Abstract
Background
Weight gain and metabolic disorders are commonly induced by antipsychotics. Orlistat is a lipase inhibitor used for weight control. The effect of orlistat on weight gain and metabolic disturbances in people (especially women) treated with antipsychotics has not been sufficiently studied. This study aimed to investigate the efficacy of orlistat in mitigating antipsychotic-induced weight gain and abnormal glycolipid metabolism.
Methods
Patients with schizophrenia or bipolar disorder with a weight gain ≥ 7% after taking antipsychotics were recruited. Participants were randomly allocated to two groups: one received eight weeks of orlistat (360 mg/day) and the other received a placebo. Anthropometric and fasting serum biochemical parameters were measured at baseline, week 4 and week 8.
Results
Sixty individuals (orlistat:placebo = 32:28) participated in the study. After controlling for the study center, the eight-week changes in body mass index (BMI), cholesterol (CHOL), high-density lipoprotein cholesterol (HDL-CH) and low-density lipoprotein cholesterol (LDL-CH) were significantly different between the groups. According to the mixed linear models, CHOL and LDL-CH were significantly lower in the orlistat group than in the control group at week 8. The week 0-to-8 slopes of BMI, CHOL and LDL-CH were also significantly lower in the orlistat group.
Conclusions
These findings suggested that orlistat is an effective intervention for attenuating weight gain and serum lipid disturbances in antipsychotic-treated patients.
Trial registration
ClinicalTrials.gov NCT03451734.
Similar content being viewed by others
Background
Antipsychotics are widely used as an effective therapy for various mental disorders, including schizophrenia and affective disorders. However, antipsychotics may also induce metabolic abnormalities [1,2,3,4]. Huhn et al. analyzed data from 116 studies and found that 12 antipsychotics could induce significant weight gain in comparison with a placebo [5]. Subsequent meta-analyses indicated that blood lipids and glucose could also be influenced by multiple antipsychotics [6]. Without intervention, obesity and metabolic disorders may lead to cardiovascular disease [7], which shortens [8, 9] and impairs the quality of life and adherence to antipsychotics [10, 11]. According to previous literature, chlorpromazine, olanzapine and clozapine are at high risk for metabolic disturbance [12,13,14].
Antipsychotic-treated patients prefer high-fat food [15, 16]. Increased high-fat food cravings can predict clozapine-induced weight gain [17]. Furthermore, restrictions on fat intake contribute to attenuating antipsychotic-related weight gain [18, 19]. As the only clinically approved drug for obesity management in China [20], orlistat reduces systemic absorption of dietary fat by inhibiting gastric and pancreatic lipases in the gastrointestinal tract [21, 22]. Orlistat is not absorbable and is almost completely excreted in the feces [23, 24]. Previous studies have thoroughly investigated the effects of orlistat on obesity [25,26,27,28]. Long-term orlistat treatment reduces weight by 2–3 kg in obese people [26,27,28,29]. In addition, orlistat decreases fasting serum low-density lipoprotein cholesterol (LDL-CH), glucose and insulin levels, too [30,31,32]. For obese individuals with diabetes [33,34,35,36] and polycystic ovary syndrome [37,38,39], orlistat has also been shown to be capable of controlling weight and improving glycolipid metabolism.
Orlistat is also suitable for obese people with mental disorders because it does not influence plasma levels of psychotropic drugs [40]. However, there is limited research on the effectiveness of orlistat for treating antipsychotic-related obesity. Pavlovic reported a case, in which orlistat reduced the fasting blood glucose and weight of a man with schizophrenia on clozapine [41]. Joffe et al. administered orlistat to individuals taking olanzapine or clozapine for 16 weeks [42]. The results showed that orlistat effectively reduced weight in male participants, but not in female participants. Subsequent research confirmed the efficacy of orlistat in weight control for antipsychotic-treated men [43]. In fact, compared to men, women on antipsychotics have greater risks of weight gain, diabetes, and cardiovascular events [44]. Further evidence is needed to determine whether orlistat is beneficial to individuals, especially women, who take antipsychotics. This study was designed to assess the efficacy of orlistat in mitigating antipsychotic-induced weight gain. It was hypothesized that orlistat could control weight and improve glycolipid metabolism in antipsychotic-treated individuals.
Methods
Trial design
This clinical trial used a multicenter, randomized, double-blind, placebo-controlled design. Participants were assigned to the orlistat group and the control group at a 1:1 ratio. Interviews were conducted at three different time points: before treatment (week 0), four weeks after treatment began (week 4), and at the endpoint of the eight weeks of treatment (week 8).
Demographic information and medical history were collected at week 0. Height, Weight, hip circumference and waist circumference were measured at all three visits to calculate body mass index (BMI) and the waist-to-hip ratio (WHR). Fasting blood was collected at the three time points to analyze serum biochemical parameters including cholesterol (CHOL), high-density lipoprotein cholesterol (HDL-CH), LDL-CH, the HDL-CH–to–CHOL ratio (HD/CH), triglyceride (TG), glucose (GLU) and glycosylated hemoglobin (HbA1c). Participants were asked to report adverse events and the number of their remaining capsules at each visit as an indicator of treatment adherence. Taking fewer than two capsules per day was considered a treatment interruption.
This study is part of the project, “Optimizing and Individualizing the Pharmacological Treatment of First-episode Schizophrenic Patients” (ClinicalTrials.gov NCT03451734), which has been approved by the Medical Ethics Committee of the Second Xiangya Hospital of Central South University (ref: 2016S035) [45]. This study followed the Declaration of Helsinki, and the privacy rights of participants were always observed.
