Abstract
Background
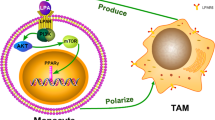
Serous ovarian carcinoma is the most common type of ovarian carcinoma. Tumor-associated macrophages (TAMs) promote ovarian cancer progression. Most macrophages are generated by monocyte differentiation. Lysophosphatidic acid (LPA) levels are high in blood, tissues and ascites of patients with ovarian cancer. This study investigated whether human monocytes can directly differentiate into TAMs in the serous ovarian carcinoma microenvironment.
Methods
Human monocytes were isolated and purified from umbilical cord blood. A serous ovarian carcinoma-like microenvironment was generated by coculturing monocytes and SKOV3 cells in 0.4-μm-pore-size Transwell chambers. Additionally, the effect of LPA was assessed. The two cultured cell types and supernatants were evaluated.
Results
The morphology and function of monocytes cocultured with SKOV3 cells and/or stimulated with LPA were significantly changed compared with those of non-stimulated monocytes. The CD14 + CD163 + and CD206 + phenotype indicated that stimulated cells were TAMs. The induced cells promoted SKOV3 cell proliferation and invasion, further proving that they were TAMs. The level of the cytokine interleukin-6R in the supernatant was significantly elevated in the treatment groups compared to the control monocyte group. Pathway enrichment analysis of ELISA results showed a strong influence of interleukin-6 family signaling, especially the JAK-STAT signaling pathway, further confirming the importance of IL-6R.
Conclusion
Monocytes can differentiate into TAMs under coculture with SKOV3 cells and/or LPA stimulation. The induced TAMs promote SKOV3 cell proliferation and invasion. The cytokine receptor IL-6sR and the JAK-STAT signaling pathway play an important role in the differentiation of monocytes into TAMs.
Similar content being viewed by others
Background
Ovarian carcinoma is the leading cause of death among gynecological cancers and the fifth leading cause of malignancy-related death in women [1, 2]. The prognosis of ovarian cancer is poor because most cases have metastasized before diagnosis and are not diagnosed until they are in an advanced stage [3]. In 2021, an estimated 21,750 new cases of ovarian carcinoma will be diagnosed and 13,770 deaths from ovarian cancer will occur in the USA [1]. Global new cases and deaths for ovarian cancer were 313,959 and 207,252 in 2020 [4]. The 1-year, 5-year and 10-year survival rates are approximately 72%, 48% and 35%, respectively [5, 6]. Serous ovarian cancer is the most common pathological type of ovarian cancer, and is the main focus of this study.
The unique tumor microenvironment (TME) of ovarian cancer promotes tumor metastasis, immunosuppression and drug resistance [7]. Tumor-associated macrophages (TAMs) are important.
components of the TME and play considerable roles in all aspects of ovarian cancer [8]. TAMs modulate the effectiveness of various antitumor treatments [9,10,11,12,13,14]. They usually show an M2-like phenotype, and ovarian cancer TAMs are reported to express high levels of CD163 and CD206 [15].
The survival and prognosis of ovarian cancer patients are strongly associated with TAMs, especially M2-like (CD14 + CD163 +) TAMs [16]. CD163 and CD206 function as immunosuppressive receptor molecules, and their expression usually indicates early recurrence and decreased relapse-free survival (RFS) times in patients with ovarian cancer [15, 17,18,19].
In humans, macrophages are derived from the differentiation of monocytes [20,21,22]. Most macrophages at disease sites arise from the polarization of circulating monocytes [22]. Monocytes are attracted by chemokines, enter sites of damage and differentiate into macrophages. Monocytes can supplement the population of resident macrophages by differentiating into macrophages and can respond to inflammatory signals at disease sites under different conditions [20,21,22]. Therefore, the TAMs in serous ovarian cancer may also be derived from circulating monocytes.
