Abstract
Background
Due to the contribution of coronary artery disease (CAD) to serious cardiovascular events, determining biomarkers that could robustly predict its risk would be of utmost importance. Thus, this research was designed to assess the value of traditional cardio-metabolic indices, and more novel atherogenicity indices and insulin resistance surrogate markers in the identification of individuals at risk of CAD.
Methods
A case‒control survey was conducted, in which 3085 individuals were enrolled. Their clinical and biochemical data were gathered at baseline. The investigated indices included the atherogenic index of plasma (AIP), triglyceride-glucose (TyG) index, TyG-body mass index (TyG-BMI), lipoprotein combine index (LCI), cholesterol index (CHOLINDEX), Castelli’s risk indices-I, II (CRI-I, CRI-II), and metabolic score for insulin resistance (METS − IR). To examine the relationship between these variables and CAD risk, multiple regression analyses adjusted for potential confounders were conducted.
Results
Overall, 774 angiographically confirmed CAD patients (mean age = 54 years) were compared with 3085 controls (mean age = 51 years). Higher triglyceride, total cholesterol and fasting blood sugar levels and lower HDL-C levels were related to an elevated risk of CAD (P-for-trend < 0.001), while the direct association between increased serum LDL-C concentrations and a greater risk of CAD only became apparent when excluding those with diabetes, and statin users. Among novel indices, greater values of the majority of these markers, including AIP, CRI-I, and -II, CHOLINDEX, LCI, and TyG-index, in comparison to the lower values, significantly elevated CAD risk (P-for-trend < 0.001).
Conclusion
According to the current findings, novel atherogenicity indices and insulin resistance surrogate markers, in particular, AIP, CRI-I and II, CHOLINDEX, LCI, and TyG-index, may be useful in predicting CAD risk.
Similar content being viewed by others
Background
In 2019, Cardiovascular diseases (CVDs) were attributed to nearly 32% of worldwide fatalities, i.e., approximately 17.9 million deaths [1]. These diseases have effectively established a “pandemic” in the world [2]. As a major CVD, coronary artery disease (CAD) has been established as the primary cause of mortality and morbidity, a public health burden, and a major contributor to the decline of general well-being worldwide [3, 4]. CAD is known to be an inflammatory disorder generated as a result of multifactorial and complex processes such as atherosclerosis and atherosclerotic thrombosis of the coronary arteries [5, 6]. Over the course of the past years, numerous mechanisms have been suggested as the underlying pathogenesis of CAD, including accumulated glucose and lipid levels, reduced levels or disturbed function of vasodilator factors (e.g., prostacyclin and nitric oxide (NO)), elevated homocysteine levels, endothelial dysfunction, hypercoagulation, and the thrombogenic hypothesis [7,8,9,10]. The lipid theory ascribed atherosclerosis to progressive lipid aggregation in the arterial vessel due to the formation of acid mucopolysaccharide complexes and the imbalance between lipid removal and deposition [11,12,13]. Of note, lipid accumulation and oxidation in the arteries, development of fatty streak and progression of the atherosclerotic lesion have been acknowledged as certain events accelerating the formation and progression of atherosclerosis and contributing to its complications [11,12,13,14,15]. There is also extensive literature supporting the role of triglycerides and cholesterol esters as the two most important circulating lipids involved in atherosclerosis [13, 16,17,18]. Likewise, insulin resistance could also advance atherosclerosis progression by disrupting lipid metabolism and triggering endothelial dysfunction [8, 9, 19]. Following these metabolic disturbances, vascular occlusion and atherosclerotic plaque rupture may ensue and ultimately culminate in serious embolic and thrombotic cardiovascular (CV) events, including limb ischemia, angina, myocardial infarction, or sudden death due to a CV cause [20, 21]. In this regard, coronary catheterization with an angiogram has been accredited as the CAD prognosis gold standard examination. However, the invasive nature of the procedure prohibits its use as a screening tool [22]. Accordingly, exploring various risk factors that predict CAD occurrence can help researchers and physicians recognize patients who are at an elevated risk for its complications and may therefore need more extensive assessment or intensive treatment. Identifying such candidate biomarkers may also advance the development of novel effective treatment strategies [22]. Detecting perturbed levels of biomarkers indicative of the main pathophysiological disturbances associated with atherosclerosis and CAD, including dyslipidemia, dysglycemia and insulin resistance, may be potentially effective in decreasing the risk of developing more serious CV events. Conventionally, the measurement of serum levels of atherogenic cardiometabolic factors, such as total cholesterol, fasting blood sugar (FBS), triglycerides, and low-density lipoprotein-cholesterol (LDL-C), and anti-atherogenic biomarkers, such as high-density lipoprotein-cholesterol (HDL-C), has been applied to both research and clinical settings to predict the progression of atherosclerotic lesions [23]. However, these ‘conventional’ markers may not be capable of portraying the extent of atherosclerosis severity, and therefore CAD treatment targets based on the levels of these biomarkers may be suboptimal [23]. For instance, reaching a target goal for LDL-C was thought to be associated with adequate prevention of CV events. However, emerging evidence indicates that a high risk for CV events may persist even after normalizing LDL-C levels [24].
