Abstract
Background
Generally, low-density lipoprotein (LDL) particle size can be inferred from the LDL cholesterol concentration to total apolipoprotein B concentration ratio (LDL-C/ApoB ratio, hereinafter called LAR), which is a good predictor of cardiovascular disease. However, the predictive ability of LAR for mortality risk in the general population is still unclear. This study aimed to explore the association between LAR and cardiovascular as well as all-cause mortality among American adults.
Methods
The present study was a secondary analysis of existing data from the National Health and Nutrition Examination Survey (NHANES). The final analysis included 12,440 participants from 2005 to 2014. Survival differences between groups were visualized using Kaplan‒Meier curves and the log-rank test. The association of LAR with cardiovascular and all-cause mortality was evaluated using multivariate Cox regression and restricted cubic spline analysis. Age, sex, coronary artery disease, diabetes, lipid-lowering medication use and hypertriglyceridemia were analyzed in subgroup analyses.
Results
The median age in the study cohort was 46.0 years [interquartile range (IQR): 31.0–62.0], and 6,034 (48.5%) participants were male. During the follow-up period, there were 872 (7.0%) all-cause deaths and 150 (1.2%) cardiovascular deaths. Compared with individuals without cardiovascular events, those who experienced cardiovascular deaths had a lower LAR (1.13 vs. 1.25) (P < 0.001). The adjusted Cox regression model indicated that lower LAR was an independent risk factor for both cardiovascular [hazard ratio (HR) = 0.304, 95% confidence interval (CI): 0.114–0.812] and all-cause mortality (HR = 0.408, 95% CI: 0.270–0.617). Moreover, a significant age interaction was observed (P for interaction < 0.05), and there was a strong association between LAR and mortality among participants over 65 years of age. Further analysis showed an inverse association between LAR and both cardiovascular and all-cause mortality.
Conclusions
LAR can independently predict cardiovascular and all-cause mortality in the general population.
Similar content being viewed by others
Background
Abnormal lipid metabolism has been closely linked to cardiovascular disease (CVD), which is currently the leading cause of death worldwide [1, 2]. It is widely known that an increase in low-density lipoprotein cholesterol (LDL-C) levels is a significant risk factor for the development and progression of atherosclerosis and coronary artery disease (CAD), making LDL-C the primary target for cholesterol-lowering therapy [3,4,5]. However, in clinical practice, a considerable proportion of patients with normal LDL-C concentrations still experience atherosclerosis, which leads to careful consideration of LDL-C concentrations as a sole indicator [6]. Sachdeva et al. [7] followed up with CAD patients who had been hospitalized continuously for 6 years and found that nearly half of them had relatively normal LDL-C concentrations of < 100 mg/dL. An investigation by Superko and Gadesam [8] demonstrated that particle size was another important index that affected the atherogenicity of LDL in addition to the serum concentration. When serum LDL-C concentrations were fixed, it was observed that the smaller the particle size was, the higher the risk of long-term ischemic heart disease [9]. With age, LDL subfraction profile would shift more and more from a healthy “pattern A” (major LDL peak > 255 A) to “pattern B” (representing small, dense LDL (sdLDL) particles) [10]. Several prospective cohort studies have shown a significant correlation between a high proportion of sdLDL particles and an increased risk of CVD [11,12,13].
Nuclear magnetic resonance spectroscopy is a valid technology for the measuring of lipoprotein profile, and the results are not easily compromised by lipoprotein composition. However, stringent assay conditions and expensive instruments make it difficult to popularize [14, 15]. Ion mobility analysis and vertical auto profile are also feasible alternative methods [16]. Given the complexity of other traditional testing technologies, such as polyacrylamide gel electrophoresis and density ultracentrifugation, the LDL-C concentration to total apolipoprotein B (ApoB) concentration ratio (LDL-C/ApoB ratio, hereinafter called LAR) could be a more accessible and substantially cheaper tool to estimate LDL particle size [17]. ApoB is the main protein component of LDL and plays a critical role in the transport and clearance of cholesterol in the vascular wall. When LAR is below 1.2 (LDL particle size is approximately 25.5 nm or less), proatherogenic sdLDL is abundantly present [18].