Various mediators can affect the activation and function of TAMs [8]. Lysophosphatidic acid (LPA), a potential marker for ovarian cancer initially called ovarian cancer growth factor and ovarian cancer activator, enhances tumorigenesis and ascites formation [23,24,25,26]. LPA levels are significantly increased in the blood, tissues and ascites of patients with epithelial ovarian cancer [27]; the pathological concentration is 2.0–22 μmol/L, and the LPA levels have been reported to be related to the formation and malignant behavior of ovarian cancer stem cells [28, 29]. Moreover, LPA regulates macrophage polarization [30]. One study reported that LPA can induce murine monocytes to differentiate into macrophages that express high levels of CD206, but CD163 expression was not assessed in that study [30].
Based on the above background information, the following hypothesis was proposed: human monocytes can directly differentiate into TAMs in the serous ovarian cancer TME where the LPA level is high. Our article proposing this hypothesis has been published [31].
In this study, we further explored the role of LPA and tumor cells in the differentiation of TAMs. To this end, a coculture system of primary monocytes and SKOV3 serous ovarian carcinoma cells was established to simulate the in vivo microenvironment of serous ovarian cancer. We used a 0.4-μm pore size Transwell where monocytes and SKOV3 cells were cultured in distinct chambers in medium either with or without LPA. Whereas cells cannot pass through the membrane, the exchange of cytokines is not affected. Our study had three goals based on the use of this system. First, we explored the effects of coculture with SKOV3 cells and/or LPA stimulation on monocytes. Second, we explored the effects of coculture with monocytes and/or LPA stimulation on SKOV3 cells. Third, we explored the changes in cytokines in the medium.
Results
Morphology and function of induced monocyte-derived cells under the conditions of coculture with SKOV3 cells and/or LPA stimulation were changed significantly compared to those of monocytes
After 7 days, as shown in Fig. 1, the morphology of monocyte-derived cells in the 3 treatment groups (LPA5, SM and SM5) changed significantly compared to that of cells in the MO group. The MO group contained cells growing in suspension and with a small, round and regular shape. The other 3 treatment groups contained adherent cells with an increased size, and morphological diversity. Pseudopodia and protrusions were also observed in these groups. The irregular shapes indicate the active functions (Fig. 2).
The morphology of induced monocyte-derived cells under the conditions of coculture with SKOV3 cells and/or LPA stimulation was changed significantly compared to those of monocytes. Monocyte-derived cells cocultured with SKOV3 cells and/or stimulated with LPA (LPA5, SM and SM5) showed adherent cell growth, an increased size, an irregular shape, pseudopodia and protrusions. Notes: 1) MO: monocytes were cultured alone in LPA-free medium; 2) LPA5: monocytes were cultured alone in medium with 5 μM LPA; 3) SM: monocytes were cocultured with SKOV3 cells in LPA-free medium; and 4) SM5: monocytes were cocultured with SKOV3 cells in medium with 5 μM LPA
These cells were subsequently analyzed by flow cytometry after 7 days of treatment. The distribution under the same voltage showed that monocyte-derived cells in the 3 treatment groups (LPA5, SM and SM5) were much larger and more dispersed than those in the MO group (Fig. 3A). Moreover, monocyte-derived cells in the 3 treatment groups (LPA5, SM and SM5) showed positive expression of CD163 and CD206 and co-expression of CD14 and CD163 (CD163 + , CD206 + , CD14 + CD163 +) (Fig. 3B). Statistical analysis of the percentage of positive cells revealed significant differences between each of the 3 treatment groups (LPA5, SM and SM5) and the MO group (p < 0.0001, Fig. 3C). However, there was no difference among the 3 treatment groups (LPA5, SM and SM5).
The monocytes differentiated into CD163 + macrophages under the conditions of coculture with SKOV3 cells and/or LPA stimulation. A The distribution of cells under the same voltage: monocyte-derived cells in the 3 treatment groups (LPA5, SM and SM5) were much larger and more dispersed than those in the MO group. B, C Monocyte-derived cells in the 3 treatment groups (LPA5, SM and SM5) showed positive expression of CD163 and CD206 and coexpression of CD14 and CD163 (CD163 + , CD206 + , CD14 + CD163 +). Notes: *, p < 0.0001 compared to monocytes
These results indicated that monocytes differentiated into CD163 + macrophages under the 3 tested conditions (LPA5, SM and SM5).
