Abstract
Background
Quality of life is as important as survival in heart failure (HF) patients. Controversies exist with regards to echocardiographic determinants of exercise capacity in HF, particularly in patients with preserved ejection fraction (HFpEF). The aim of this study was to prospectively examine echocardiographic parameters that correlate and predict functional exercise capacity assessed by 6 min walk test (6-MWT) in patients with HFpEF.
Methods
In 111 HF patients (mean age 63 ± 10 years, 47% female), an echo-Doppler study and a 6-MWT were performed in the same day. Patients were divided into two groups based on the 6-MWT distance (Group I: ≤ 300 m and Group II: >300 m).
Results
Group I were older (p = 0.008), had higher prevalence of diabetes (p = 0.027), higher baseline heart rate (p = 0.004), larger left atrium - LA (p = 0.001), longer LV filling time - FT (p = 0.019), shorter isovolumic relaxation time (p = 0.037), shorter pulmonary artery acceleration time - PA acceleration time (p = 0.006), lower left atrial lateral wall myocardial velocity (a’) (p = 0.018) and lower septal systolic myocardial velocity (s’) (p = 0.023), compared with Group II.
Patients with HF and reduced EF (HFrEF) had lower hemoglobin (p = 0.007), higher baseline heart rate (p = 0.005), higher NT-ProBNP (p = 0.001), larger LA (p = 0.004), lower septal s’, e’, a’ waves, and septal mitral annular plane systolic excursion (MAPSE), shorter PA acceleration time (p < 0.001 for all), lower lateral MAPSE, higher E/A & E/e’, and shorter LVFT (p = 0.001 for all), lower lateral e’ (p = 0.009), s’ (p = 0.006), right ventricular e’ and LA emptying fraction (p = 0.012 for both), compared with HFpEF patients.
In multivariate analysis, only LA diameter [2.676 (1.242–5.766), p = 0.012], and diabetes [0.274 (0.084–0.898), p = 0.033] independently predicted poor 6-MWT performance in the group as a whole. In HFrEF, age [1.073 (1.012–1.137), p = 0.018] and LA diameter [3.685 (1.348–10.071), p = 0.011], but in HFpEF, lateral s’ [0.295 (0.099–0.882), p = 0.029], and hemoglobin level [0.497 (0.248–0.998), p = 0.049] independently predicted poor 6-MWT performance.
Conclusions
In HF patients determinants of exercise capacity differ according to severity of overall LV systolic function, with left atrial enlargement in HFrEF and longitudinal systolic shortening in HFpEF as the the main determinants.
Similar content being viewed by others
Background
Despite advances in the diagnosis and treatment of heart failure (HF), it still presents a major public health problem [1], with increased incidence [2, 3] and poor prognosis [4,5,6]. In patients with HF and reduced left ventricular (LV) ejection fraction (HFrEF) several echo parameters correlated with functional capacity [7,8,9,10,11,12,13,14,15,16]. In contrast, in patients with HF and preserved LV EF (HFpEF), determinants of functional capacity are not well investigated [15,16,17,18]. Exercise capacity has been objectively assessed in the setting of HF using the six-min walk test (6-MWT) [19]. While several echocardiographic markers at rest predicted limited exercise capacity in patients with HFrEF [7,8,9,10,11,12,13,14,15,16], none of them correlate with functional capacity in those with HFpEF [15, 17]. The aim of this study was to prospectively examine whether 6-MWT results correlate with cardiac function parameters in a consecutive group of patients with HF and to identify possible determinants of exercise capacity in those with HFpEF.
Methods
Study population
We studied 111 patients (mean age 63 ± 10 years, 47% female), with clinical diagnosis of HF, and New York Heart Association (NYHA) functional class I-III, secondary to ischemic or non-ischemic etiology. Patients were referred to the Clinic of Cardiology, University Clinical Centre of Kosova, between May 2013 and June 2016. At the time of the study all patients were on optimum HF medications, optimized at least 2 weeks prior to enrollment, based on patient’s symptoms and renal function: 82% were receiving ACE inhibitors or ARB, 78% beta-blockers, 12% calcium-blockers, 10% digoxin, 52% spironolactone, 62% diuretics. Patients with HFrEF had ischemic aetiology in 45%, hypertensive in 38%, and unknown aetiology in 17%. Patients with HFpEF had ischemic aetiology in 41% and hypertensive in 59%. All patients were in sinus rhythm. Patients with clinical evidence for cardiac decompensation, limited physical activity due to factors other than cardiac symptoms (e.g. arthritis), more than moderate mitral regurgitation, more than mild renal failure, chronic obstructive pulmonary disease or those with recent acute coronary syndrome, stroke or anemia were excluded. Patients gave a written informed consent to participate in the study, which was approved by the local Ethics Committee.