Previous studies on the role of LAR in diseases have mainly focused on evaluating atherosclerotic lipid profiles and predicting the risk of suffering from CVD [19,20,21]. A recent prospective cohort study [22] found that LAR can predict adverse cardiovascular outcomes among individuals with established atherosclerosis. Similarly, the LURIC Study [23] also showed that LDLapoB/LDL-C ratios were independently associated with cardiovascular mortality. However, to our knowledge, there are currently no studies that focus on LAR to predict mortality events in the general population. Accordingly, our study aimed to investigate the association between LAR and mortality events, i.e., its potential predictive ability for prognosis among general adults.
Methods
Study population
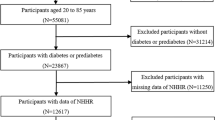
The study population was selected from the official website of the National Health and Nutrition Examination Survey (NHANES), which is a sequence of multistage surveys that represent the noninstitutionalized populace of the United States. The detailed protocol is provided in NHANES procedure manual [24]. From 2005 to 2014, a total of 50,965 participants were included in the health survey. All subjects with available data on LDL-C and ApoB were enrolled in this study (n = 15,416). In this study, 2,359 cases aged < 18 years, 604 cases with cancer and 13 cases who were lost to follow-up were excluded (Fig. 1). The remaining subjects (n = 12,440) were successfully included for further analysis. This study passed the ethical review process, and all participants provided written informed consent.
Assessment of exposure
All participants were instructed to provide fasting blood samples according to the standard protocol. The determination of total cholesterol (TC) and triglyceride (TG) concentrations was based on enzymatic methods. Serum high-density lipoprotein cholesterol (HDL-C) concentrations were determined by either the heparin-manganese precipitation method or direct immunoassay, while ApoB concentrations were measured by immunonephelometry [25]. LDL-C was estimated via the Friedewald formula. The assays were conducted on a Hitachi 704/717 Analyzer or Roche Modular P Chemistry Analyzer [26].
Assessment of covariates
Participants were interviewed either in person or via computer-assisted personal interview (CAPI). All respondents completed a questionnaire providing demographic and health information. Demographic information, such as date of birth, sex, race, health information (including medical history and smoking and drinking habits), and medications at the time of enrollment, was collected. Information on a history of the following medical conditions was collected according to self-reported data: hypertension, diabetes mellitus, CAD and stroke. Smokers were defined as those who had smoked for more than 6 months or over 100 cigarettes accumulatively, and drinkers were defined as those who had at least 12 drinks in the past 12 months [27]. Participants with systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg were categorized as having hypertension, and individuals with a fasting blood glucose concentration ≥ 7 mmol/L or glycated hemoglobin (HbA1c) concentration ≥ 6.5% were considered to have diabetes [25].
Outcome ascertainment
The etiologies of mortality were classified according to the tenth revision of the International Classification of Diseases (ICD-10) [28]. The study endpoint was all-cause and cardiovascular mortality, defined as any cardiovascular disease-related death (ICD-10 codes I00-I99). Data on deaths were obtained by cross-linking the NHANES datasets to the National Death Index [29]. Each participant’s follow-up duration commenced on the date of their survey participation and ended on either the date of their death or the end of the follow-up period (December 31, 2015).
Statistical analysis
The Shapiro‒Wilk test was conducted to evaluate the normality of the distribution of continuous variables. Nonnormally distributed variables were presented as medians (interquartile ranges, IQRs), while categorical variables were presented as proportions. The Mann‒Whitney U test or χ2 test was utilized for group comparisons. Kaplan‒Meier curves and log-rank tests were used to compare survival differences between groups. The Cox proportional hazards model was used to examine the independent association between LAR and all-cause and cardiovascular mortality. Three risk models were created: Model 1 was a crude model without adjustment for confounders. Age, sex, race, alcohol consumption, and smoking status were included as covariates in Model 2. Model 3 included all covariates in Model 2, as well as other conventional cardiovascular risk factors such as hypertension, diabetes, CAD, serum TG levels, and lipid-lowering medication use.