Participants
Participants were recruited from two study centers: the Second Xiangya Hospital of Central South University (Changsha, Hunan) and the Affiliated Kangning Hospital of Ningbo University (Ningbo, Zhejiang). Individuals with schizophrenia or bipolar disorder were introduced to orlistat and the study protocol in detail. Only patients who were eligible for this study were recorded.
The inclusion criteria of this study are listed below: (a) aged between 16 and 60 years; (b) diagnosed with schizophrenia or bipolar disorder based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); (c) receiving an antipsychotic at a constant dose for at least one month, with no plan to change the therapeutic regimen; (d) gaining weight after initiation of antipsychotic treatment and a current weight gain of ≥ 7% compared to the pre antipsychotic weight; and (e) providing written consent for themselves and their guardians.
Participants with the following conditions were excluded: (a) had a contraindication for orlistat, including an allergy and being overweight or obese due to physical disease; (b) were diagnosed with a major physical disease (including hyperthyroidism, hypothyroidism, hypertension, chronic liver disease, chronic kidney disease, immune system disorder and tumor); (c) were on a hypoglycemic drug, a hypolipemic drug, an anti-obesity drug, or any other therapy for weight loss (including systematic dietary control and high-intensity exercise) currently or during the past three months; (d) were pregnant or lactating; (e) had a history of psychoactive substance abuse or addiction; (f) were diagnosed with an eating disorder; and (g) were not taking medicine or interviewed as needed.
The study was monitored by an experienced psychiatrist. Participants’ mental status and adverse events were evaluated through interviews every four weeks. In addition, the research team was accessible to the participants and their guardians so that they could report any adverse events or changes in their conditions and consult with the researchers at any time. If participants experienced a health condition that required treatment violating the study protocol, researchers would help them access the necessary medical resources and exclude them from the study. Participants who met an exclusion criterion could still be interviewed if they were willing, and related data were analyzed by the intention-to-treat (ITT) method.
Interventions
The orlistat group received orlistat capsules (0.12 g per capsule) and the control group received placebo capsules. The orlistat and placebo capsules were free of charge to participants. While continuing their original treatments, participants were required to take one capsule (orlistat or placebo) they received during or within one hour after each meal, three times a day for eight weeks.
Outcomes
The primary outcomes were weight and BMI and the changes in them from baseline to the endpoint. To calculate BMI, weight (in kg) was divided by height (in m) squared. The secondary outcomes were levels of WHR, TG, CHOL, HDL-CH, LDL-CH, HD/CH, GLU and HbA1c, as well as the changes in these indicators from baseline to the endpoint.
Sample size
Power and sample size calculations were conducted using the Power and Sample Size tool (http://powerandsamplesize.com/). The calculations were based on the results of a pre-experiment and designed to demonstrate an intergroup difference of 0.4 in changes in BMI from week 0 to week 8, with a power of 90% and a two-tailed significance level of 0.05. In addition, a standard deviation (SD) of 0.6 and an anticipated 20% loss to follow-up were also factored in. Eventually, it was determined that each treatment group would require a sample size of 26.
Randomization and masking
Participants were assigned according to tables of random numbers generated by SPSS version 26 (IBM Corp, Armonk, NY, US). Orlistat and placebo capsules used in this trial were produced and packed by Hangzhou Zhongmeihuadong Pharmaceutical Co. The capsules looked the same and could only be distinguished from each other by production batch numbers. The lists recording the actual content of each batch were sealed in envelopes by the producer and were confidential to the researchers and participants during recruitment and visiting.
Data analyses
Differences in variables over eight weeks were compared between groups by linear regression models. ITT analyses were achieved by filling in missing values via the last-observation-carried-forward (LOCF) method or by conducting mixed linear models. The study center was included in the linear regression models and mixed linear models as a covariate. In sensitivity analyses, taking a mood stabilizer was included as a covariate along with the study center. Stata version 17 (StataCorp LLC, College Station, TX, US) was used to construct mixed linear models. Other statistical analyses were conducted with SPSS version 26. The results were considered significant when P < 0.05 or 95% confidence intervals (CIs) did not include 0.
Results
Demographic data
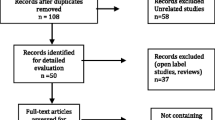
Sixty eligible patients who used antipsychotics participated in this study from June 2019 to October 2021. Demographic and clinical information along with baseline outcomes are shown in Table 1. Briefly, 26 (43.3%) patients were diagnosed with schizophrenia and 34 (56.7%) were diagnosed with bipolar disorder. The illness duration was approximately 61.11 months (SD = 82.56). Thirty-eight (63.3%) participants were treated with olanzapine or clozapine, and none of the participants were treated with chlorpromazine. Other participants were on quetiapine, amisulpride, risperidone, lurasidone, aripiprazole or phenazopyridine (36.7%). Nineteen (31.7%) participants were taking two antipsychotics. The average age of the participants was 25.68 years (SD = 9.72). The participants were mainly (90.0%) females, and the overwhelming majority (93.3%) were Han Chinese. The demographic characteristics and the baseline outcomes were not significantly different between the groups (P > 0.05).