On the basis of these considerations, several recently published epidemiological studies have suggested novel atherogenicity-related biomarkers, including the atherogenic index of plasma (AIP), Castelli’s risk indices-I, II (CRI-I, CRI-II), cholesterol index (CHOLINDEX), lipoprotein combine index (LCI), and surrogate markers for insulin resistance such as triglyceride glucose index (TyG), metabolic score for insulin resistance (METS − IR), and triglyceride glucose-body mass index (TyG-BMI). Such markers can be employed as more informative predictors of CV risk and atherosclerosis severity [25,26,27,28,29,30,31]. Nevertheless, as of now, there is conflicting evidence about the screening, diagnostic, and prognostic roles of these indices in regard to CAD, specifically among patients who suffer from multiple metabolic comorbidities. It is possible that unique arrays of these biomarkers will be useful for different CAD subpopulations [32,33,34,35]. Thus, this research was designed to assess the value of traditional cardio-metabolic indices, such as triglycerides, FBS, HDL-C, total cholesterol, LDL-C, and more novel atherogenicity indices and insulin resistance surrogate markers, including AIP, CRI-I, CRI-II, LCI, CHOLINDEX, TyG, METS − IR, and TyG-BMI, in the identification of individuals at risk of CAD.
Methods
Study population
Data collection for the present retrospective case‒control survey was performed between June 2017 and September 2019. During this time period, approximately 20,000 subjects visited the cardiology outpatient clinic at Dr. Heshmat Hospital in Rasht, whether it was due to having clinical signs and/or symptoms of heart disease or for routine check-ups. These subjects were examined by expert cardiologists to rule in or out cardiac etiologies that could explain their symptomology. It was initially assumed to randomly recruit approximately three sex (assigned at birth)-matched non-CAD individuals for each CAD case. Therefore, the medical records of 4400 individuals, of whom 3300 subjects were identified as free of CAD and 1100 were referred to the catheterization laboratory and diagnosed as angiographically confirmed CAD cases, were reviewed by our study staff. After assessing the eligibility for inclusion in the current research and the availability of these individuals’ laboratory and anthropometric data, approximately 3085 subjects in the non-CAD group were recruited to the control arm (controls), and 774 angiographically confirmed chronic CAD patients were allocated to the case group (cases). For the case group, chronic CAD diagnosis was made in accordance with “ESC 2019 guidelines for the diagnosis and management of chronic coronary syndromes” [36]. The CAD cases experienced clinical signs such as chest pain and shortness of breath, and showed abnormal findings according to the carried-out (exercise stress test, and echocardiography) and angiography. The control group consisted of subjects with no clinical or laboratory signs of coronary artery disturbances or atherosclerotic disorders. The diagnosis of CAD for controls was ruled out based on the patients’ symptoms (not having typical signs of angina pectoris) and according to the findings of non-invasive investigations, including exercise stress tests and/or echocardiography.
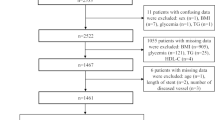
To be included in the study, all study participants had to have a BMI between 18.5 and 39 kg/m2, and an age between 20 and 75 years old. Conversely, individuals who had a history of unstable angina, cerebrovascular events, myocardial infarction, heart failure, valvular disorders, arrhythmias, cardiomyopathies, malignancies, thyroid dysfunction, acute or chronic liver/kidney diseases, and/or inflammatory/infectious disorders prior to the study or who had previously undergone a coronary angiography (CAG) and/or revascularization procedure were not eligible for the study. Moreover, a lack of clinical and biochemical laboratory data relevant to the participant’s metabolic profile, including classic lipid parameters, glycemic indices or anthropometric measures (e.g., height and weight), was also considered as an exclusion criterion. The study participants’ recruitment procedure is depicted in Fig. 1.
