109/08958378.2012.758197.
Hickling LM, Perez-Iglesias R, McNeill A, Dawkins L, Moxham J, Ruffell T, Sendt KV, McGuire P. A pre-post pilot study of electronic cigarettes to reduce smoking in people with severe mental illness. Psychol Med. 2019. https://doi.org/10.1017/s0033291718001782.
Kerr DMI, Brooksbank KJM, Taylor RG, Pinel K, Rios FJ, Touyz RM, Delles C. Acute effects of electronic and tobacco cigarettes on vascular and respiratory function in healthy volunteers: a cross-over study. J Hypertens. 2019. https://doi.org/10.1097/hjh.0000000000001890.
Kotoulas SC, Pataka A, Domvri K, Spyratos D, Katsaounou P, Porpodis K, Fouka E, Markopoulou A, Passa-Fekete K, Grigoriou I, et al. Acute effects of e-cigarette vaping on pulmonary function and airway inflammation in healthy individuals and in patients with asthma. Respirology. 2020. https://doi.org/10.1111/resp.13806.
Palamidas A, Tsikrika S, Katsaounou PA, Vakali S, Gennimata SA, Kaltsakas G, Gratziou C, Koulouris N. Acute effects of short term use of e-cigarettes on airways physiology and respiratory symptoms in smokers with and without airway obstructive diseases and in healthy non smokers. Tob Prev Cessat. 2017. https://doi.org/10.18332/tpc/67799.
Polosa R, Morjaria JB, Prosperini U, Busà B, Pennisi A, Malerba M, Maglia M, Caponnetto P. COPD smokers who switched to e-cigarettes: health outcomes at 5-year follow up. Ther Ad Chronic Dis. 2020. https://doi.org/10.1177/2040622320961617.
Polosa R, Morjaria JB, Caponnetto P, Caruso M, Campagna D, Amaradio MD, Ciampi G, Russo C, Fisichella A. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med. 2016;21(114):99–108.
Arnold MJ, Nollen NL, Mayo MS, Ahluwalia JS, Leavens EL, Zhang G, Rice M, Pulvers K. Harm reduction associated with dual use of cigarettes and e-cigarettes in Black and Latino smokers: secondary analyses from a randomized controlled e-cigarette switching trial. Nicotine Tob Res. 2021. https://doi.org/10.1093/ntr/ntab069.
Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012. https://doi.org/10.1378/chest.11-2443.
Veldheer S, Yingst J, Midya V, Hummer B, Lester C, Krebs N, Hrabovsky S, Wilhelm A, Liao J, Yen MS, et al. Pulmonary and other health effects of electronic cigarette use among adult smokers participating in a randomized controlled smoking reduction trial. Addict Behav. 2019. https://doi.org/10.1016/j.addbeh.2018.10.041.
Walele T, Bush J, Koch A, Savioz R, Martin C, O’Connell G. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real-life setting. Regul Toxicol Pharmacol. 2018. https://doi.org/10.1016/j.yrtph.2017.12.010.
Pulvers K, Nollen NL, Rice M, Schmid CH, Qu K, Benozitz NL, Ahluwalia JS. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Open. 2020. https://doi.org/10.1001/jamanetworkopen.2020.26324.
Polosa R, Morjaria J, Caponnetto P, Caruso M, Strano S, Battaglia E, Russo C. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: evidence for harm reversal. Int J Environ Res Public Health. 2014. https://doi.org/10.3390/ijerph110504965.
Polosa R, Morjaria JB, Prosperini U, Russo S, Pennisi A, Puleo R, Caruso M, Caponetto P. Health effects in COPD smokers who switch to electronic cigarettes: a retrospective-prospective 3-year follow-up. Int J Chron Obstruct Pulmon Dis. 2018. https://doi.org/10.2147/COPD.S161138.
Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A, Bruno CM. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir Res. 2016. https://doi.org/10.1186/s12931-016-0481-x.
Glaab T, Vogelmeier C, Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res. 2010. https://doi.org/10.1186/1465-9921-11-79.
Eberly LE, Ockene J, Sherwin R, Yang L, Kuller L. Pulmonary function as a predictor of lung cancer mortality in continuing cigarette smokers and in quitters. Int J Epidemiol. 2003. https://doi.org/10.1093/ije/dyg177.
Barreiro T, Perillo I. An approach to interpreting spirometry. AFP. 2004;69(5):1107–14.
Kwon DS, Choi YJ, Kim TH, Byun MK, Cho JH, Kim HJ, Park HJ. FEF(25–75%) values in patients with normal lung function can predict the development of chronic obstructive pulmonary disease. Int J Chronic Obstruct Pulmon Dis. 2020. https://doi.org/10.2147/COPD.S261732.
Neuspiel DR, Mosenifar Z. Peak expiratory flow rate measurement. https://emedicine.medscape.com/article/1413347-overview. Accessed 17 Mar 2022.