Data collection
Detailed history and clinical assessment were obtained in all patients, in whom routine biochemical tests were also performed including hemoglobin, lipid profile, blood glucose level, and kidney function tests. Estimated body mass index (BMI) was calculated from weight and height measurements. Waist, hip measurements were also made and waist/hip ratio was calculated.
Echocardiographic examination
A single operator performed all echocardiographic examinations using a Philips Intelligent E-33 system with a multi-frequency transducer, and harmonic imaging as appropriate. Images were obtained with the patient in the left lateral decubitus position and during quiet expiration. Measurements of interventricular septal thickness, posterior wall thickness, and LV dimensions were made at end-diastole and end-systole, as recommended by the American Society of Echocardiography [19]. LV mass (LVM) was calculated using the Devereux formula [20] and normalized to body surface area (LV mass index [LVMI]).
LV volumes and EF were calculated from the apical 2 and 4 chamber views using the modified Simpson’s method. Ventricular long axis motion was studied by placing the M-mode cursor at the lateral and septal angles of the mitral ring and the lateral angle of the tricuspid ring. Total amplitude of long axis motion was measured as previously described [21] from peak inward to peak outward points. LV and right ventricular (RV) long axis myocardial velocities were also studied using Doppler myocardial imaging technique. From the apical 4-chamber view, longitudinal velocities were recorded with the sample volume placed at the basal part of LV lateral and septal segments as well as RV free wall. Systolic (s’), as well as early and late (e’ and a’) diastolic myocardial velocities were measured with the gain optimally adjusted. Mean value of lateral and septal LV velocities were calculated.
Left atrial diameter was measured from aortic root recordings with the M-mode cursor positioned at the level of the aortic valve leaflets. LA volumes were measured using area-length method from the apical four chamber views, according to the guidelines of the American Society of Echocardiography and European Association of Echocardiography [22]. Left atrial maximal volume (LAV max) was measured at the end of LV systole, just before the opening of the mitral valve, LA minimal volume (LAV min) was measured at end diastole, right after mitral valve closure. LA emptying fraction (LA EF) was calculated with the formula [22, 23]:
Diastolic LV and RV function was assessed from filling velocities using spectral pulsed wave Doppler with the sample volume positioned at the tips of the mitral and tricuspid valve leaflets, respectively, during a brief apnea. Peak LV and RV early (E wave) and late (A wave) diastolic velocities were measured and E/A ratios were calculated. E wave deceleration time (DT) was also measured from peak E wave to the end of its deceleration in all study patients. The E/e’ ratio was calculated from the transmitral E wave and the mean lateral and septal segments e’ wave velocities. The isovolumic relaxation time was also measured from aortic valve closure to mitral valve opening, on the pulsed wave Doppler recording. LV filling pattern was considered ‘restrictive’ when E/A ratio was >2.0, E wave deceleration time < 140 ms and the left atrium dilated of more than 40 mm in transverse diameter [24]. Total LV filling time was measured from the onset of the E wave to the end of the A wave and ejection time from the onset to the end of the aortic Doppler flow velocity.
Mitral regurgitation severity was assessed by colour and continuous wave Doppler and was graded as mild, moderate, or severe according to the relative jet area to that of the left atrium as well as the flow velocity profile, in line with the recommendations of the American and European Society of Echocardiography [25, 26]. Likewise, tricuspid regurgitation was assessed by colour Doppler and continuous-wave Doppler. Retrograde trans-tricuspid pressure drop > 35 mmHg was taken as an evidence for pulmonary hypertension [26, 27]. All M-mode and Doppler recordings were made at a fast speed of 100 mm/s with a superimposed ECG (lead II).