As illustrated in Fig. 1, 32 participants were randomly assigned to the orlistat group and 28 were assigned to the placebo group. In the orlistat group, one patient did not take orlistat continuously for eight weeks but was followed up by week 4 and week 8; one patient started using atorvastatin after week 0 but was followed up by week 4 and week 8; and two patients stopped taking orlistat before week 4 but were followed up by week 4 and were lost by week 8. The data obtained from the visits mentioned above were included in the ITT analyses but were excluded when calculating differences in variables over eight weeks. Two patients in the placebo group were unwilling to further participate in this study after their last visit. Finally, 32 participants (53.3%) completed their 8-week treatments and all visits, including 19 (59.4%) from the orlistat group and 13 (46.4%) from the placebo group (P = 0.316).
Comparisons of differences over eight weeks between groups
After eight weeks of treatments, weight decreased by 1.32 ± 2.16 kg and BMI decreased by 0.48 ± 0.77 kg/m2 in the orlistat group while weight increased by 0.47 ± 1.72 kg and BMI increased by 0.20 ± 0.65 kg/m2 in the placebo group. For the secondary outcomes, CHOL, HDL-CH, LDL-CH and GLU levels were also increased by placebo and reduced by orlistat treatment. After controlling for the study center in linear regression models, 8-week changes in weight, BMI, CHOL, LDL-CH and HDL-CH significantly differed between the two groups (b = -1.77 to -0.2, upper bounds of 95% CIs < 0, Table 2). Linear regression models were performed again after filling in missing values by the LOCF method, and the results remained almost the same (Table S1).
ITT analyses
Considering the high proportion of participants who dropped out, ITT analyses were conducted to further verify the effects of orlistat on metabolism-related indices. As shown in Fig. 2, the margin of BMI increased from 27.11 (95% CI: [25.51, 28.71]) to 27.34 (95% CI: [25.70, 28.98]) kg/m2 in the placebo group (slope: 0.23, 95% CI: [-0.20, 0.66]) and decreased from 28.31 (95% CI: [26.84, 29.77]) to 27.89 (95% CI: [26.40, 29.38]) kg/m2 in the orlistat group (slope: -0.41, 95% CI: [-0.75, -0.08]). Similarly, the margins of CHOL, LDL-CH and GLU also decreased in the orlistat group and increased in the placebo group. No variables significantly differed between the groups at week 0 and week 4. However, at week 8, the CHOL (contrast = -0.66, 95% CI: [-1.18, -0.15]) and LDL-CH (contrast = -0.54, 95% CI: [-0.99, -0.09]) levels in the orlistat group were significantly lower in comparison with those in the placebo group. The week-0-to-8 slopes of BMI (contrast = -0.64, 95% CI: [-1.19, -0.10]), CHOL (contrast = -0.69, 95% CI: [-1.09, -0.28]) and LDL-CH (contrast = -0.61, 95% CI: [-0.97, -0.26]) were significantly lower in the group treated with orlistat. ITT analyses were also repeated without data from the invalid visits mentioned in § 3.1, and similar results were obtained (Figure S1).
Mixed linear models for comparisons of metabolic parameters. Data from all visits were analyzed using the intent-to-treat method. (a–j) show results for weight, BMI, WHR, TG, CHOL, HDL-CH, LDL-CH, HD/CH, GLU and HbA1c, respectively. The dots and error bars represent margins and 95% confidence intervals. ✱ Significant difference between groups (95% confidence interval for the contrast between margins does not include 0). Abbreviations: BMI: body mass index, WHR: waist-to-hip ratio, TG: triglyceride, CHOL: cholesterol, HDL-CH: high-density lipoprotein cholesterol, LDL-CH: low-density lipoprotein cholesterol, HD/CH: HDL-CH–to–CHOL ratio, GLU: glucose, HbA1c: glycosylated hemoglobin
Subgroup analyses
Sex
Previous studies have indicated that orlistat is not effective for females [42, 43]. Therefore, the metabolic parameters of the female participants were analyzed again (Figure S2). The outcomes were similar to the previous outcomes. CHOL and LDL-CH levels did not differ between groups at baseline but were lower in the orlistat group after eight weeks (CHOL: contrast = -0.75, 95% CI: [-1.26, -0.24]; LDL-CH: contrast = -0.68, 95% CI: [-1.13, -0.23]). Weight, BMI, CHOL, LDL-CH, HDL-CH and GLU increased in the placebo group but decreased in the orlistat group. The differences in the week-0-to-8 slopes between the groups were significant for weight (contrast = -1.55, 95% CI: [-3.01, -0.09]), BMI (contrast = -0.61, 95% CI: [-1.16, -0.06]), CHOL (contrast = -0.72, 95% CI: [-1.15, -0.28]), HDL-CH (contrast = -0.20, 95% CI: [-0.34, -0.07]) and LDL-CH (contrast = -0.62, 95% CI: [-1.00, -0.24]). The number of males was too low so ITT analyses were not repeated for male participants.
Diagnosis
In participants with schizophrenia, orlistat was ineffective to weight or BMI, but the slopes of CHOL (contrast = -0.93, 95% CI: [-1.56, -0.30]) and LDL-CH (contrast = -0.87, 95% CI: [-1.37, -0.36]) were still lower in the orlistat group. In participants with bipolar disorder, the slopes of weight (contrast = -1.32, 95% CI: [-4.90, -0.00]) and BMI (contrast = -0.94, 95% CI: [-1.83, -0.05]) were significantly lower for those treated with orlistat; CHOL did not differ between groups at week 0 but the orlistat group had a significantly lower CHOL level at week 8 (contrast = -0.79, 95% CI: [-1.54, -0.03]) (Figure S3).