Clinical data collection
Study staff who were professional healthcare providers reviewed all patient medical records that were relevant to the study. At baseline, demographic and clinical features of participants and their medication consumption history were obtained. Information on anti-hyperlipidemia drugs, particularly statin medications (mainly atorvastatin, simvastatin, and rosuvastatin), was also collected. Considering anthropometric measurements, patients’ weight and height were determined through a Seca 755 medical scale and a standard stadiometer (rounded to the nearest 0·5 kg and 0.1 cm, in order), respectively. BMI was then computed by dividing the weight in kilograms by height in meters squared.
The study procedures were executed on the basis of the guidelines outlined in the 2013 version of the Declaration of Helsinki. The protocol of the current investigation was evaluated and confirmed by the Cardiovascular Diseases Research Center institutional review board, affiliated with Guilan University of Medical Sciences (GUMS) and Ethics Committee of GUMS (registered with research number = 4258, and ethics code = “IR.GUMS.REC.1401.173”).
Coronary angiography and echocardiography
All the subjects in the case arm of the study were examined by two interventional cardiologists to perform CAG. CAG was performed on the basis of the Judkins technique with 6F catheters by the femoral artery approach. Then, the abovementioned cardiologists, who were blinded to the research protocol, interpreted the angiogram findings, including atherosclerosis severity and level of stenosis. If the narrowing of the vessel was < 50%, stenosis was described as minimal, while a vessel diameter reduction between 50 and 70% was described as moderate stenosis. A diameter decrease of greater than 70% was considered severe (or significant) stenosis. The presence of stenosis in one, two or three major coronary arteries was defined as single-, two- or three-vessel CAD, respectively. Of note, the diagnostic entity of nonobstructive CAD was considered when no culprit lesion was found on the angiogram, and luminal stenosis of epicardial vessels was estimated to be less than 50% [37].
A certified echocardiographer conducted echocardiograms on all CAD patients by means of a standard commercial ultrasound machine and within 72 h of admission to the hospital to estimate left ventricular systolic ejection fraction (LVEF). All echocardiography results were reassessed by two independent cardiologists according to the international Simpson method.
Laboratory analysis
At the study baseline, venous blood samples (approximately five ml) were obtained following a minimum of 8 h of overnight fasting. The samples were then segregated within 10 min and frozen at − 20 °C until the tests were carried out. Total cholesterol and FBS concentrations were measured by commercial kits (obtained from MAN Co. in Tehran, Iran)) that apply the enzymatic method through cholesterol oxidase and cholesterol esterase, and glucose oxidase enzymes, respectively. HDL-C levels were estimated on the basis of the enzymatic method (MAN Co., Tehran, Iran). An auto Analyzer (Hitachi, Japan) was used to measure these biochemical molecules. The same enzymatic method was used for evaluating triglyceride levels, albeit via glycerol phosphate oxidase and on the Bionic Corporation platform. Ultimately, the Friedewald formula was applied to estimate LDL-C values.
Explanation of the novel atherogenicity indices and insulin resistance surrogate markers
The formulas that were implemented to calculate the novel atherogenicity and surrogate insulin resistance indices are indicated below: [24, 38,39,40,41,42,43,44,45]
(All patients had triglyceride levels < 400 mg/dL).
Statistical analyses
To verify whether the collected data were normally distributed, the Kolmogorov–Smirnov test was used. Continuous variables were described as the mean ± standard deviation (SD). The differences in the mean values of continuous factors between the studied groups were then determined via independent samples T tests. Regarding categorical factors, corresponding data were demonstrated as frequency and percentage, and the Chi-square or Fisher's exact tests were run to compare groups. Comparisons of the traditional markers and novel indices, according to CAG-derived clinical features of CAD patients (i.e., type of CAD and severity of atherosclerosis), were made by applying one-way analysis of variance (ANOVA) followed by Least Significant Difference (LSD) post hoc tests.
Crude and adjusted logistic regression analyses were additionally executed to evaluate the relationship between the targeted variables and CAD risk, and the odds ratios (OR, or adjusted odds ratio, aOR) along with the corresponding 95% confidence interval (95% CI) were estimated for the relationship between independent variables and CAD risk. To test for linear trends across quartiles of lipid parameters and atherogenic indices in relation to CAD risk, the median value of each quartile was considered as a continuous variable.