The induced monocyte-derived cells promoted the proliferation and invasion of SKOV3 cells, further proving that these cells were TAMs
Two different conditions (coculture and monoculture) were established for the proliferation assay. “Coculture” indicates continuous coculture of cells in the SM group and SM5 group after 7 days of treatment. “Monoculture” indicates that SKOV3 cells in the SM group and SM5 group were cultured alone after 7 days of treatment. Under continuous coculture conditions, as shown in Fig. 4, the number of SKOV3 cells in the coculture groups increased significantly beginning on the second day. A difference was observed under monoculture conditions beginning on the third day. This coculture environment greatly influences tumor cell proliferation.
The induced monocyte-derived cells promoted the proliferation of SKOV3 cells. Under continuous coculture conditions (A, C, E), the number of SKOV3 cells in the coculture groups increased significantly beginning on the second day. A difference was observed under monoculture conditions (B, D, F) beginning on the third day. Notes: Coculture (A, C, E): continuous coculture of cells in the SM group and SM5 group after 7 days of treatment. Monoculture (B, D, F): SKOV3 cells in the SM group and SM5 group were cultured alone after 7 days of treatment. a, p < 0.05 compared to blank; b, p < 0.05 compared to LPA5
The invasion of SKOV3 cells was examined next. Invasion was evaluated by using 8-μm Transwell inserts and Matrigel according to the protocol and instructions. Here, the SKOV3 cells in the SM group and SM5 group were continuously cocultured after 7 days of treatment. The invasion ability of SKOV3 cells in the coculture groups, especially in the SM5 group, was significantly enhanced compared with that of SKOV3 cells cultured alone (Fig. 5).
The induced monocyte-derived cells promoted the invasion of SKOV3 cells. The invasion ability of SKOV3 cells in the coculture groups (SM and SM5), especially in the SM5 group, was significantly enhanced compared with that of SKOV3 cells cultured alone (SKOV3 and LPA5). Notes: the SKOV3 cells in the SM group and SM5 group were continuously cocultured after 7 days of treatment. a p < 0.05 compared to blank; b p < 0.05 compared to LPA5; c p < 0.05 compared to MS
SKOV3 cells, a human ovarian adenocarcinoma, showed increased proliferation and increased invasion after coculture with monocytes or monocytes plus LPA, further proving that these induced monocyte-derived cells were TAMs. This result could also indicate that in vivo, TAMs could exert a similar stimulatory effect on ovarian cancer cells to promote tumor progression.
The cytokine IL-6sR and the Janus kinase/signal transducer and activator of transcription (JAK-STAT) signaling pathway played an important role in the differentiation of monocytes into TAMs
The culture supernatant was collected from the four groups after 7 days of treatment. The cytokine concentrations were then assessed by EILSA array. Based on the outcomes of differential protein expression analysis, IL-6sR was significantly elevated in the 3 treatment groups (LPA5, SM and SM5) compared to the MO group (Fig. 6A, 6B). The results of GO enrichment analysis revealed that the main molecular functions involved were cytokine activity and growth factor receptor binding and that the main biological processes involved were positive regulation of the STAT cascade and positive regulation of the JAK-STAT cascade (Fig. 6C-F). KEGG pathway enrichment analysis also showed enrichment of the JAK-STAT signaling pathway and inflammatory bowel disease pathway (Fig. 6G). These results indicate that IL-6sR and the JAK-STAT signaling pathway play an important role in the differentiation of monocytes into TAMs.