Metabolic risk levels of antipsychotics
In participants taking olanzapine or clozapine, the slopes of weight (contrast = -2.55, 95% CI: [ -4.36, -0.75]), BMI (contrast = -0.97, 95% CI: [-1.63, -0.31]), CHOL (contrast = -0.74, 95% CI: [-1.20, -0.28]) and LDL-CH (contrast = -0.58, 95% CI: [-1.01, -0.14]) were significantly reduced by orlistat. In participants treated with other antipsychotics, week-0-to-8 slopes were not different between groups; CHOL (contrast = -1.17, 95% CI: [-2.07, -0.27]), LDL-CH (contrast = -0.83, 95% CI: [-1.55, -0.12]) and GLU (contrast = -1.20, [-2.28, -0.12]) were significantly lower in the orlistat group after interventions, while no indicators differed between the groups at baseline (Figure S4).
Numbers of antipsychotics
For participants treated with one antipsychotic, the week-0-to-8 slopes of BMI (contrast = -0.76, 95% CI: [-1.48, -0.04]), CHOL (contrast = -0.86, 95% CI: [-1.34, -0.37]) and LDL-CH (contrast = -0.71, 95% CI: [-1.14, -0.27]) in the orlistat group were significantly lower; CHOL and LDL-CH were comparable for the two groups at baseline but were significantly lower in the orlistat group at week 8 (CHOL: contrast = -0.65, 95% CI: [-1.29, -0.02]; LDL-CH: contrast = -0.53, 95% CI: [-1.05, -0.00]). In participants treated with two antipsychotics, metabolic parameters did not differ between groups at any time point; slopes were not different between groups (Figure S5).
Sensitivity analyses
Twenty-five (41.7%) participants were taking lithium, valproate, or lamotrigine. The effect of mood stabilizers on body weight is controversial and was therefore included in sensitivity analyses [46]. Week-0-to-8 slopes of weight (contrast = -1.66, 95% CI: [-3.15, -0.18]), BMI (contrast = -0.64, 95% CI: [-1.19, -0.10]), CHOL (contrast = -0.69, 95% CI: [-1.09, -0.29]) and LDL-CH (contrast = -0.61, 95% CI: [-0.97, -0.26]) were significantly different between groups. CHOL (contrast = -0.63, 95% CI: [-1.17, -0.10]) and LDL-CH (contrast = -0.52, 95% CI: [-0.99, -0.05]) were significantly lower in the orlistat group after the intervention while no variable was significantly different between the groups at baseline (Figure S6).
Adverse events
Sixteen participants (50.0%) in the orlistat group and two (7.1%) in the placebo group reported newly-emerged diarrhea, oily feces, or oil leakage from the anus during the study (P < 0.001). No other severe side effects related to orlistat were reported.
Discussion
This study is a randomized, placebo-controlled, and double-blind clinical trial focusing on the efficiency of orlistat on metabolic side effects caused by antipsychotics. The results indicate that orlistat contributes to weight control and blood lipid homeostasis in antipsychotic-treated people, which is consistent with previous studies conducted in obese people [25,26,27,28,29,30,31, 47, 48]. However, the effects of orlistat on glucose metabolism are not credible, which is inconsistent with previous reports [29, 31, 35, 48,49,50]. The level of HbA1c is relatively stable, so an 8-week intervention with orlistat may be too short to influence its level. For fasting serum glucose, the mixed linear model tended to decrease after orlistat treatment, but the difference was not statistically significant; this may be explained by the dispersion of the data. Further research should be carried out with larger sample sizes to provide confirmative evidence that orlistat repairs antipsychotic-related glucose metabolic disturbances.
Few studies have examined the efficiency of orlistat on antipsychotic-related weight gain. Previous articles reported that in olanzapine- or clozapine-treated people, orlistat was effective in weight control for men but not women [42, 43]. However, among individuals taking antipsychotics, women are at a greater risk of metabolic syndrome than men are [51,52,53,54]. Women are also more interested in becoming slimmer [55, 56], which makes the effectiveness of orlistat even more meaningful for them. Although the majority of the samples were from females, protective effects of orlistat on metabolism were still observed, which persisted when only females were analyzed. According to previous articles, orlistat can also decrease body weight and serum lipids and improve insulin sensitivity in obese females diagnosed with polycystic ovary syndrome [37, 39, 57]. Overall, orlistat may still be beneficial for females who are overweight or obese due to taking antipsychotics.
Metabolic syndrome is equally prevalent among patients with bipolar disorder and patients with schizophrenia [58, 59]. However, it is not clear whether the prognosis of antipsychotic-related metabolic abnormalities differs between schizophrenia and bipolar disorder [60,61,62]. Furthermore, the use of olanzapine and clozapine results in a greater risk for metabolic disturbance than other antipsychotics [13, 58, 62]. This study presents an exploration of the effects of diagnosis and treatment regimen on the efficacy of orlistat by subgroup analyses. The results revealed that orlistat was able to control weight and serum lipids in antipsychotic-treated participants with schizophrenia and bipolar disorder. Orlistat also effectively decreased the weight gain and increased serum lipids induced by antipsychotics with high metabolic risk, and increased serum lipids and glucose levels induced by antipsychotics with medium and low metabolic risk. In addition, orlistat was ineffective for participants who took two antipsychotics. However, considering the sample size, the results of subgroup analyses may not be representative. Mood stabilizers may also lead to weight gain [63]. Compared to antipsychotics, mood stabilizers have less of an effect on weight [64,65,66]. It has also been argued that there is insufficient evidence to prove that mood stabilizers (such as lithium, valproate and lamotrigine) can significantly increase weight [46, 67, 68]. Sensitivity analyses that included the presence or absence of mood stabilizers as a covariate were conducted in mixed linear models. The results still showed that orlistat alleviated antipsychotic-induced weight gain and dyslipidemia.