The reported P values are two-tailed. The tests were regarded as significant if the obtained P values were less than or equal to 0.05. The Statistical Package for the Social Sciences (SPSS) software (version 21 (Chicago: SPSS Inc. IBM Corp.) was employed for conducting these analyses.
Sensitivity analysis
To test the robustness and stability of the present findings, two sensitivity analyses were carried out by excluding the subjects who reported consuming statins (first sensitivity analysis) and those with diabetes (second sensitivity analysis) at study baseline to attenuate the potential effects of these likely confounders.
Results
Baseline characteristics
Overall, 3085 non-CAD subjects (42.6% males) and 774 CAD patients (40.2% males) were enrolled in the current study. Table 1 demonstrates the baseline demographic, clinical, and anthropometric features of the study participants. Non-CAD individuals (mean (SD) of age = 51 ± 12) were significantly younger than CAD patients (mean (SD) of age = 54 ± 11) (P < 0.001). The BMIs of non-CAD and CAD participants were comparable (27.65 ± 4.63 and 27.62 ± 4.49 kg/m2, respectively). CAD patients were more likely to report a family history of CVDs and a personal history of diabetes and hypertension (P value < 0.001). In addition, there tended to be more statin users and more smokers among CAD cases than the controls (P value < 0.001). Additionally, the patients diagnosed with CAD had significantly elevated concentrations of triglycerides, total cholesterol, and FBS, and reduced concentrations of HDL-C (P value < 0.001). However, LDL-C levels did not differ significantly among controls and CAD patients. Additionally, this group showed greater values of novel atherogicity indices and insulin resistance surrogate markers than the non-CAD controls (P value < 0.001) (Table 1).
Echocardiogram and coronary angiogram findings in subjects diagnosed with CAD
Clinical features derived from CAG and echocardiography procedures conducted for CAD study cases are illustrated in Table 2. The majority of CAD patients (~ 64%) were revealed to have mild systolic dysfunction (i.e., LVEF between 41%-55%). The angiographic findings of CAD cases also showed that approximately 42% and 44% of these patients were diagnosed with nonobstructive CAD and suffered from severe stenosis, respectively (Table 2).
Comparisons of traditional cardio-metabolic factors, atherogenicity indices, and surrogate markers of insulin resistance according to CAD patients’ clinical features that were derived from CAG (i.e., type of CAD and severity of atherosclerosis) are shown in Supplementary Tables 1 and 2. Those with three-vessel coronary disease tended to have higher FBS and TyG, and lower BMI and TyG-BMI levels than patients with nonobstructive CAD. In addition, the CAD patients with severe and moderate stenosis showed higher levels of HDL-C, FBS, and TyG, and lower values of BMI, TyG-BMI, CRI-I and METS-IR than those with minimal stenosis (P value < 0.05).
The relationship between traditional cardio-metabolic indices and CAD risk
The odds ratios (95% CIs) for CAD based on the quartiles of the studied variables are demonstrated in Table 3, using crude and adjusted multiple regression models. According to the crude model, patients in quartiles 2, 3 and 4 of triglycerides showed a 39%, 82% and 165% elevated CAD risk compared to 1st quartile patients, respectively (2nd quartile OR = 1.39, 95% CI: 1.07–1.79; 3rd quartile OR = 1.82, 95% CI: 1.42–2.31; 4th quartile OR = 2.65, 95% CI: 2.09–3.35; P-for-trend < 0.001). Following adjusting for age, sex, BMI, smoking, family history of heart disease, HTN, type 2 diabetes mellitus, and statin use, an approximately 66%, 90% and 172% elevated risk of CAD was shown for those in the 2nd, 3rd, and 4th serum triglyceride quartiles as opposed to 1st quartile patients (2nd quartile aOR = 1.66, 95% CI: 1.16–2.38; 3rd quartile aOR = 1.90, 95% CI: 1.33–2.72; 4th quartile aOR = 2.72, 95% CI: 1.90–3.89; P-for-trend < 0.001). Each mmol/L increment in serum triglyceride concentration was also associated with an approximately 98% elevated risk of CAD (aOR = 1.98, 95% CI: 1.65–2.37; P value < 0.001). Although no significant associations were found based on the crude regression model, when taking into account the abovementioned potential confounders, the patients in the fourth quartile of total cholesterol demonstrated a higher risk of CAD by approximately 51% compared to those in the lowest quartile (aOR = 1.51 95% CI: 1.08–2.12; P-for-trend = 0.031). In addition, approximately 30% and 35% elevations in CAD risk were noted for each mmol/L increment in total cholesterol and LDL-C levels, respectively (aOR = 1.30, 95% CI: 1.15–1.45; aOR = 1.35, 95% CI: 1.12–1.53; P value < 0.001). Nevertheless, those in the second quartile of LDL-C levels demonstrated a lower risk of CAD by approximately 40% than those in the lowest quartile (2nd quartile aOR = 0.60, 95% CI: 0.42–0.85; P-for-trend = 0.009).