The cytokine IL-6sR and JAK-STAT signaling pathway played an important role in the differentiation of monocytes into TAMs. A, B IL-6sR was significantly elevated in the 3 treatment groups (LPA5, SM and SM5) compared to the MO group. C, D, E, F GO enrichment analysis: the main molecular functions involved were cytokine activity and growth factor receptor binding and that the main biological processes involved were positive regulation of the STAT cascade and positive regulation of the JAK-STAT cascade. G KEGG pathway enrichment analysis: enrichment of the JAK-STAT signaling pathway and inflammatory bowel disease pathway
Discussion
The TME is the environment surrounding the tumor and includes immune cells, signaling molecules, ECM, blood vessels and fibroblasts [32,33,34]. TAMs, a type of immune cell in the TME, link inflammation with cancer and affect the behavior of tumor cells [35,36,37,38]. TAMs are associated with worse survival and prognosis in ovarian carcinoma [39]. They affect many aspects of ovarian cancer, including tumor progression, prognosis, drug resistance and immunosuppression [8]. Serous carcinoma is the most common pathological type of epithelial ovarian carcinoma [3, 40]. SKOV3 is an ovarian carcinoma cell line derived from a patient with serous ovarian cystadenocarcinoma [41]. In this study, SKOV3 cells and primary monocytes were cocultured in a system with 0.4-µm pore size Transwell chambers; thus, the transport, diffusion, secretion and communication of cytokines and other molecules were not affected, providing cells with a microenvironment similar to that found in vivo.
TAMs have an M2-like phenotype. CD163 and CD206 have been suggested to be highly expressed on TAMs in ovarian carcinoma and can be used to predict its recurrence [15, 17, 18, 42]. In addition, the LPA level is high in the blood, tissues and ascites of patients with ovarian cancer; thus, LPA is a potential marker for ovarian carcinoma [23,24,25]. A recent study suggested that murine macrophages differentiated from LPA-induced monocytes express CD14, CD64, CD68 and CD206, where the expression of CD163 was not evaluated in that study [30]. In this research, human monocyte-derived cells cocultured with SKOV3 cells and/or stimulated with LPA showed adherent cell growth, an increased size, an irregular shape, pseudopodia and protrusions, and positive expression of CD163, CD206, CD14 and CD163, indicating differentiation of monocytes into CD163 + macrophages (M2-like TAMs) in the simulated serous ovarian cancer microenvironment. Therefore, a similar phenomenon may also occur in the in vivo serous ovarian cancer microenvironment in humans.
TAMs have been reported to affect biological behaviors of cancer cells, including proliferation and invasion [37, 38]. In the current study, SKOV3 cells showed increased proliferation and invasion abilities after coculture with monocytes or monocytes plus LPA, especially after continuous coculture. These findings also inversely prove that the monocyte-derived cells were TAMs and that this coculture environment greatly influences tumor cell proliferation and invasion. The effect of the TME on the differentiation of monocytes into TAMs and the proliferation and invasion of ovarian cancer cells cannot be underestimated. These findings could also indicate that in vivo, TAMs may exert a similar stimulatory effect on ovarian cancer cells to promote tumor progression.
Cytokines and related signaling pathways play a vital role in tumor immunity and cell differentiation. IL-6R, also called CD126, is a type I cytokine receptor. IL-6R has two forms, IL-6sR and membrane IL-6R (IL-6mR) [43,44,45,46] IL-6sR is an agonist of IL-6 activity [47]. IL-6sR is responsible for the proinflammatory nature of IL6 and is an important participant in the development of chronic inflammatory diseases [47]. Binding of IL6 to IL-6sR is necessary to induce VEGF production [48]. The IL-6sR:IL-6 complex binds to IL-6ST/gp130 on the cell surface and induces signal transduction, even in cells that do not express IL-6mR, a process called trans-signaling [49, 50]. Then, the JAK-STAT signaling pathway is activated, [49,50,51,52] which promotes the biological functions of differentiation, proliferation, immune regulation, oxidative stress responses, etc. [53] In this study, we found that the IL-6sR and the JAK-STAT signaling pathway were significantly upregulated under conditions of monocyte coculture with SKOV3 cells and/or stimulation with LPA. IL-6sR and the JAK-STAT signaling pathway appear to played a prominent role in the differentiation of monocytes into TAMs. However, their specific role and mechanism in this process need to be further studied.