For subgroup analysis, the fully adjusted models were stratified by age, sex, CAD, diabetes, use of lipid-lowering medication and hypertriglyceridemia, and interactions were assessed. Then, we also evaluated the overall effect and linear trend between LAR and mortality risk by restricted cubic spline models. We accounted for the complex survey design and the probability weights were used as recommended by the NCHS in our analysis [30, 31]. Multiple imputation was used to replace missing values. Statistical analysis was performed using R version 3.5.3, with two-tailed tests used for all analyses. A P value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Among 12,440 participants, the overall median age was 46.0 years (IQR 31.0–62.0), and 6,034 (48.5%) were male. The median (IQR) LAR percentile was 1.24 (1.12–1.35), and the percentile distribution ranged from 0.92 to 1.51 (5-95th percentile) and from 0.74 to 1.63 (1-99th percentile). During a median follow-up of 68 months, 872 (7.0%) all-cause deaths occurred, 150 (17.2%) of which were CVD-related deaths. According to whether cardiovascular deaths occurred, participants were divided into two groups. Table 1 provides a detailed description of the baseline characteristics of the two groups.
Compared with the participants without cardiovascular deaths, those who experienced cardiovascular deaths were older, predominantly male, and had a greater proportion of smokers (P < 0.05). The occurrence of any coexisting cardio-cerebrovascular diseases, including hypertension, diabetes, CAD and stroke, was higher in the population with cardiovascular deaths, as was the use of lipid-lowering medication (all P values < 0.05). Moreover, serum TG concentrations were significantly higher in individuals who experienced cardiovascular death (P < 0.05). Importantly, LAR displayed obvious downward trends in participants who experienced cardiovascular death (P < 0.001).
Association of LAR with cardiovascular mortality
Participants were categorized into two groups based on whether their baseline LAR was above or below 1.2. Kaplan‒Meier survival curves showed a notable difference in cardiovascular death risk between the groups (P for log-rank test < 0.001, Fig. 2A). After adjusting for possible confounding variables, the final Cox proportional hazards regression model demonstrated a statistically significant association between a decline in LAR and an elevated risk of cardiovascular mortality (HR = 0.304, 95% CI: 0.114–0.812, Table 2). The restricted cubic spline model showed that there was a linear association of LAR with cardiovascular mortality (P for nonlinearity = 0.998, Fig. 3A).
Restricted cubic spline plots of the association between LAR with cardiovascular mortality (A) and all-cause mortality (B) in the general population. Analysis was adjusted for age, sex, race, drinking, smoking, hypertension, diabetes, coronary artery disease, serum triglyceride level and lipid-lowering drugs. HR, hazard ratio
Association of LAR with all-cause mortality
The Kaplan–Meier curves indicated a significant association between different LAR groups and overall survival rate (P < 0.001, Fig. 2B). LAR demonstrated a clear association with all-cause mortality in the Cox model without adjustment (HR = 0.151, 95% CI: 0.108–0.211). To exclude the influence of the confounders, potential confounders were incorporated as adjustment factors into multivariate regression. LAR remained an independent predictor of all-cause mortality (HR = 0.408, 95% CI: 0.270–0.617). The risk of all-cause mortality in the low ratio group (LAR < 1.2) was approximately 1.22 times greater than that in the high ratio group (HR = 1.223, 95% CI: 1.054–1.419, Table 2). As shown by restricted cubic spline analyses (Fig. 3B), HRs for all-cause mortality gradually increased with decreasing LAR (P for nonlinearity = 0.822).