Dempsey TM, Scanlon PD. Pulmonary function tests for the generalist: a brief review. Mayo Clin Proc. 2018. https://doi.org/10.1016/j.mayocp.2018.04.009.
Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin N Am. 2012. https://doi.org/10.1016/j.mcna.2012.04.011.
Desiraju K, Agrawal A. Impulse oscillometry: the state-of-art for lung function testing. Lung India. 2016. https://doi.org/10.4103/0970-2113.184875.
Ponce MC, Sharma S. Pulmonary function tests. Treasure Island, FL: StatPearls Publishing; 2021.
United States Department of Health and Human Services. Smoking cessation: a report of the Surgeon General. Rockville: US Department of Health and Human Services; 2020.
Gratzious C, Rovina N. Physiological consequences of smoking cessation: benefits for respiratory and cardiovascular system. In: Hayashi I, editor. Smoking: health effects, psychological aspects and cessation. New York: Nova Science Publishers; 2012. p. 59–73.
Tsai AG, Christie JD, Gaughan CA, Palma WR, Margolis ML. Change in forced expiratory time and spirometric performance during a single pulmonary function testing session. Respir Care. 2006;51(3):246–51.
Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM, Siegel MD. Fishman’s pulmonary diseases and disorders. 5th ed. New York: McGraw Hill; 2015.
Lauterstein D, Savidge M, Chen Y, et al. Nonanimal toxicology testing approaches for traditional and deemed tobacco products in a complex regulatory environment: limitations, possibilities, and future directions. Toxicol In Vitro. 2020. https://doi.org/10.1016/j.tiv.2019.104684.
Erasmus A, Holman B, Ioannidis J. Data-dredging bias. In: Catalogue of bias; 2020 https://catalogofbias.org/biases/data-dredging-bias/. Accessed 12 Jan 2021.
Chevassus-au-Louis N. Fraud in the lab: the high stakes of scientific research. Cambridge, MA: Harvard University Press; 2019.
Ritchie S. Science fictions: how fraud, bias, negligence, and hype undermine the search for truth. New York: Metropolitan Books; 2020.
Krishna R, Maithreyi R, Surapaneni KM. Research bias: a review for medical students. J Clin Diagn Res. 2010;4(2):2320–4.
McGauran N, Wieseler B, Kreis J, Schüler Y-B, Kölsch H, Kaiser T. Reporting bias in medical research—a narrative review. Trials. 2010. https://doi.org/10.1186/1745-6215-11-37.
Kirkham JJ, Altman DG, Chan A-W, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ. 2018. https://doi.org/10.1136/bmj.k3802.
Ayorinde AA, Williams I, Mannion R, Song F, Skrybant M, Lilford RJ, Chen YF. Assessment of publication bias and outcome reporting bias in systematic reviews of health services and delivery research: a meta-epidemiological study. PLoS ONE. 2020. https://doi.org/10.1371/journal.pone.0227580.
Al-Marzouki S, Roberts I, Marshall T, Evans S. The effect of scientific misconduct on the results of clinical trials: a Delphi survey. Contemp Clin Trials. 2005. https://doi.org/10.1016/j.cct.2005.01.011.
Rising K, Bacchetti P, Bero L. Reporting bias in drug trials submitted to the Food and Drug Administration: review of publication and presentation. PLoS Med. 2008. https://doi.org/10.1371/journal.pmed.0050217.
Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285(4):437–43.
Sackett DL. Bias in analytic research. Int J Chronic Dis. 1979;32:51–63.
de Hoog N, Stroebe W, de Wit JBF. The processing of fear-arousing communications: how biased processing leads to persuasion. Soc Influ. 2008. https://doi.org/10.1080/15534510802185836.
Brennner LA, Koehler DJ, Tversky A. On the evaluation of one-sided evidence. J Behav Decis Mak. 1996;9:59–70.
D’Antoni D, Auyeung V, Weinman J. The effect of framed health messages on intention to take antivirals for pandemic influenza: a vignette-based randomised controlled trial. J Health Commun. 2019. https://doi.org/10.1080/10810730.2019.1631914.
Gallagher KM, Updegraff JA. Health message framing effects on attitudes, intentions, and behavior: a meta-analytic review. Ann Behav Med. 2012. https://doi.org/10.1007/s12160-011-9308-7.
Keyworth C, Nelson PA, Bundy C, Pye SR, Griffiths CEM, Cordingley L. Does message framing affect changes in behavioural intentions in people with psoriasis? A randomized exploratory study examining health risk communication. Psychol Health Med. 2018. https://doi.org/10.1080/13548506.2018.1427876.
Turrentine M. It’s all how you “spin” it: interpretive bias in research findings in the obstetrics and gynecology literature. Obstet Gynecol. 2017. https://doi.org/10.1097/aog.0000000000001818.