Macrophages are derived from circulating monocytes or yolk sac progenitor cells, but the exact origin of TAMs remains controversial [54]. However, macrophages at disease sites have been reported to originate primarily from the differentiation of circulating monocytes [22]. The type of macrophages in the TME depends on the type, location, and nature of the tumor, meaning that TAMs are tissue-specific and tumor-specific [55]. The differentiation of circulating monocytes in ovarian cancer patients after arriving at sites of ovarian cancer tissue remains incompletely understood. In the microenvironment established in this study, which was similar to that in vivo, monocytes differentiated into M2-like TAMs when cocultured with SKOV3 cells. This finding indicates that circulating monocytes can directly differentiate into TAMs after arriving at sites of serous ovarian cancer, and that TAMs in serous ovarian carcinoma tissue can be derived from circulating monocytes.
Conclusions
In summary, this study demonstrated the following: the morphology and function of induced human monocyte-derived cells under conditions of coculture with SKOV3 cells and/or LPA stimulation were significantly changed compared with those of non-induced monocytes; the induced cells exhibited a CD14 + CD163 + and CD206 + phenotype, indicating that they were TAMs; the induced cells promoted the proliferation and invasion of SKOV3 cells, further proving that these cells were TAMs; and the cytokine IL-6sR and the JAK-STAT signaling pathway played an important role in the differentiation of monocytes into TAMs. Therefore, the following conclusions can be made: human monocytes can be differentiated into TAMs by coculture with SKOV3 cells and/or stimulation with LPA, monocytes in the blood can directly differentiate into TAMs after arriving at sites of serous ovarian cancer tissue, and TAMs in serous ovarian carcinoma tissue can be derived from circulating monocytes. The level of the cytokine IL-6sR was significantly increased in the medium, but the specific role and mechanism of IL-6sR and the JAK-STAT signaling pathway in this process need to be further studied.
Materials and methods
The experimental flowchart shown in Fig. 1 gives an overview of this study. The details are as follows.
Cell culture and ethics statement
Isolation of primary human monocytes is a core technique used in this research. The specific method was described in our previously published article [56]. Umbilical cord blood from healthy full-term deliveries (50 ml) was diluted with PBS at a ratio of 1:1, pipetted slowly onto Ficoll Paque Plus (TBD, Tianjin, China) at a ratio of 1:1, centrifuged at 1000 × g for 30 min at room temperature (acceleration and deceleration were set at zero), and peripheral blood mononuclear cells (PBMCs) were collected. Then, monocytes were isolated from PBMCs by using magnetic-activated cell sorting (MACS) with CD14 + microbeads (Miltenyi Biotec, USA) and were cultured in RPMI-1640 medium (Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco) and 1% penicillin–streptomycin. The purity of the isolated cells was greater than 95%. This study was approved by the Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. Written informed consent was obtained from all patients before surgery.
The SKOV3 cell line was obtained from the Medical Research Center of Beijing Chaoyang Hospital, Capital Medical University. The use of the cell line was approved by the ethics and institutional review board. Cells were grown in RPMI-1640 medium containing 10% FBS under standard conditions (5% CO2, 37 °C).
Coculture system and LPA stimulation
The coculture system was established by inserting 0.4-μm pore size Transwell chambers (Corning, USA) in 6-well plates, where cells could not pass through the membrane and the exchange of cytokines was not affected, to simulate the in vivo microenvironment of serous ovarian cancer. In this system, when monocytes were the focus, monocytes (1 × 106) were cultured in the lower chamber, and SKOV3 cells (5 × 104) were cultured in the upper chamber. When harvesting of SKOV3 cells was required, monocytes were cultured in the upper chamber, and SKOV3 cells were cultured in the lower chamber. Cells were cultured in RPMI-1640 medium containing 10% FBS and 1% penicillin–streptomycin.