Subgroup analysis
To further investigate the association of LAR with cardiovascular and all-cause mortality, stratified analyses by age, sex, CAD, diabetes, use of lipid-lowering medication and hypertriglyceridemia were performed (Table 3). Among participants of different sexes or with and without CAD, diabetes, lipid-lowering medication and hypertriglyceridemia, there was no significant difference in the association of LAR with mortality (P for interaction > 0.05). In addition, there was a significant interaction between LAR and age (P for interaction < 0.001) in terms of mortality, and the association of LAR with cardiovascular and all-cause mortality was more pronounced in participants over 65 years of age (P < 0.05). When using restricted cubic spline plots to depict LAR as a continuous variable, a linear correlation was observed between LAR and both cardiovascular and all-cause mortality rates in subgroups stratified by age, sex, CAD, diabetes, lipid-lowering medication use and hypertriglyceridemia (Figs. 4 and 5).
Discussion
LAR is commonly used as an indirect marker of LDL particle size and is associated with the development of atherosclerosis and adverse cardiovascular outcomes. This is the first study to evaluate the long-term prognostic efficacy of LAR among general adults. This study demonstrated that a decreased LAR was linked to higher all-cause and cardiovascular mortality in the general population, irrespective of other conventional risk factors. In the population studied, there was no significant difference in the association of LAR with the risk of mortality among subjects of different genders, history of CAD, diabetes and hypertriglyceridemia, and a history of lipid-lowering medication. Further analysis suggested that there was a negative linear association between LAR and mortality.
The majority of international guidelines agree that reducing circulating LDL concentrations is one of the main goals for the prevention of atherosclerotic cardiovascular disease [32,33,34]. LDL is a heterogeneous population of particles with relatively discrete rather than continuously variable size and density [35]. Specifically, LDL is subdivided into seven subcategories (LDL-1 to LDL-7) in nondenaturing gradient gel electrophoresis, ranging from largest and most buoyant to smallest and densest [36]. LDL-3 to 7 is also referred to as sdLDL. A previous study [13] found that sdLDL was the most atherogenic parameter, even more than LDL, which is related to its biochemical properties. The smaller size makes it easier for LDL particles to penetrate the arterial wall and escape receptor-mediated uptake, leading to increased atherogenic risk [37, 38]. Moreover, its longer operational half-life predisposes atherogenic modification [39]. High concentrations of sdLDL have been unequivocally demonstrated as a risk factor for CVD [38, 40, 41]. A study among older men with stable CAD by Sakai et al. [42] also indicated that sdLDL-C was a superior biomarker to LDL-C to predict future cardiovascular events. ApoB-100, a 550 kDa glycoprotein synthesized in the liver, is the major apolipoprotein contained in LDL particles and serves as the binding domain for the LDL receptor [43]. It has been substantiated that ApoB is comparable to non-HDL cholesterol in predicting future CAD risk, while it may be even superior to non-HDL cholesterol in predicting cardiovascular events [44,45,46]. Studies [47, 48] have suggested that each LDL particle has a single ApoB molecule and more than 90% of all ApoB is found in LDL; of course, a minor fraction of ApoB is also carried on other atherogenic particles (e.g., very-low density lipoproteins and intermediate density lipoproteins); thus, plasma ApoB broadly reflects the quantity of LDL particles. Hence, the significance of LAR has been discussed, as it can indicate the approximate size of LDL particles and assess the relative predominance of sdLDL. In individuals with low ratio values, sdLDL has a higher predominance in LDL particles in plasma.
The measurement of LAR has gained increased attention in recent years. Multiple studies [49,50,51,52] have indicated that LAR is associated with cardiovascular diseases and the development of cardiovascular diseases later in life. Drexel et al. [22] recently reported that LAR can predict major adverse cardiovascular events in patients with preexisting atherosclerotic cardiovascular diseases. However, the enrolled population of the study was relatively small and limited. Therefore, the present study was conducted in the general population. The sample had wide coverage, and the study population was nationally representative. The results indicated that this ratio was able to predict cardiovascular mortality not only among patients with CAD but also in the general population. LAR of 1.2 serves as a critical value for the classification of sdLDL and large, buoyant LDL [18, 53]. When the ratio is below 1.2, there is a substantial increase in the risk of all-cause mortality.