On the other hand, an increased serum level of HDL-C was shown to be protective against CAD risk in both bivariable (4th quartile OR = 0.63, 95% CI: 0.50–0.80; P-for-trend = 0.001) and multiple regression analyses (4th quartile aOR = 0.41, 95% CI: 0.29–0.59; P-for-trend < 0.001) (Table 3). Each mmol/L increment in serum HDL-C concentration was also linked to an approximately 74% reduced risk of CAD (aOR = 0.26, 95% CI: 0.16–0.41; P value < 0.001).
Additionally, when comparing higher to lowest FBS quartiles, CAD risk was demonstrated to be augmented (2nd quartile OR = 1.98, 95% CI: 1.34–2.93; 3rd quartile OR = 5.31 95% CI: 3.72–7.58; 4th quartile OR = 26.29, 95% CI: 18.67–37.03; P-for-trend < 0.001). This relationship between FBS quartiles and CAD risk persisted in the adjusted regression models (2nd aOR = 2.81, 95% CI: 1.71–4.62; 3rd quartile aOR = 6.34, 95% CI: 4.00–10.04; 4th quartile aOR = 21.56, 95% CI: 13.54–34.33; P-for-trend < 0.001). Furthermore, each mmol/L increment in serum FBS levels was accompanied by an approximately 92% elevated risk of CAD (aOR = 1.92, 95% CI: 1.72–2.14; P value < 0.001) (Table 3).
The relationship between novel atherogenicity and insulin resistance surrogate indices, and CAD risk
Significant relationships between various atherogenicity indices including AIP (2nd quartile OR = 1.98, 95% CI: 1.52–2.58; 3rd quartile OR = 3.00, 95% CI: 2.33–3.88; 4th quartile OR = 3.19, 95% CI: 2.47–4.11; P-for-trend < 0.001), CRI-I (2nd quartile OR = 1.27, 95% CI: 1.02–1.59; 4th quartile OR = 1.35, 95% CI: 1.08–1.68; P-for-trend = 0.042), LCI (2nd quartile OR = 1.62, 95% CI: 1.28–2.06; 3rd quartile OR = 1.65, 95% CI: 1.31–2.10; 4th quartile OR = 1.95, 95% CI: 1.55–2.47; P-for-trend < 0.001), METS − IR (2nd quartile OR = 1.67, 95% CI: 1.31–2.14; 3rd quartile OR = 2.23, 95% CI: 1.75–2.83; 4th quartile OR = 2.00, 95% CI: 1.57–2.55; P-for-trend < 0.001), TyG index (2nd quartile OR = 1.68, 95% CI: 1.24–2.26; 3rd quartile OR = 2.96, 95% CI: 2.24–3.92; 4th quartile OR = 7.44, 95% CI: 5.70–9.70; P-for-trend < 0.001), and TyG-BMI (2nd quartile OR = 1.73, 95% CI: 1.36–2.20; 3rd quartile OR = 1.84, 95% CI: 1.45–2.37; 4th quartile OR = 1.99, 95% CI: 1.53–2.46; P-for-trend < 0.001), and CAD risk were found based on crude regression analyses (Table 3). Considering the multiple regression models adjusted for age, sex, BMI, smoking, family history of heart disease, HTN, type 2 diabetes mellitus, and statin use, parallel results were observed for all of the abovementioned novel biomarkers, except for METS-IR and TyG-BMI. Compared to the lowest quartile, elevated levels of AIP significantly multiplied the odds of CAD by approximately 1.9–3.8 times (2nd quartile aOR = 1.92, 95% CI: 1.32–2.79, 3rd quartile aOR = 3.05, 95% CI: 2.10–4.42, 4th quartile aOR = 3.80, 95% CI; 2.62–5.53; P-for-trend < 0.001). In addition, each unit increment in the AIP was accompanied by an approximately 8.92-fold elevated risk of CAD (aOR = 8.92, 95% CI: 5.23–15.23; P value < 0.001). Also, those in the 2nd and 4th quartiles of the CRI-I, with regard to the lowest values, showed significantly elevated odds of CAD by approximately 73% and 158% (2nd quartile aOR = 1.73, 95% CI: 1.22–2.45; 4th quartile aOR = 2.58, 95% CI: 1.82–3.66; P-for-trend < 0.001). Similarly, those in the highest quartile of the CRI-II showed an approximately 68% greater CAD risk than those in the lowest quartile (4th quartile aOR = 1.68, 95% CI: 1.21–2.34; P-for-trend < 0.001). Furthermore, each unit increment in the CRI-I and II was associated with an approximately 1.4-fold increased risk of CAD (aOR = 1.37, 95% CI: 1.26–1.49; aOR = 1.43, 95% CI: 1.30–1.57, respectively; P value < 0.001). Moreover, the