LPA (18:1) (Avanti Polar Lipids, USA) was solubilized, and a stock solution was prepared according to the method provided in the instructions from the manufacturer. Liquid LPA was added to the culture medium to study its effect on monocytes. Preliminary experimental results showed that 5 μM LPA was the optimal concentration.
Accordingly, four groups were established: 1) MO: monocytes were cultured alone in LPA-free medium; 2) LPA5: monocytes were cultured alone in medium with 5 μM LPA; 3) SM: monocytes were cocultured with SKOV3 cells in LPA-free medium; and 4) SM5: monocytes were cocultured with SKOV3 cells in medium with 5 μM LPA.
Cells were visualized with a microscope (Olympus, CX-14, Japan) every day. The morphology of monocyte-derived cells changed significantly after 7 days. The duration of 7 days was also determined by a previous literature report [30].
Flow cytometry
Monocyte-derived cells were harvested after 7 days of treatments. These cells were washed and stained with APC-CD14 (Biosciences, USA), PE-CD163 (Biosciences, USA), and BV-421-CD206 antibodies (Biosciences, USA) and were then analyzed by flow cytometry (FACSCanto II, BD Biosciences, USA) and FlowJo software (version 10; Tree Star, USA) was used to analyze the outcomes.
Cell proliferation assay
SKOV3 cells were cultured in the lower chamber and monocytes were cultured in the upper chamber in the same coculture system. SKOV3 cells were harvested after 7 days. The proliferation of SKOV3 cells were evaluated by using a CCK8 kit (KeyGEN Bio TECH, China). Cell densities were calculated, and cells (3 × 103) were seeded in 96-well plates. Cell viability and growth were evaluated after culture for 0 h, 24 h, 48 h and 72 h. The optical density (OD) values at 450 nm were measured in a microplate reader. “Coculture” indicates that SKOV3cells in the SM group and SM5 group were in continuous coculture after 7 days of treatment. “Monoculture” indicates that SKOV3 cells in the SM group and SM5 group were cultured alone after 7 days of treatment.
Cell invasion assay
SKOV3 cells were cultured in the lower chamber and monocytes were cultured in the upper chamber in the coculture system. SKOV3 cells were harvested after seven days of treatment. The invasion of SKOV3 cells was evaluated by using 8-μm Transwell inserts (Corning, USA) and Matrigel (BD Bioscience, USA) according to the protocols and instructions. The membranes of the upper chambers were coated with 50 g/ml Matrigel. Cells (4 × 104) suspended in medium containing 0.1% FBS were seeded in the upper chambers. Complete medium (10% FBS) was added to the lower chambers. After 24 h, the cells on the polycarbonate membranes were fixed with paraformaldehyde and stained with crystal violet. Those cells on the upper surface of membranes were wiped off with cotton-tipped applicators. Blue-stained cells refer to cells attached to the lower chamber side of the membrane after passing through the Matrigel and membrane. The invasion ability was calculated by counting the number of blue-stained cells entering the lower chamber with ImageJ software. Based on outcomes of the proliferation experiment, the SKOV3 cells in the SM group and SM5 group were continuously cocultured after 7 days of treatment in this experiment.
Assessment of cytokines in the cell supernatant
The cell culture supernatant was collected after 7 days of treatment and stored at − 80 °C for subsequent evaluation. The concentrations of 34 Th1-, Th2- and Th17–related cytokines were detected by a RayBio® Human Th1/Th2/Th17 Antibody Array (G-Series, RayBiotech, USA).The array contained the following cytokines: CD30, CD40 ligand, CD40, granulocyte colony-stimulating factor (G-CSF), glucocorticoid-induced TNFR-related protein (GITR), granulocyte–macrophage colony-stimulating factor (GM-CSF), IFN-gamma, interleukin (IL)-1 sRI, IL-1 sRII, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-17A, IL-17F, IL17R, IL-1β, IL-2, IL-21, IL-21R, IL-22, IL-23p19, IL-28A, IL-4, IL-5, IL-6, soluble IL-6 receptor (IL-6sR), macrophage inflammatory protein (MIP)-3α, sgp130, transforming growth factor (TGF)-β1, TGF-β3, TGF-α, TGF-β and TNF-related activation-induced cytokine (TRANCE). The images and signals were aquired using a GenePix 4000B scanner (Molecular Devices, LLC, USA). RayBio® Analysis Tool software was used to analyze the data. Subsequently, differential protein expression analysis, gene ontology (GO) enrichment analysis (including molecular function (MF), biological process (BP) and cellular component (CC) terms) and KEGG pathway enrichment analysis were performed.