TGs are considered to be the most powerful determinant of LDL particle size [54]. Most plasma triglycerides are found in lipoproteins that are rich in triglycerides (TRLs), including chylomicrons and very low-density lipoprotein (VLDL), and their metabolism is inextricably linked to cholesterol metabolism [55]. Tani et al. conducted a cross-sectional study [56] and found that the estimated LDL particle size (based on LAR) showed significant inverse correlations with most markers related to TG-rich lipoproteins, including TG concentration, especially among CAD patients with diabetes mellitus. Experimental studies suggest that sdLDL, stimulated by hypertriglyceridemia, may be more susceptible to oxidative modification and concomitant dysfunctional HDL and may adversely affect atherosclerosis [56,57,58]. Considering that abnormal TG metabolism seems to lead to a reduction in LDL particle size, hypertriglyceridemia was included as a confounding factor in the multivariate regression analysis to eliminate its effect. The effective predictive ability of LAR for all-cause and cardiovascular mortality persisted.
Statins are the most commonly used drugs in the clinic to control hyperlipidemia and prevent cardiovascular disease. However, a recent study [59] have suggested that statins likely play a more complex role than simply lowering serum lipid concentrations. The reported benefits of statin therapy might be overestimated and exaggerated, and their clinical efficacy has also been questioned by some scholars [60]. Statins may have implications for many other biological pathways, and they have been reported to have effects on LDL particle size [61, 62]. In a multicenter randomized study [59], patients with familial combined hyperlipidemia were treated with two statins. However, different responses were observed: atorvastatin increased the average LDL particle size, while pravastatin reduced the particle size. Therefore, in our study, multivariate analyses were utilized to account for the use of lipid-lowering drugs and demonstrated that the significance of LAR as a predictor of mortality persisted in our particular context. Furthermore, stratified analysis and the test for interaction showed that the association between LAR and mortality did not change with the use of lipid-lowering medications. Given that different drugs affect LDL particle size differently, further detailed research is needed to clarify the influence of different lipid-lowering drugs on the relationship between LAR and mortality.
Comparisons with other studies and what does the current work add to the existing knowledge
Previous studies [19,20,21] have mainly focused on the association between LAR and the development of cardiovascular disease. To our knowledge, only the study by Drexel et al. [22] and Silbernagel et al. [23] have evaluated the prognostic value of LAR for death. In contrast, their studies were only conducted in CAD patients, while we expanded firstly the scope to the general population. This further demonstrated the importance of this indicator. Our findings might provide a theoretical basis for the predictive value of LAR in the general population, which is worth further promoting and utilization in clinical practice.
Study strengths and limitations
The present study had several key points and strengths. First, the sample was large and had extensive coverage. The study population was largely representative of the general US adult population. Second, our retrospective cohort study presented firm evidence of the prognostic efficiency of LAR for both cardiovascular and all-cause mortality in the general population. Prior to this study, similar studies were only conducted among patients with CAD. Finally, we used a restricted cubic spline model to visually demonstrate the relationship between LAR and the long-term risk of mortality for the first time.
There were also several potential limitations. Principally, LAR was a rough estimate of LDL particle size rather than a direct measurement tool. And measurement of LDL-C, ApoB and covariates was limited to the baseline in this study, but they might have changed during follow-up. The ratio could not represent the long-term levels in the population. Second, as medication intake and physical activity could not be completely followed up and systematically analyzed, the possible effects of lipid-regulating agents and physical activity on the results cannot be excluded. Moreover, the Friedewald formula is still the main method for the assessment of serum LDL-C concentrations because of its low cost and convenience. With the improved accuracy of the direct method of LDL-C measurement, it can be expected that it will be substituted for the Friedewald formula. The data were derived from residents of the United States; therefore, future studies can be conducted in other countries to determine whether these results are valid worldwide.