subjects in the highest quartile of CHOLINDEX indicated an approximately 49% elevation in the risk of CAD compared to the lowest quartile (4th quartile aOR = 1.49, 95% CI: 1.09–2.05; P-for-trend < 0.001). Additionally, each unit increment in the CHOLINDEX was linked to approximately 1.5 times greater CAD risk (aOR = 1.44, 95% CI: 1.27–1.62; P value < 0.001). In addition, higher LCI levels significantly increased the odds of CAD by nearly 2–3 folds, after controlling the regression models for potential confounders (2nd quartile aOR = 2.19, 95% CI: 1.52–3.16, 3rd quartile aOR = 2.63, 95% CI: 1.82–3.79, 4th quartile OR = 3.24, 95% CI: 2.25–4.66; P-for-trend < 0.001). Furthermore, each unit increment in the LCI was accompanied by an approximately 2% increased risk of CAD (aOR = 1.02, 95% CI: 1.01–1.03; P value < 0.001).
When taking into account the potential confounders, including age, sex, smoking, family history of heart disease, HTN, type 2 diabetes mellitus, and statin use, in the multiple regression model, greater values of METS − IR showed accelerated odds of CAD by approximately 1.5 times in the second and third quartiles as opposed to the first quartile (2nd quartile aOR = 1.46; 95% CI: 1.03–2.06, 3rd quartile aOR = 1.64, 95% CI: 1.18–2.29). Likewise, raised levels of the TyG index significantly worsened the CAD risk by nearly 1.5–5 folds (2nd quartile aOR = 1.64. 95% CI: 1.10–2.45, 3rd quartile aOR = 2.51, 95% CI: 1.71–3.70, 4th quartile aOR = 4.80, 95% CI: 3.29–6.97; P-for-trend < 0.001). Furthermore, each unit increment in the TyG index was linked to approximately 4 times greater CAD risk (aOR = 4.12, 95% CI: 3.20–5.30; P value < 0.001). Nevertheless, no significant associations were found between TyG-BMI and CAD risk according to the multiple regression analysis (Table 3).
Sensitivity analysis
The ORs (and 95% CI) for CAD in the quartiles of traditional and novel atherogenicity markers, and insulin resistance surrogate markers remained consistent, when in the sensitivity analysis were conducted excluding the participants who consumed statins at study baseline (Table 4) or those suffering from diabetes (Table 5) were excluded. Interestingly, the direct association between increased serum LDL-C concentrations and CRI-I values and a greater risk of CAD became more apparent in these sensitivity analyses. Furthermore, although those in the second quartile of LDL-C levels demonstrated a lower risk of CAD in the overall analysis, this association was no longer significant when excluding those who used statins at baseline (OR = 0.66, 95% CI: 0.44–1.00).
Discussion
Collectively, the present results of a case‒control study on 774 angiographically confirmed CAD patients and 3085 non-CAD subjects highlighted that, after controlling for potential confounders, several novel atherogenicity and insulin resistance surrogate indices, including AIP, CRI-I, CRI-II, CHOLINDEX, LCI, and TyG, and to some extent, METS-IR, can significantly predict the risk of CAD. Additionally, the current results demonstrated that traditional dyslipidemia and hyperglycemia serum/plasma biomarkers, such as triglycerides, total cholesterol, HDL-C, and FBS, were able to independently predict the odds of having CAD, taking into consideration the confounding variables’ effects.
In sensitivity analyses, interestingly, the direct association between increased serum LDL-C concentrations and CRI-I values, and greater risk of CAD became more apparent when excluding those with diabetes and statin users. Furthermore, although those in the second quartile of LDL-C levels demonstrated a lower risk of CAD, this association was no longer significant when excluding those who used statins at baseline.