Statistical analysis
Data were collected from at least 3 independent experiments. SPSS 23.0 (IBM Corp., USA) and GraphPad Prism software (GraphPad, Inc., USA) were used for statistical analysis. A t-test and ANOVA were used to evaluate differences between groups. Quantitative data are shown as the mean ± SEM values. Statistical significance was defined as a two-sided p-value of less than 0.05.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
National Comprehensive Cancer Network® (NCCN®). Ovarian Cancer. Including Fallopian Tube Cancer and Primary Peritoneal Cancer. NCCN Guidelines Version 2.2020. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). 2020;Version 2.2020 — January 12, 2021.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–53.
Hyuna Sung JF, Rebecca L Siegel, Mathieu Laversanne, Isabelle Soerjomataram, Ahmedin Jemal, Freddie Bray Global Cancer Statistics 2020 GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–49.
Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review (CSR) 1975–2015, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. Bethesda, MD: National Cancer Institute. Updated September 10, 2018.
Ovarian cancer survival statistics. https://wwwcancerresearchukorg/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer/survival?_ga=23005318318576163891578627784-14268553771578627784#heading-Zero.Retrieved. 2020–01–10.
Pogge von Strandmann E, Reinartz S, Wager U, Muller R. Tumor-Host Cell Interactions in Ovarian Cancer: Pathways to Therapy Failure. Trends Cancer. 2017;3(2):137–48.
Worzfeld T, Pogge von Strandmann E, Huber M, Adhikary T, Wagner U, Reinartz S, et al. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front Oncol. 2017;7:24.
Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9(389).
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74(18):5057–69.
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–37.
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67.
Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res. 2015;3(5):518–25.
Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70(14):5679–85.
Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134(1):32–42.
Maccio A, Gramignano G, Cherchi MC, Tanca L, Melis L, Madeddu C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep. 2020;10(1):6096.
Burt BM, Rodig SJ, Tilleman TR, Elbardissi AW, Bueno R, Sugarbaker DJ. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 2011;117(22):5234–44.
Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. American journal of translational research. 2012;4(4):376–89.
Reinartz S, Finkernagel F, Adhikary T, Rohnalter V, Schumann T, Schober Y, et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol. 2016;17(1):108.
Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17(1):2–8.
Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44(3):439–49.
Pittet MJ, Nahrendorf M, Swirski FK. The journey from stem cell to macrophage. Ann N Y Acad Sci. 2014;1319:1–18.
Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 1995;1(10):1223–32.
Xu Y, Casey G, Mills GB. Effect of lysophospholipids on signaling in the human Jurkat T cell line. J Cell Physiol. 1995;163(3):441–50.
Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280(8):719–23.
Li H, Zhao Z, Wei G, Yan L, Wang D, Zhang H, et al. Group VIA phospholipase A2 in both host and tumor cells is involved in ovarian cancer development. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(10):4103–16.
Xu Y. Lysophospholipid Signaling in the Epithelial Ovarian Cancer Tumor Microenvironment. Cancers. 2018;10(7).
Fan Q, Cai Q, Li P, Wang W, Wang J, Gerry E, et al. The novel ZIP4 regulation and its role in ovarian cancer. Oncotarget. 2017;8(52):90090–107.