Larue F, Tashib T, Ribeiro PAB, Lavoie KL, Dolan E, Bacon SL. Immediate physiological effects of acute electronic cigarette use in humans: a systematic review and meta-analysis. Respir Med. 2021. https://doi.org/10.1016/j.rmed.2021.106684.
Goniewicz ML, Miller CR, Sutanto E, Li D. How effective are electronic cigarettes for reducing respiratory and cardiovascular risk in smokers? A systematic review. Harm Reduct J. 2020. https://doi.org/10.1186/s12954-020-00440-w.
Chand BR, Hosseinzadeh H. Association between e-cigarette use and asthma: a systematic review and meta-analysis. J Asthma. 2021. https://doi.org/10.1080/02770903.2021.1971703.
Wills TA, Soneji SS, Choi K, Jaspers I, Tam EK. E-cigarette use and respiratory disorders: an integrative review of converging evidence from epidemiological and laboratory studies. Eur Respir J. 2021. https://doi.org/10.1183/13993003.01815-2019.
Fuchs O, Bahmer T, Rabe KF, Mutius EV. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017. https://doi.org/10.1016/S2213-2600(16)30187-4.
Bozier J, Chivers EK, Chapman DG, Larcombe AN, Bastian NA, Masso-Silva JA, Byun MK, McDonald CF, Crotty Alexander LE, Ween MP. The evolving landscape of e-cigarettes: a systematic review of recent evidence. Chest. 2020. https://doi.org/10.1016/j.chest.2019.12.042.
Bravo-Gutiérrez OA, Falfán-Valencia R, Ramírez-Venegas A, Sansores RH, Ponciano-Rodríguez G, Pérez-Rubio G. Lung damage caused by heated tobacco products and electronic nicotine delivery systems: a systematic review. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18084079.
Mowat V, Alexander DJ, Pilling AM. A comparison of rodent and nonrodent laryngeal and tracheal bifurcation sensitivities in inhalation toxicity studies and their relevance for human exposure. Toxicol Pathol. 2017. https://doi.org/10.1177/0192623316678695.
Acknowledgements
We thank Prof. Gaetano Bertino for his support and project oversight as Scientific Director. We thank Damian Odimegwu, Dr. rer.nat. for his work on the searches and data extraction of studies.
Funding
This investigator-initiated study was sponsored by ECLAT srl, a research-based spin-off company of the University of Catania, with the help of a grant from the Foundation for a Smoke-Free World, Inc a US nonprofit 501(3)(c) private foundation [no grant number], and MQ, GL, and RV were supported by a bursary from the University of Catania Italy [Bando N° 1355 2020 Borsa di Ricerca]. The funders had no role in the design of the study or the collection, analysis, and interpretation of data, or in the writing of the manuscript. NOTE: The contents, selection, and presentation of facts, as well as any opinions expressed in this systematic review, are the sole responsibility of the authors and under no circumstances shall be regarded as reflecting the positions of the Foundation for a Smoke-Free World, Inc.
Author information
Authors and Affiliations
Contributions
MQ contributed to formal analysis, investigation, methodology, validation, writing—original draft, and writing—reviewing and editing. RV contributed to formal analysis, investigation, methodology, validation, writing—original draft, and writing—reviewing and editing. GL contributed to formal analysis, investigation, methodology, validation, writing—original draft, and writing—reviewing and editing. RP contributed to conceptualization, funding acquisition, resources, and writing—reviewing and editing. RO contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, and writing reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
GL and RV declare no conflict of interest. RO is supported by a contract with ECLAT; Srl. ECLAT has received funding from the Foundation for a Smoke-Free World. She declares no conflicts of interest. MQ has received a grant from Foundation for a Smoke-Free World outside the submitted work. She declares no conflict of interest. RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. He has served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., and Health Diplomats. He has served on the Medical and Scientific Advisory Board (MSAB) of Cordex Pharma, Inc., CV Therapeutics, Duska Therapeutics Inc, Pfizer, and PharmaCielo. Lecture fees from a number of European EC industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vaper advocacy non-profit organizations. RP is the founder of the Center for Tobacco prevention and treatment (CPCT) at the University of Catania and the Center of Excellence for the acceleration of Harm Reduction (CoEHAR) at the same University, which has received support from Foundation for a Smoke Free World to conduct 8 independent investigator-initiated research projects on harm reduction. RP is currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA) and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: conclusion section has been updated.
Supplementary Information
Additional file 1
. Supplementary matertals.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qureshi, M.A., Vernooij, R.W.M., La Rosa, G.R.M. et al. Respiratory health effects of e-cigarette substitution for tobacco cigarettes: a systematic review. Harm Reduct J 20, 143 (2023). https://doi.org/10.1186/s12954-023-00877-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12954-023-00877-9