The present research findings on the relationship between novel atherogenicity indices and surrogate markers of insulin resistance, and CAD risk further support the results of previous research efforts [19, 24,25,26,27,28,29,30,31, 40,41,42, 46,47,48,49]. Treating the studied variables as continuous in the multiple regression analysis revealed that higher levels of “AIP (= log10(triglycerides/HDL-C))” were linked to an elevated risk of CAD by nearly 9 times. Additionally, subjects with the greatest values of “CRI-I (= total cholestrol/HDL-C)”, CRI-II (= LDL-C/HDL-C), and CHOLINDEX (= LDL-C-HDL-C) demonstrated higher CAD risk by approximately 1.4–1.5 times. Additionally, higher levels of “LCI (= total cholesterol × triglycerides × LDL-C/HDL-C)” significantly increased the CAD risk by approximately 2%. Cai et al. conducted a case–control study in 2017 on patients with CAD (n = 2936) compared to control subjects (n = 2451), aiming to explore the association between the novel and comprehensive atherogenicity indices, and CAD risk among the Chinese Han population, following CAG [24]. Similar to the current study, they revealed that subjects suffering from CAD demonstrated greater levels of atherogenic lipid markers while having reduced HDL-C concentrations. Novel indices, such as the non-HDL-C to HDL-C ratio, non-HDL-C, total cholesterol to HDL-C ratio (i.e., CRI-I), LDL-C to HDL-C ratio (i.e., CRI-II), LCI and AIP, were all demonstrated to be elevated among patients suffering from CAD. Specifically, it was also mentioned that AIP contributed to a higher risk of CAD regardless of other factors [24]. Additional studies have also indicated that AIP could positively correlate with atherosclerosis severity among subjects diagnosed with CAD [19, 47]. In 2020, Qin et al. conducted an observational study in which they identified AIP as a reliable predictor of post-percutaneous coronary intervention (PCI) prognosis, and developed treatment targets among subjects suffering from type 2 diabetes. They also found that greater levels of AIP (especially > 0.318) were accompanied by a higher risk of developing cerebrovascular adverse events [48]. Several other reports have also found a direct and significant link between AIP and carotid intima-media thickness (as an indirect factor showing atherosclerotic lesion development and progression) [50,51,52]. AIP has additionally been linked to symptomatic carotid artery stenosis [53]. Interestingly, these relationships seem to persist even after accounting for conventional CV risk markers [52, 53]. In accordance with the current study findings and based on the observations of these researchers, it can be speculated that isolated lipid parameters such as LDL-C cannot be considered as comprehensive and reliable predictors of atherosclerotic lesion severity and patient prognosis.
In terms of alternative markers for insulin resistance, the current study revealed that raised levels of the TyG index (= Ln [fasting serum triglycerides × fasting blood glucose/2]) significantly worsened the risk of CAD by nearly fourfold. In addition, those in the second and third quartiles of “METS − IR (= Ln ((2 × fasting blood glucose (mg/dL)) + fasting serum triglycerides (mg/dL)) × (BMI (kg/m2))/((Ln (HDL-C (mg/dL))))” showed an accelerated risk of CAD by approximately 1.5 times. However, analyzing the overall association between METS-IR and TyG-BMI (= TyG × BMI) values, and CAD risk did not detect significant findings when treating the variables as continuous in the multiple regression models.
In line with prior reports [54, 55] and the present study findings, a recently published systematic review documented that among asymptomatic individuals, a greater TyG index might be individualistically linked to a greater incidence of atherosclerotic CVDs, including CAD and stroke [26]. Furthermore, similar to more well-known markers for insulin resistance, such as “homeostasis model assessment for insulin resistance (HOMA-IR)”, these novel biomarkers have also been significantly associated with CVD risk factors, such as hypertension, arterial stiffness, coronary stenosis, metabolic syndrome, type 2 diabetes, and all-cause and/or CV mortality [25, 43,44,45, 56,57,58,59].