Measurement of amino-terminal pro BNP
Fasting venous blood was collected from study participants after they had rested in a supine position for 20 min. Samples were placed in disposable EDTA containers (1 g/L of plasma), and N-terminal proBNP was measured by a Cobas Elecsys E 411 analyzer (measuring range 5–35000 pg/mL) using a chemiluminescent immunoassay kit (Roche Diagnostics, Grenach-Wyhlen, Germany).
Six minute walk test
Within 24 h of the echocardiographic examination a 6-MWT was performed on a level hallway surface, administered by a specialized nurse who was blinded to the results of the echocardiogram. According to the method of Gyatt et al. [28] patients were informed of the purpose and protocol of the 6 MWT which was conducted in a standardized fashion while patients on their regular medications [29, 30]. A 15 m flat, obstacle-free corridor was used and patients were instructed to walk as far as they can, turning 180° after they have reached the end of the corridor, during the allocated time of 6 min. Patients walked unaccompanied so not to influence walking speed. At the end of the 6 min the supervising nurse measured the total distance walked by the patient.
Using the norm-reference equation developed by Troosters [31] for the prediction of 6MWT distance according to age, height, weight, and gender that has been proposed for healthy patients, we derived the percentage of the predicted value (PPV). PPV is computed by dividing the actual 6MWT distance by the expected value of 6MWT distance and then multiplying by 100. Troosters’ equation is as follows: Predicted 6MWT distance = 218 + 5.14 ∗ height (cm) − 5.32 ∗ age (years) −1.8 ∗ weight (kg) + 51.31 ∗ sex (1–male, 0–female).
Statistical analysis
Data are presented as mean ± SD or proportions (% of patients). Continuous data was compared with two-tailed unpaired Student’s t test and discrete data with Chi-square test. Correlations were tested with Pearson coefficients. Determinants of 6 MWT distance were identified with univariate analysis and multivariate logistic regression was performed using the step-wise method. A significant difference was defined as p < 0.05 (2-tailed). Patients were divided according to their ability to walk >300 m into good and limited exercise performance groups [30], and were compared using unpaired Student t-test. Also, patients with HFpEF (>45%) were compared with those with HFrEF (<45%) using unpaired t-test.
Results
Patients with Limited vs. Good 6 MWT performance (Table 1)
Patients with limited exercise capacity were older (p = 0.008) and had higher prevalence of diabetes (p = 0.027) compared with those with good exercise capacity. Patients with limited 6-MWT had larger left atrium (p = 0.001), increased LVMI (p = 0.006), shorter LV filling time (p = 0.019), shorter isovolumic relaxation time (p = 0.037) and shorter PA acceleration time (p = 0.006), lower septal a’ (p = 0.018) and s’ (p = 0.023), compared to those with good 6-MWT performance. The rest of the clinical and echocardiographic indices were not different between groups.
Patients with HFpEF vs. HFrEF (Table 2)
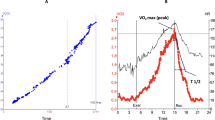
The whole group of study patients walked a distance of 310 ± 116 m during 6-MWT. Patients with HFrEF walked significantly shorter distance compared those with HFpEF (p = 0.01, Fig. 1).
Mean PPV of 6-MWT distance for the whole group was 50 ± 20%, and was lower in HFrEF compared to HFpEF patients (p = 0.004). Patients with HFrEF had lower hemoglobin (p = 0.007), higher baseline heart rate (p = 0.005), higher NT-ProBNP (p = 0.001), larger LAV max (p = 0.005), larger LAV min (p < 0.001), larger LA (p = 0.004), increased LVM and LVMI (p < 0.001, for both), shorter pulmonary acceleration time, lower septal s’, e’ and a’ velocities, and lower septal and lateral mitral annular plane systolic excursion (MAPSE) (p < 0.001 for all), higher E/A, shorter LVFT and higher E/e’ (p = 0.001 for all), shorter E wave DT (p = 0.002), lower lateral e’ (p = 0.009) and s’ (p = 0.006), RV e’ and LA EF (p = 0.012 for both) compared to HFpEF patients. Eleven of the 55 HFpEF patients and 23 of 56 HFrEF patients had mild-moderate mitral regurgitation. Seven of the 55 HFpEF patients and 17 of 56 HFrEF patients had mild or more tricuspid regurgitation.