Conclusions
In conclusion, the current study provided evidence of an independent association of LAR, serving as an estimation indicator of LDL particle size, with both cardiovascular and all-cause mortality. In addition to serum LDL concentrations, LAR may also function as another valuable indicator of poor prognosis, which may be relevant for residual cardiovascular risk.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ApoB:
-
Apolipoprotein B
- CAD:
-
Coronary artery disease
- CVD:
-
Cardiovascular disease
- HDL-C:
-
High-density lipoprotein cholesterol
- LAR:
-
The LDL cholesterol concentration to apolipoprotein B concentration ratio
- LDL-C:
-
Low-density lipoprotein cholesterol
- NHANES:
-
National Health and Nutrition Examination Survey
- sdLDL:
-
Small dense low-density lipoprotein
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
References
Emerging Risk Factors Collaboration. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307(23):2499–506.
Wan EYF, Yu EYT, Chin WY, Barrett JK, Mok AHY, Lau CST, et al. Greater variability in lipid measurements associated with cardiovascular disease and mortality: a 10-year diabetes cohort study. Diabetes Obes Metab. 2020;22(10):1777–88.
Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–30.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. 2019;381(16):1557–67.
Superko H, Garrett B. Small dense LDL: scientific background, clinical relevance, and recent evidence still a risk even with ‘normal’ LDL-C levels. Biomedicines. 2022;10(4):829.
Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC, Dai D, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in get with the guidelines. Am Heart J. 2009;157(1):111–7.e2.
Superko HR, Gadesam RR. Is it LDL particle size or number that correlates with risk for cardiovascular disease? Curr Atheroscler Rep. 2008;10(5):377–85.
St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard PM, Després JP, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec cardiovascular study. Arterioscler Thromb Vasc Biol. 2005;25(3):553–9.
Abate N, Vega GL, Grundy SM. Variability in cholesterol content and physical properties of lipoproteins containing apolipoprotein B-100. Atherosclerosis. 1993;104(1–2):159–71.
Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20(2):195–203.
Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q, Li YF, et al. Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19(1):45.
Ikezaki H, Lim E, Cupples LA, Liu CT, Asztalos BF, Schaefer EJ. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham Offspring Study. J Am Heart Assoc. 2021;10(5):e019140.
Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106(15):1930–7.
Tsai MY, Georgopoulos A, Otvos JD, Ordovas JM, Hanson NQ, Peacock JM, et al. Comparison of ultracentrifugation and nuclear magnetic resonance spectroscopy in the quantification of triglyceride-rich lipoproteins after an oral fat load. Clin Chem. 2004;50(7):1201–4.
Kanonidou C. Small dense low-density lipoprotein: analytical review. Clin Chim Acta. 2021;520:172–8.
Viktorinova A, Malickova D, Svitekova K, Choudhury S, Krizko M. Low-density lipoprotein cholesterol-to-apolipoprotein B ratio as a potential indicator of LDL particle size and plasma atherogenicity in type 2 diabetes. Diabetes Res Clin Pract. 2021;176:108858.
Hirano T, Ito Y, Yoshino G. Measurement of small dense low-density lipoprotein particles. J Atheroscler Thromb. 2005;12(2):67–72.
Kaneva AM, Potolitsyna NN, Bojko ER. Usefulness of the LDL-C/apoB ratio in the overall evaluation of atherogenicity of lipid profile. Arch Physiol Biochem. 2017;123(1):16–22.
Rabizadeh S, Rajab A, Mechanick JI, Moosaie F, Rahimi Y, Nakhjavani M, et al. LDL/apo B ratio predict coronary heart disease in type 2 diabetes independent of ASCVD risk score: a case-cohort study. Nutr Metab Cardiovasc Dis. 2021;31(5):1477–85.
Jung HW, Ra M, Bae HJ, Hong SP. The LDL-C/Apo B predicts coronary atherosclerotic heart disease in non-diabetic patients without high LDL-C. Med (Baltim). 2023;102(1):e32596.
Drexel H, Larcher B, Mader A, Vonbank A, Heinzle CF, Moser B, et al. The LDL-C/ApoB ratio predicts major cardiovascular events in patients with established atherosclerotic cardiovascular disease. Atherosclerosis. 2021;329:44–9.