Seo EJ, Kwon YW, Jang IH, Kim DK, Lee SI, Choi EJ, et al. Autotaxin Regulates Maintenance of Ovarian Cancer Stem Cells through Lysophosphatidic Acid-Mediated Autocrine Mechanism. Stem cells. 2016;34(3):551–64.
Ray R, Rai V. Lysophosphatidic acid converts monocytes into macrophages in both mice and humans. Blood. 2017;129(9):1177–83.
Feng Y, Xiao M, Zhang Z, Cui R, Jiang X, Wang S, et al. Potential interaction between lysophosphatidic acid and tumor-associated macrophages in ovarian carcinoma. J Inflamm (Lond). 2020;17:23.
Alfarouk KO, Muddathir AK, Shayoub ME. Tumor acidity as evolutionary spite. Cancers (Basel). 2011;3(1):408–14.
Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80.
Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–8.
Korneev KV, Atretkhany KN, Drutskaya MS, Grivennikov SI, Kuprash DV, Nedospasov SA. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine. 2017;89:127–35.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9.
Network NCC. Ovarian Cancer. Including Fallopian Tube Cancer and Primary Peritoneal Cancer. NCCN Guidelines Version 3.2019 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). 2019;Version 3.2019 — November 26, 2019.
Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59(1):221–6.
Adhikary T, Wortmann A, Finkernagel F, Lieber S, Nist A, Stiewe T, et al. Interferon signaling in ascites-associated macrophages is linked to a favorable clinical outcome in a subgroup of ovarian carcinoma patients. 2017;18(1):243.
Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5): 105954.
Campbell IL, Erta M, Lim SL, Frausto R, May U, Rose-John S, et al. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J Neurosci. 2014;34(7):2503–13.
Briso EM, Dienz O, Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J Immunol. 2008;180(11):7102–6.
Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175(6):3463–8.
Garbers C, Thaiss W, Jones GW, Waetzig GH, Lorenzen I, Guilhot F, et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem. 2011;286(50):42959–70.
Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1521–9.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–48.
Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18(4):374–84.
Zegeye MM, Lindkvist M, Falker K, Kumawat AK, Paramel G, Grenegard M, et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun Signal. 2018;16(1):55.
Su H, Lei CT, Zhang C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front Immunol. 2017;8:405.
Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18(12):773–89.
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99(Pt B):180–5.
Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–39.
Xiao M, Feng Y, Cao G, Liu C, Zhang Z. A novel MtHSP70-FPR1 fusion protein enhances cytotoxic T lymphocyte responses to cervical cancer cells by activating human monocyte-derived dendritic cells via the p38 MAPK signaling pathway. Biochem Biophys Res Commun. 2018;503(3):2108–16.
Acknowledgements
We thank Zhiren Li in the Department of Gastroenterology and Medical Research Center, Beijing Chao‑Yang Hospital, Capital Medical University for their excellent technical assistance work; and Zhixiang Wang in the Department of Radiation Oncology (Maastro), GROW-School for Oncology, Maastricht University Medical Centre+, Maastricht, Netherlands for the help of picture modification.
Funding
This study was supported by Foreign Cooperation Project of the Ministry of Science and Technology of China (Project No. 2012DFA30490), National Key Research and Development Program of China (Project No. 2014AA020606) and Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding-YangFan Project (Project No. ZYLX201713).
Author information
Authors and Affiliations
Contributions
Each author made substantial contributions to the conception and design of the work. Ying Feng was the major contributor in writing the manuscript; Sofia Xanthoulea, Andrea Romano, Chongdong Liu and Zhenyu Zhang substantively revised it; Stan Zijtveld, Bert Delvoux, Meizhu Xiao, Guangming Cao, Hao Liu, Yanfang Li and Shuzhen Wang helped the acquisition, analysis and interpretation of the work; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Feng, Y., Xiao, M., Cao, G. et al. Human monocytes differentiate into tumor-associated macrophages upon SKOV3 cells coculture and/or lysophosphatidic acid stimulation. J Inflamm 19, 11 (2022). https://doi.org/10.1186/s12950-022-00307-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12950-022-00307-w