Eating behaviors are related to the efficiency of orlistat [69]. Orlistat treatment is usually combined with a low-fat diet to fully exert its effects [30,31,32, 38, 39, 70,71,72]. However, low compliance is common in weight loss programs [73]. People with negative or depressive symptoms may lack the motivation to follow a weight-control diet, especially when their appetite is elevated by antipsychotics. In this study, there was no requirement for physical exercise or diet, which may have limited the efficiency of orlistat but allowed the study to reflect the real situation. Taghizadeh et al. also observed a decrease in body weight in a group of overweight people who received orlistat treatment while maintaining their lifestyles [74]. In two other large studies, orlistat remained capable of controlling body weight after switching from a hypocaloric diet to a weight-maintenance diet [30, 31]. For people who cannot change their lifestyle, orlistat alone is still helpful for weight control.
Orlistat is generally safe because its absorption and accumulation are negligible [24, 31]. In this study, orlistat rarely induced side effects other than gastrointestinal adverse events, which is consistent with previous findings [30,31,32, 70, 75]. Even gastrointestinal events tend to occur early in orlistat treatment and may resolve spontaneously [30, 31, 76]. In addition, orlistat does not influence plasma concentrations of psychotropic drugs [40]. While benefitting body weight and lipid metabolism, orlistat does not interfere with antipsychotic treatment [77]. Therefore, orlistat is suitable for overweight or obese people taking antipsychotics.
Study strengths and limitations
The efficacy of orlistat for antipsychotic metabolic side effects has not been adequately studied. Previous articles reported that orlistat was able to alleviate olanzapine- and clozapine-induced weight gain and abnormal serum lipid and glucose levels, but was only effective in men with schizophrenia [41,42,43]. In contrast, this study proved that orlistat is effective in antipsychotic-treated women and patients with bipolar disorder. The present study also confirmed that orlistat is effective for treating metabolic abnormalities caused by antipsychotics other than olanzapine and clozapine. Furthermore, the study revealed that orlistat is effective only in patients treated with a single antipsychotic. In summary, this study fills the gap of previous studies and affirms the efficacy of orlistat in managing the metabolic side effects induced by antipsychotics.
This study has a few limitations. First, the sample size was small. During the coronavirus disease (COVID-19) pandemic, recruitment was suspended for months because of pandemic prevention and control policies and the delayed supply of study drugs. Further, more than 1/3 of the participants dropped out, which is similar to the findings of some long-term studies on orlistat [30, 31, 40, 78]. Some participants refused to leave home for follow-up visits to avoid the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Meanwhile, the characteristic side effects of orlistat caused difficulties in blinding. Many participants were convinced that they were taking a placebo due to the absence of weight loss and steatorrhea and were unwilling to continue the study. As a result, more individuals completed the study in the orlistat group than in the placebo group, which was consistent with the higher incidence of gastrointestinal adverse events in the orlistat group. In previous studies with large samples, the drop-out ratios in placebo groups were also greater [30, 31, 36]. A placebo lead-in period may help screen out participants with low adherence, thus reducing attrition [79]. However, this was a short-term study, and the availability of study drugs was limited, so there was no lead-in period. The sample size also limited the quality of subgroup analyses. In summary, the effects of orlistat on antipsychotic-induced metabolic disorders should be validated in larger samples exclusive to individuals with poor compliance.
Conclusions
In antipsychotic-treated patients, eight weeks of orlistat treatment can control body weight and improve fasting lipid levels. Orlistat is also effective in antipsychotic-treated women. Patients with bipolar disorder and patients with schizophrenia can both benefit from orlistat treatment. In addition, orlistat is effective at alleviating metabolic disturbances induced by antipsychotics, regardless of metabolic risk level. However, orlistat is not effective in patients taking multiple antipsychotics.
Data availability
Data are accessible through corresponding authors upon reasonable request.
Abbreviations
- BMI:
-
Body Mass Index
- WHR:
-
Waist-to-Hip Ratio
- TG:
-
Triglyceride
- CHOL:
-
Cholesterol
- HDL-CH:
-
High-Density Lipoprotein Cholesterol
- LDL-CH:
-
Low-Density Lipoprotein Cholesterol
- HD/CH:
-
HDL-CH–to–CHOL ratio
- GLU:
-
Glucose
- HbA1c:
-
glycosylated Hemoglobin
- ITT:
-
Intention-To-Treat
- SD:
-
Standard Deviation
- CI:
-
Confidence Interval
- LOCF:
-
Last-Observation-Carried-Forward
References
Barton BB, Segger F, Fischer K, Obermeier M, Musil R. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19:295–314.
Tek C, Kucukgoncu S, Guloksuz S, Woods SW, Srihari VH, Annamalai A. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiat. 2016;10:193–202.
Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients. Neuropsychopharmacology. 2010;35:1997–2004.
Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: differences between antipsychotic-naive and treated patients. J Pharmacol Pharmacother. 2013;4:176–86.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L, Rothe P, Cipriani A, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–51.
Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, Beck K, Natesan S, Efthimiou O, Cipriani A, Howes OD. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64–77.
Ratliff JC, Palmese LB, Reutenauer EL, Srihari VH, Tek C. Obese schizophrenia spectrum patients have significantly higher 10-year general cardiovascular risk and vascular ages than obese individuals without severe mental illness. Psychosomatics. 2013;54:67–73.
Jayatilleke N, Hayes RD, Dutta R, Shetty H, Hotopf M, Chang CK, Stewart R. Contributions of specific causes of death to lost life expectancy in severe mental illness. Eur Psychiatry. 2017;43:109–15.
Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with Schizophrenia in the United States. JAMA Psychiatry. 2015;72:1172–81.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23.
Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. 2004;66:51–7.
Li C, Mittal D, Owen RR. Impact of patients’ preexisting metabolic risk factors on the choice of antipsychotics by Office-Based Physicians. Psychiatric Serv. 2011;62:1477–84.
Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17:341–56.
Burschinski A, Schneider-Thoma J, Chiocchia V, Schestag K, Wang D, Siafis S, Bighelli I, Wu H, Hansen WP, Priller J, et al. Metabolic side effects in persons with schizophrenia during mid- to long-term treatment with antipsychotics: a network meta-analysis of randomized controlled trials. World Psychiatry. 2023;22:116–28.
Mutwalli H, Keeler JL, Bektas S, Dhopatkar N, Treasure J, Himmerich H. Eating cognitions, emotions and behaviour under treatment with second generation antipsychotics: a systematic review and meta-analysis. J Psychiatr Res. 2023;160:137–62.
Bussell K, Reeves G, Hager E, Zhu S, Correll CU, Riddle MA, Sikich L. Dietary consumption among Youth with antipsychotic-Induced Weight Gain and Changes following healthy Lifestyle Education. J Child Adolesc Psychopharmacol. 2021;31:364–75.
Garriga M, Mallorquí A, Serrano L, Ríos J, Salamero M, Parellada E, Gómez-Ramiro M, Oliveira C, Amoretti S, Vieta E, et al. Food craving and consumption evolution in patients starting treatment with clozapine. Psychopharmacology. 2019;236:3317–27.
Ntalkitsi S, Efthymiou D, Bozikas V, Vassilopoulou E. Halting the Metabolic Complications of Antipsychotic Medication in patients with a first episode of psychosis: how far can we go with the Mediterranean Diet? A pilot study. Nutrients 2022, 14.
Green CA, Yarborough BJ, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, Perrin NA, Nichols GA, Stevens VJ. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry. 2015;172:71–81.
Zeng Q, Li N, Pan XF, Chen L, Pan A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol. 2021;9:393–405.
Hogan S, Fleury A, Hadvary P, Lengsfeld H, Meier MK, Triscari J, Sullivan AC. Studies on the antiobesity activity of tetrahydrolipstatin, a potent and selective inhibitor of pancreatic lipase. Int J Obes. 1987;11(Suppl 3):35–42.
Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20:270–9.
Zhi J, Melia AT, Funk C, Viger-Chougnet A, Hopfgartner G, Lausecker B, Wang K, Fulton JS, Gabriel L, Mulligan TE. Metabolic profiles of minimally absorbed orlistat in obese/overweight volunteers. J Clin Pharmacol. 1996;36:1006–11.
Zhi J, Melia AT, Eggers H, Joly R, Patel IH. Review of limited systemic absorption of Orlistat, a lipase inhibitor, in healthy human volunteers. J Clin Pharmacol. 1995;35:1103–8.
Shi Q, Wang Y, Hao Q, Vandvik PO, Guyatt G, Li J, Chen Z, Xu S, Shen Y, Ge L, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet. 2022;399:259–69.
Singh AK, Singh R. Pharmacotherapy in obesity: a systematic review and meta-analysis of randomized controlled trials of anti-obesity drugs. Expert Rev Clin Pharmacol. 2020;13:53–64.
Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of Pharmacological Treatments for Obesity with Weight Loss and adverse events: a systematic review and Meta-analysis. JAMA. 2016;315:2424–34.
Sahebkar A, Simental-Mendía LE, Reiner Ž, Kovanen PT, Simental-Mendía M, Bianconi V, Pirro M. Effect of orlistat on plasma lipids and body weight: a systematic review and meta-analysis of 33 randomized controlled trials. Pharmacol Res. 2017;122:53–65.
Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2004;2003:CD004094.
Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, Heimburger DC, Lucas CP, Robbins DC, Chung J, Heymsfield SB. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235–42.
Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–72.
Drent ML, Larsson I, William-Olsson T, Quaade F, Czubayko F, von Bergmann K, Strobel W, Sjostrom L, van der Veen EA. Orlistat (Ro 18–0647), a lipase inhibitor, in the treatment of human obesity: a multiple dose study. Int J Obes Relat Metab Disord. 1995;19:221–6.
Derosa G, Cicero AF, D’Angelo A, Fogari E, Maffioli P. Effects of 1-year orlistat treatment compared to placebo on insulin resistance parameters in patients with type 2 diabetes. J Clin Pharm Ther. 2012;37:187–95.
Aldekhail NM, Logue J, McLoone P, Morrison DS. Effect of orlistat on glycaemic control in overweight and obese patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2015;16:1071–80.