Silbernagel G, Scharnagl H, Saely CH, Reinthaler M, Rief M, Kleber ME, et al. The LDL apolipoprotein B-to-LDL cholesterol ratio: association with cardiovascular mortality and a biomarker of small, dense LDLs. Biomedicines. 2022;10(6):1302.
Centers for Disease Control and Prevention/National Center for Health Statistics. Nhanes survey methods and analytic guidelines. 2023. https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx.
Zhang K, Qi X, Zhu F, Dong Q, Gou Z, Wang F, et al. Remnant cholesterol is associated with cardiovascular mortality. Front Cardiovasc Med. 2022;9:984711.
Doran B, Guo Y, Xu J, Weintraub H, Mora S, Maron DJ, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and Nutrition Examination Survey III (NHANES-III). Circulation. 2014;130(7):546–53.
Ryan H, Trosclair A, Gfroerer J. Adult current smoking: differences in definitions and prevalence estimates–NHIS and NSDUH, 2008. J Environ Public Health. 2012;2012:918368.
Sun S, Kuja-Halkola R, Faraone SV, D’Onofrio BM, Dalsgaard S, Chang Z, et al. Association of psychiatric comorbidity with the risk of premature death among children and adults with attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2019;76(11):1141–9.
Centers for Disease Control and Prevention/National Center for Health Statistics. NCHS data linkage - mortality data. 2023. https://www.cdc.gov/nchs/data-linkage/mortality.htm.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2(161):1–24.
Centers for Disease Control and Prevention. National health and nutrition examination survey: analytic guidelines, 2011–2012. 2013. http://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf.
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA /ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143.
Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43(9):1363–79.
Duan R, Xue W, Wang K, Yin N, Hao H, Chu H, et al. Estimation of the LDL subclasses in ischemic stroke as a risk factor in a Chinese population. BMC Neurol. 2020;20(1):414.
Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev. 2017;2017:1273042.
Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res. 1998;39(6):1263–73.
Diffenderfer MR, Schaefer EJ. The composition and metabolism of large and small LDL. Curr Opin Lipidol. 2014;25(3):221–6.
Huang J, Gu JX, Bao HZ, Li SS, Yao XQ, Yang M, et al. Elevated serum small dense low-density lipoprotein cholesterol may increase the risk and severity of coronary heart disease and predict cardiovascular events in patients with type 2 diabetes mellitus. Dis Markers. 2021;2021:5597028.
Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. 2020;75(17):2122–35.
Sakai K, Koba S, Nakamura Y, Yokota Y, Tsunoda F, Shoji M, et al. Small dense low-density lipoprotein cholesterol is a promising biomarker for secondary prevention in older men with stable coronary artery disease. Geriatr Gerontol Int. 2018;18(6):965–72.
Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019;4(12):1287–95.
Sondermeijer BM, Rana JS, Arsenault BJ, Shah PK, Kastelein JJ, Wareham NJ, et al. Non-HDL cholesterol vs. apo B for risk of coronary heart disease in healthy individuals: the EPIC-Norfolk prospective population study. Eur J Clin Invest. 2013;43(10):1009–15.
Aggarwal DJ, Kathariya MG, Verma DPK. LDL-C, NON-HDL-C and APO-B for cardiovascular risk assessment: looking for the ideal marker. Indian Heart J. 2021;73(5):544–8.
Grundy SM, Vega GL, Tomassini JE, Tershakovec AM. Comparisons of apolipoprotein B levels estimated by immunoassay, nuclear magnetic resonance, vertical auto profile, and non-high-density lipoprotein cholesterol in subjects with hypertriglyceridemia (SAFARI Trial). Am J Cardiol. 2011;108(1):40–6.
Dhingra S, Bansal MP. Hypercholesterolemia and apolipoprotein B expression: regulation by selenium status. Lipids Health Dis. 2005;4:28.
Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, et al. Association of triglyceride-lowering LPL variants and LDL-C-Lowering LDLR variants with risk of Coronary Heart Disease. JAMA. 2019;321(4):364–73.
Chang TY, Chen JD. Low-density lipoprotein cholesterol/apolipoprotein B ratio is superior to apolipoprotein B alone in the diagnosis of coronary artery calcification. Coron Artery Dis. 2021;32(6):561–6.