Determinants of limited 6 MWT distance (Table 3)
Determinants of limited 6 MWT distance in all HF patients
High baseline heart rate (p = 0.008), age (p = 0.011), diabetes (p = 0.017) and NYHA class (p = 0.015) predicted limited 6-MWT distance. Also, enlarged LA (p = 0.001), increased LVMI (p = 0.004), increased E wave velocity (p = 0.019), short LV filling time (p = 0.036) and pulmonary artery acceleration time (p = 0.01), raised E/e’ (p = 0.028), low septal a’ and s’ (p = 0.032 and p = 0.041, respectively), predicted limited 6 MWT distance. In multivariate analysis [odds ratio 95% confidence interval], only enlarged LA diameter [2.676 (1.242–5.766), p = 0.012], and diabetes [0.274 (0.084–0.898), p = 0.033], independently predicted the limited 6-MWT distance.
Determinants of limited 6 MWT distance in HFpEF patients
In univariate analysis, body surface area - BSA (p = 0.012), low hemoglobin level (p = 0.031), diabetes (p = 0.037), and NYHA class > 1 (p = 0.049), increased LVMI (p = 0.025), low lateral s’ (p = 0.018) and a’ (p = 0.039) predicted limited 6-MWT distance. In multivariate analysis, lateral s’ [0.295 (0.099–0.882), p = 0.029, Fig. 2], and hemoglobin level [0.497 (0.248–0.998), p = 0.049], independently predicted the limited 6-MWT distance.
Determinants of limited 6 MWT distance in HFrEF patients
In univariate analysis, age (p = 0.02) and enlarged LA (p = 0.009) predicted limited exercise distance, which also remained as independent determinants in multivariate analysis: age [1.073 (1.012–1.137), p = 0.018] and LA diameter [3.685 (1.348–10.071), p = 0.011, Fig. 3].
Discussion
Findings
The results of this study show that in general HF patients with limited exercise capacity are older and have worse left ventricular function and raised filling pressures than those with satisfactory exercise performance. However, determinants of exercise capacity differed significantly according to ejection fraction. While patients with reduced ejection fraction have the enlarged left atrium and advanced age as the independent determinants of exercise capacity, it was the low hemoglobin and reduced lateral s’ the respective determinants in those with preserved ejection fraction.
Data interpretation
Patients with heart failure due to reduced ejection fraction are known to have worse segmental and overall ventricular function, with additional signs of myocardial stiffness and raised filling pressures in many of them [32]. These perpetual changes result in left atrial enlargement due to the raised pressure, either because of venous hypertension, additional mitral regurgitation or the combination of both [33]. Indeed left atrial enlargement has previously been shown to be the most important prognostic marker in heart failure patients, irrespective of the development of atrial fibrillation [34]. It has also been taken as a reflection of the severity of LV myocardial stiffness, which is an end-stage dysfunction, thus an irreversible damage. On the other hand, many factors contribute to the pathophysiology of heart failure with preserved ejection fraction [35], including atrial fibrillation, hypertension and kidney disease. Although none of our patients was in atrial fibrillation, the low hemoglobin levels were the main determinant of compromised exercise capacity. This reflects the need for acknowledging differences in the strategic management of these patients when compared with those of HFrEF. Finally, our findings discard ejection fraction, as the commonest marker of ventricular function as a determinant of exercise capacity.
Limitations
The main limitation of our study is that we did not investigate the response of echocardiographic measurements to exercise, at the time of symptoms development. However the objective of this study was to determine determinants of ordinary walking exercise limitation rather than heavy exercise in HF patients. The other limitation was the lack of invasive measurements of left atrial pressures, but the study was based on Doppler measurements which have been shown to be reproducible and correlate closely with invasive pressure measurements [36]. The small sample size was another limitation, but we believe that future studies with larger sample size should strength our findings.