Jacob S, Rabbia M, Meier MK, Hauptman J. Orlistat 120 mg improves glycaemic control in type 2 diabetic patients with or without concurrent weight loss. Diabetes Obes Metab. 2009;11:361–71.
Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, Weiss SR, Crockett SE, Kaplan RA, Comstock J, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care. 1998;21:1288–94.
Ghandi S, Aflatoonian A, Tabibnejad N, Moghaddam MH. The effects of metformin or orlistat on obese women with polycystic ovary syndrome: a prospective randomized open-label study. J Assist Reprod Genet. 2011;28:591–6.
Panidis D, Tziomalos K, Papadakis E, Chatzis P, Kandaraki EA, Tsourdi EA, Katsikis I. The role of orlistat combined with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin Endocrinol. 2014;80:432–8.
Moini A, Kanani M, Kashani L, Hosseini R, Hosseini L. Effect of orlistat on weight loss, hormonal and metabolic profiles in women with polycystic ovarian syndrome: a randomized double-blind placebo-controlled trial. Endocrine. 2015;49:286–9.
Hilger E, Quiner S, Ginzel I, Walter H, Saria L, Barnas C. The effect of orlistat on plasma levels of psychotropic drugs in patients with long-term psychopharmacotherapy. J Clin Psychopharmacol. 2002;22:68–70.
Pavlovic ZM. Orlistat in the treatment of clozapine-induced hyperglycemia and weight gain. Eur Psychiatry. 2005;20:520.
Joffe G, Takala P, Tchoukhine E, Hakko H, Raidma M, Putkonen H, Eronen M, Rasanen P. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:706–11.
Tchoukhine E, Takala P, Hakko H, Raidma M, Putkonen H, Rasanen P, Terevnikov V, Stenberg JH, Eronen M, Joffe G. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week open-label extension phase and both phases of a randomized controlled trial. J Clin Psychiatry. 2011;72:326–30.
Seeman MV. Secondary effects of antipsychotics: women at greater risk than men. Schizophr Bull. 2009;35:937–48.
Xiao J, Huang J, Long Y, Wang X, Wang Y, Yang Y, Hei G, Sun M, Zhao J, Li L, et al. Optimizing and individualizing the pharmacological treatment of first-episode schizophrenic patients: study protocol for a Multicenter Clinical Trial. Front Psychiatry. 2021;12:611070.
Domecq JP, Prutsky G, Leppin A, Sonbol MB, Altayar O, Undavalli C, Wang Z, Elraiyah T, Brito JP, Mauck KF, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363–70.
Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. Am J Clin Nutr. 2004;80:1461–8.
Zhou YH, Ma XQ, Wu C, Lu J, Zhang SS, Guo J, Wu SQ, Ye XF, Xu JF, He J. Effect of anti-obesity drug on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2012;7:e39062.
Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, D’Angelo A, Fogari E, Palumbo I, Randazzo S, Cicero AF. Comparison of orlistat treatment and placebo in obese type 2 diabetic patients. Expert Opin Pharmacother. 2010;11:1971–82.
Kopelman P, Groot GH, Rissanen A, Rossner S, Toubro S, Palmer R, Hallam R, Bryson A, Hickling RI. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obes (Silver Spring). 2010;18:108–15.
Ingimarsson O, MacCabe JH, Haraldsson M, Jonsdottir H, Sigurdsson E. Risk of diabetes and dyslipidemia during clozapine and other antipsychotic drug treatment of schizophrenia in Iceland. Nord J Psychiatry. 2017;71:496–502.
Afzal M, Siddiqi N, Ahmad B, Afsheen N, Aslam F, Ali A, Ayesha R, Bryant M, Holt R, Khalid H, et al. Prevalence of overweight and obesity in people with severe Mental illness: systematic review and Meta-analysis. Front Endocrinol (Lausanne). 2021;12:769309.
Woldekidan NA, Mohammed AS, Degu A, Tadiwos Y. Prevalence of metabolic syndrome and associated factors among psychiatric patients at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE. 2021;16:e0256195.
Allison DB, Fontaine KR, Heo M, Mentore JL, Cappelleri JC, Chandler LP, Weiden PJ, Cheskin LJ. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60:215–20.
Gualdi-Russo E, Rinaldo N, Masotti S, Bramanti B, Zaccagni L. Sex differences in body image perception and ideals: analysis of possible determinants. Int J Environ Res Public Health 2022, 19.
Soares FLC, Batista RFL, Cardoso VC, Simoes VMF, Santos AM, Coelho S, Silva AAM. Body image dissatisfaction and symptoms of depression disorder in adolescents. Braz J Med Biol Res. 2020;54:e10397.
Cho LW, Kilpatrick ES, Keevil BG, Coady AM, Atkin SL. Effect of metformin, orlistat and pioglitazone treatment on mean insulin resistance and its biological variability in polycystic ovary syndrome. Clin Endocrinol. 2009;70:233–7.
Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, Rosenbaum S, Correll CU. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14:339–47.
Tao H, Shen D, Zhou Y, Sun F, Li G, Jin W. A systematic review and Meta-analysis of metabolic syndrome prevalence in Chinese inpatients with bipolar disorder. Horm Metab Res. 2022;54:587–92.