Onat A, Can G, Ciçek G, Ayhan E, Doğan Y. Predictive value of serum apolipoprotein B/LDL-cholesterol ratio in cardiometabolic risk: population-based cohort study. Clin Biochem. 2010;43(18):1381–6.
Kwon CH, Kim BJ, Kim BS, Kang JH. Low-density lipoprotein cholesterol to apolipoprotein B ratio is independently associated with metabolic syndrome in Korean men. Metabolism. 2011;60(8):1136–41.
Tajik B, Voutilainen A, Kauhanen J, Mazidi M, Lip GYH, Tuomainen TP, et al. Lipid profile, lipid ratios, apolipoproteins, and risk of cardiometabolic multimorbidity in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Lipids. 2022;57(2):141–9.
Akutsu N, Hori K, Mizobuchi S, Ogaku A, Koyama Y, Fujito H, et al. Clinical importance of the LDL-C/Apolipoprotein B ratio for neointimal formation after everolimus-eluting stent implantations. J Atheroscler Thromb. 2022;29(4):536–50.
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–333.
Packard CJ, Boren J, Taskinen MR. Causes and consequences of Hypertriglyceridemia. Front Endocrinol (Lausanne). 2020;11:252.
Tani S, Yagi T, Atsumi W, Kawauchi K, Matsuo R, Hirayama A. Relation between low-density lipoprotein cholesterol/apolipoprotein B ratio and triglyceride-rich lipoproteins in patients with coronary artery disease and type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol. 2017;16(1):123.
Skeggs JW, Morton RE. LDL and HDL enriched in triglyceride promote abnormal cholesterol transport. J Lipid Res. 2002;43(8):1264–74.
Kwiterovich PO Jr. Clinical relevance of the biochemical, metabolic, and genetic factors that influence low-density lipoprotein heterogeneity. Am J Cardiol. 2002;90(8a):30i–47i.
Kucera M, Balaz D, Kruzliak P, Ciccocioppo R, Oravec S, Rodrigo L, et al. The effects of atorvastatin treatment on the mean platelet volume and red cell distribution width in patients with dyslipoproteinemia and comparison with plasma atherogenicity indicators–A pilot study. Clin Biochem. 2015;48(9):557–61.
Ravnskov U, de Lorgeril M, Diamond DM, Hama R, Hamazaki T, Hammarskjöld B, et al. LDL-C does not cause cardiovascular disease: a comprehensive review of the current literature. Expert Rev Clin Pharmacol. 2018;11(10):959–70.
Sirtori CR, Calabresi L, Pisciotta L, Cattin L, Pauciullo P, Montagnani M, et al. Effect of statins on LDL particle size in patients with familial combined hyperlipidemia: a comparison between atorvastatin and pravastatin. Nutr Metab Cardiovasc Dis. 2005;15(1):47–55.
Chu AY, Giulianini F, Barratt BJ, Ding B, Nyberg F, Mora S, et al. Differential genetic effects on statin-induced changes across low-density lipoprotein-related measures. Circ Cardiovasc Genet. 2015;8(5):688–95.
Acknowledgements
We thank AJE for providing professional English language editing services.
Funding
This study was supported by the Youth Program of National Natural Science Foundation of China (82000379), the Science Foundation of Nanjing Medical University (NMUB20210274), the Project of Suzhou Science and Technology Development Plan (SKJY2021128) and the Suzhou Gusu health talent Program (GSWS2022071).
Author information
Authors and Affiliations
Contributions
CW and ZG designed the study and conceived the paper. LX and KZ performed statistical analysis and drafted the manuscript. FW, MW and QH arranged the data and assisted with figure preparation. ZG critically revised the manuscript, and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiao, L., Zhang, K., Wang, F. et al. The LDL-C/ApoB ratio predicts cardiovascular and all-cause mortality in the general population. Lipids Health Dis 22, 104 (2023). https://doi.org/10.1186/s12944-023-01869-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01869-1