Clinical implications
Patients with HF have significantly limited exercise tolerance. Although ejection fraction is considered as the most useful index of LV function and the corner stone for recruiting patients for various treatment modalities, the other echo parameters should be considered as part of the conventional protocol of the follow-up of such patients, depending on overall LV systolic function: enlarged left atrium in HFrEF and impaired longitudinal systolic shortening and reduced hemoglobin in those with HFpEF. While management of patients with HFrEF could be standardized, and follow one protocol, that of patients with HFpEF is likely to be individualized.
Conclusions
In HF patients determinants of exercise capacity differ according to severity of overall LV systolic function, with left atrial enlargement in HFrEF and longitudinal systolic shortening and low hemoglobin in HFpEF as the the main determinants.
Abbreviations
- 6-MWT:
-
Minute walk test
- A:
-
Atrial diastolic velovity
- a’:
-
Atrial myocardial velocity
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- DT:
-
Deceleration time
- e’:
-
Early diastolic myocardial velocity
- E:
-
Early diastolic velocity
- EF:
-
Ejection fraction
- FT:
-
Filling time
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LA EF:
-
Left atrial emptying fraction
- LA:
-
Left atrium
- LAV max:
-
Left atrial maximal volume
- LAV min:
-
Left atrial minimal volume
- LV:
-
Left ventricle
- LVM:
-
Left ventricular mass
- LVMI:
-
Left ventricular mass index
- LVPWd:
-
Left ventricular posterior wall in diastole
- MAPSE:
-
Mitral annular plane systolic excursion
- NYHA:
-
New York Heart Association
- PA:
-
Pulmonary artery
- PPV:
-
Percentage of the predicted value
- RV:
-
Right ventricle
- s’:
-
Systolic myocardial velocity
- TAPSE:
-
Tricuspidal annular plane systolic excursion
References
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360.
Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41.
Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–59.
Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15.
Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–45.
Bytyçi I, Bajraktari G. Mortality in heart failure patients. Anatol J Cardiol. 2015;15(1):63–8. doi:10.5152/akd.2014.5731.
Ciampi Q, Pratali L, Porta MD, Petruzziello B, Manganiello V, Villari B, Picano E, Sicari R. Tissue Doppler systolic velocity change during dobutamine stress echocardiography predicts contractile reserve and exercise tolerance in patients with heart failure. Eur Heart J Cardiovasc Imaging. 2013;14(2):102–9.
Gardin JM, Leifer ES, Fleg JL, Whellan D, Kokkinos P, Leblanc MH, Wolfel E, Kitzman DW, HF-ACTION Investigators. Relationship of Doppler-Echocardiographic left ventricular diastolic function to exercise performance in systolic heart failure: the HF-ACTION study. Am Heart J. 2009;158:S45–52.
Chattopadhyay S, Alamgir MF, Nikitin NP, Rigby AS, Clark AL, Cleland JG. Lack of diastolic reserve in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2010;3:35–43.
Berisha V, Bajraktari G, Dobra D, Haliti E, Bajrami R, Elezi S. Echocardiography and 6-minute walk test in left ventricular systolic dysfunction. Arq Bras Cardiol. 2009;92(2):121–34.
Leong DP, Grover S, Molaee P, Chakrabarty A, Shirazi M, Cheng YH, Penhall A, Perry R, Greville H, Joseph MX, Selvanayagam JB. Nonvolumetric echocardiographic indices of right ventricular systolic function: validation with cardiovascular magnetic resonance and relationship with functional capacity. Echocardiography. 2012;29:455–63.
Bajraktari G, Elezi S, Berisha V, Lindqvist P, Rexhepaj N, Henein MY. Left ventricular asynchrony and raised filling pressure predict limited exercise performance assessed by 6 minute walk test. Int J Cardiol. 2011;146(3):385–9. doi:10.1016/j.ijcard.2009.07.018.
Rubis P, Podolec P, Tomkiewicz-Pajak L, Kopec G, Olszowska M, Tracz W. Usefulness of the evaluation of isovolumic and ejection phase myocardial signals during stress echocardiography in predicting exercise capacity in heart failure patients. Echocardiography. 2009;26:1050–9.
Ohara T, Iwano H, Thohan V, Kitzman DW, Upadhya B, Pu M, Little WC. Role of Diastolic Function in Preserved Exercise Capacity in Patients with Reduced Ejection Fractions. J Am Soc Echocardiogr. 2015;28(10):1184–93.