De Carlo V, Grancini B, Cassina N, Casati L, Piccoli E, Vismara M, Gobbo D, Zanaschi R, Lupo S, Olivieri S, Dell’Osso B. Cardiovascular risk factors and metabolic syndrome in patients treated with long-acting injectables antipsychotics: a retrospective study. Int Clin Psychopharmacol. 2023;38:160–8.
Kahn RS, Kane JM, Correll CU, Arevalo C, Simmons A, Graham C, Yagoda S, Hu B, McDonnell D. Olanzapine/Samidorphan in young adults with Schizophrenia, Schizophreniform Disorder, or bipolar I disorder who are early in their illness: results of the Randomized, controlled ENLIGHTEN-Early Study. J Clin Psychiatry 2023, 84.
Miyakoshi T, Ishikawa S, Okubo R, Hashimoto N, Sato N, Kusumi I, Ito YM. Risk factors for abnormal glucose metabolism during antipsychotic treatment: a prospective cohort study. J Psychiatr Res. 2023;168:149–56.
Torrent C, Amann B, Sánchez-Moreno J, Colom F, Reinares M, Comes M, Rosa AR, Scott J, Vieta E. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18.
McIntyre RS, Kwan ATH, Rosenblat JD, Teopiz KM, Mansur RB. Psychotropic drug-related weight gain and its treatment. Am J Psychiatry. 2024;181:26–38.
McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721–8.
Choong E, Bondolfi G, Etter M, Jermann F, Aubry JM, Bartolomei J, Gholam-Rezaee M, Eap CB. Psychotropic drug-induced weight gain and other metabolic complications in a Swiss psychiatric population. J Psychiatr Res. 2012;46:540–8.
Wang Y, Xia J, Helfer B, Li C, Leucht S. Valproate for schizophrenia. Cochrane Database Syst Rev. 2016;11:CD004028.
McKnight RF, de La Motte DBDV, Chesney E, Amit BH, Geddes J, Cipriani A. Lithium for acute mania. Cochrane Database Syst Rev. 2019;6:CD004048.
Kim KK, Suh HS, Hwang IC, Ko KD. Influence of eating behaviors on short-term weight loss by orlistat and anorectic agent. Eat Behav. 2014;15:87–90.
Ahn SM, Kim H, Ji E, Han N, Oh JM. The effect of orlistat on weight reduction in obese and overweight Korean patients. Arch Pharm Res. 2014;37:512–9.
Olszanecka-Glinianowicz M, D browski P, KoceB ak P, Janowska J, Smertka M, Jonderko K, Chudek J. Long-term inhibition of intestinal lipase by orlistat improves release of gut hormones increasing satiety in obese women. Pharmacol Rep. 2013;65:666–71.
Alanazi J, Unnisa A, Ahmad S, Itumalla R, Alanazi M, Alharby TN, Anwar S, Younes KM, Hussain T, Hussain A, et al. Significance of Orlistat in management of dyslipidemia, systolic blood pressure and body mass index. Eur Rev Med Pharmacol Sci. 2022;26:8326–32.
Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016;10:1547–59.
Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A. Effect of the cumin cyminum L. Intake on Weight loss, metabolic profiles and biomarkers of oxidative stress in overweight subjects: a Randomized double-blind placebo-controlled clinical trial. ANNALS Nutr METABOLISM. 2015;66:117–24.
Arzola-Paniagua MA, Garcia-Salgado LER, Calvo-Vargas CG, Guevara-Cruz M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: a randomized controlled trial. Obes (Silver Spring). 2016;24:1454–63.
Rossner S, Sjostrom L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res. 2000;8:49–61.
Chukhin E, Terevnikov V, Takala P, Hakko H, Putkonen H, Rasanen P, Stenberg JH, Eronen M, Joffe G. Is there an interrelationship between the effects of antipsychotics on psychopathology and on metabolism? Nord J Psychiatry. 2016;70:190–4.
Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000;9:160–7.
Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Gravallese EA, Erondu NE, Heymsfield SB, Nguyen AM. Attrition from randomized controlled trials of pharmacological weight loss agents: a systematic review and analysis. Obes Rev. 2009;10:333–41.
Acknowledgements
Orlistat and placebo were generously provided by Hangzhou Zhongmeihuadong Pharmaceutical Co. We appreciate all participants’ devotion to the study.
Funding
The study was supported by the National Natural Science Foundation of China (grant numbers 82325020, 82271545, 82072096 and 82101578), the Key Research and Development Program of Hunan Province (grant number 2022SK2043), and the Hunan Provincial Natural Science Foundation of China (grant number 2024JJ9117).
Author information
Authors and Affiliations
Contributions
R.W. designed the study. R.W., T.S. and P.X. acquired resources. P.X., T.S., W.X., Y.Y. and Y.H. recruited and interviewed participants. Y.Liu and P.X. analyzed data. P.X. and Y.Long composed the original manuscript. P.X., Y.Long, Q.D. and H.T. made revisions. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study is a part of the project, “Optimizing and Individualizing the Pharmacological Treatment of First-episode Schizophrenic Patients”, which has been approved by the Medical Ethics Committee of the Second Xiangya Hospital of Central South University (ref: 2016S035). The conduction of this study followed the Declaration of Helsinki. All participants provided written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, P., Shao, T., Long, Y. et al. Orlistat for the treatment of antipsychotic-induced weight gain: an eight-week multicenter, randomized, placebo-controlled, double-blind trial. Lipids Health Dis 23, 225 (2024). https://doi.org/10.1186/s12944-024-02214-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02214-w