Bajraktari G, Batalli A, Poniku A, Ahmeti A, Olloni R, Hyseni V, et al. Left ventricular markers of global dyssynchrony predict limited exercise capacity in heart failure, but not in patients with preserved ejection fraction. Cardiovasc Ultrasound. 2012;10(1):36. doi:10.1186/1476-7120-10-36.
Hasselberg NE, Haugaa KH, Sarvari SI, Gullestad L, Andreassen AK, Smiseth OA, Edvardsen T. Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16(2):217–24.
Mohammed SF, Borlaug BA, McNulty S, Lewis GD, Lin G, Zakeri R, et al. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7(4):580–9.
Kosmala W, Rojek A, Przewlocka-Kosmala M, Mysiak A, Karolko B, Marwick TH. Contributions of Nondiastolic Factors to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;67(6):659–70.
Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, et al. a report of the American College of Cardiology/American Heart Association Task Force on PracticeGuidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003;108(9):1146–62.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–8.
Höglund C, Alam M, Thorstrand C. Atrioventricular valve plane displacement in healthy persons. An echocardiographic study. Acta Med Scand. 1988;224:557–62.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–70. doi:10.1093/ehjci/jev014.
Wakatsuki Y, Funabashi N, Mikami Y, Shiina Y, Kawakubo M, Takahashi M, et al. Left atrial compensatory function in subjects with early stage primary hypertension assessed by using left atrial volumetric emptying fraction acquired by transthoracic echocardiography. Int J Cardiol. 2009;136(3):363–7.
Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426–40.
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ, American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802.
Galderisi M, Henein MY, D’hooge J, Sicari R, Badano LP, Zamorano JL, Roelandt JR, European Association of Echocardiography. Recommendations of the European Association of Echocardiography: how to use echo-Doppler in clinical trials: different modalities for different purposes. Eur J Echocardiogr. 2011;12(5):339–53. doi:10.1093/ejechocard/jer051.
Gardin JM, Adams DB, Douglas PS, Feigenbaum H, Forst DH, Fraser AG, Grayburn PA, Katz AS, Keller AM, Kerber RE, Khandheria BK, Klein AL, Lang RM, Pierard LA, Quinones MA, Schnittger I, American Society of Echocardiography. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography’s Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiogr. 2002;15:275–90.
Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk test: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23.
Guyatt GH, Thompson PJ, Berman LB, Sullivan MJ, Townsend M, Jones NL, Pugsley SO. How should we measyre function in patients with chronic heart and lung disease? J Chronic Dis. 1985;28:517–24.
Ingle L, Rigby AS, Nabb S, Jones PK, Clark AL, Cleland JG. Clinical determinants of poor six-minute walk test performance in patients with left ventricular systolic dysfunction and no major structural heart disease. Eur J Heart Fail. 2006;8(3):321–5.
Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–4.
Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110(2):155–62.
Cioffi G, Gerdts E, Cramariuc D, Tarantini L, Di Lenarda A, Pulignano G, et al. Left atrial size and force in patients with systolic chronic heart failure: Comparison with healthy controls and different cardiac diseases. Exp Clin Cardiol. 2010;15(3):e45–51.
Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail. 2009;11(10):929–36. doi:10.1093/eurjhf/hfp112.
Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2016;375(19):1868–77.
Kuppahally SS, Michaels AD, Tandar A, Gilbert EM, Litwin SE, Bader FM. Can echocardiographic evaluation of cardiopulmonary hemodynamics decrease right heart catheterizations in end-stage heart failure patients awaiting transplantation? Am J Cardiol. 2010;106:1657–62.
Acknowledgements
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors have contributed (AB, GB, PI, and MH designed the manuscript; PI, IB and GB analyzed and interpreted the data; AB, PI, AA and EH drafted the manuscript; MH, GB and SE revised critically), read and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Statement on ethics approval
Nr.3729, date 22.10.2010.
Name of the ethics committee
Ethics Committee of Medical Faculty, University of Prishtina.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Batalli, A., Ibrahimi, P., Bytyçi, I. et al. Different determinants of exercise capacity in HFpEF compared to HFrEF. Cardiovasc Ultrasound 15, 12 (2017). https://doi.org/10.1186/s12947-017-0103-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12947-017-0103-x