It has been suggested in the literature that AIP, as a reliable reflector of plasma atherogenicity status, could be applied as an alternative marker for small dense LDL-C (sdLDL-C) and insulin resistance [42, 48, 49]. Of note, sdLDL-C, an LDL-C subcomponent that tends to have greater potential for being oxidized and making foam cells, has been indicated to better represent the progression of atherosclerotic lesions in CAD. However, due to difficulties in the routine assessment of sdLDL-C, particularly in clinical practice, the use of AIP as a reliable surrogate indicator of sdLDL-C has gained considerable attention in recent years [23, 42, 48, 49, 51, 60, 61]. In addition, hypertriglyceridemia, hyperglycemia, and subsequent insulin resistance further modulate the progression of atherosclerosis [8, 9, 19].
The euglycemic-hyperinsulinemic clamp test has been recognized as the “gold-standard” test for measuring insulin resistance [62]. However, routine use of this test is costly and time consuming, especially in the clinical setting. Therefore, several attempts have been made to identify more efficient methods for measuring insulin resistance [23, 44, 56, 62]. In this context, the TyG index has also been mentioned as a reliable and convenient indicator of insulin resistance [23, 44, 56]. TyG was revealed to capture the status of lipotoxicity and glucotoxicity [44, 56]. Furthermore, the use of METS‐IR, as a valid surrogate measure for sensitivity to insulin that incorporates both laboratory and anthropometric measures, has been additionally taken into consideration to recognize subjects who are more prone to developing type 2 diabetes and metabolic syndrome earlier, which are both established CVD risk indicators [44, 63]. The contribution of insulin resistance to pathogenic mechanisms involved in atherosclerotic disorders such as CAD can be biologically plausible; the accumulation of free fatty acids (FFAs), particularly in the hepatic tissue, following a decrease in the sensitivity to insulin (i.e., insulin resistance) and therefore the lack of lipolysis-inhibitory effects of insulin, may result in an elevation in apoB and very low density lipoprotein cholesterol (VLDL-C) formation and reduction of their clearance. Moreover, HDL-C levels will be decreased. These events, together with hypertriglyceridemia, ultimately promote atheroma development [64]. It is thought that lipoproteins with massive triglyceride concentrations and hyperinsulinemia promote the development and advancement of atherosclerotic lesions by promoting the secretion of reactive oxygen species (ROS), increasing inflammation due to monocytes and proinflammatory and adhesion molecules, and reducing NO release by the endothelium. These processes eventually lead to endothelial dysfunction [65,66,67,68,69].
Comparisons with other studies and what does the current work add to the existing knowledge
Regarding novel indices of atherogenicity and insulin resistance, the available literature has mostly acknowledged the direct link between augmented AIP values, and CVD risk factors (i.e., insulin resistance, hypertension, obesity, and diabetes) and outcomes. For example, AIP has been primarily connected with the progression of metabolic syndrome, CAD, all-cause mortality, and CV events [24, 26, 40,41,42, 46,47,48,49,50, 52,53,54,55, 70]. Moreover, METS‐IR and TyG have been additionally taken into consideration for the early recognition of subjects at high risk for CVD risk factors, particularly type 2 diabetes, in addition to metabolic syndrome [23, 44, 56, 63]. However, less is understood about the link between CAD and METS − IR, TyG and TyG-BMI, as more straightforward, and less expensive methods for estimating the status of insulin resistance.
Therefore, the importance of investigating more comprehensive and efficient markers to detect those at earlier risk of CAD is well recognized. The present results lend support to the current evidence on the high value of novel indices that were demonstrated to be elevated among patients suffering from CAD [24, 26, 40, 41, 48, 50, 52,53,54,55, 70]. It seems that such indices, in particular AIP, CRI-I and II, CHOLINDEX, LCI, TyG and METS-IR, may yield more comprehensive and easy to interpret estimates of high-risk individuals for CAD beyond the isolated lipid biomarkers (i.e., triglycerides, LDL-C, HDL-C, or total cholesterol) and FBS. Thus, they can be considered in the initial screening of populations for the identification of patients at risk of CAD, particularly in clinical practice. Furthermore, modeling CAD patient response to therapeutic approaches, including CAG, drug therapies or PCI, according to their baseline levels of novel atherogenic indices and insulin resistance alternative markers would be of utmost importance. With respect to this, the potential usefulness of these indices as a treatment target in clinical trials could additionally be considered as a particular clinical relevance of these findings.
Study strengths and limitations
Strengths
There are a few advantages that should be noted; to our knowledge, this is the first investigation in a sample of Iranian subjects in which the risk of CAD is assessed based on the more novel indices for dyslipidemia and hyperglycemia. Furthermore, CAG was performed for all the included CAD patients in the present investigation by two expert interventional cardiologists who were blinded to the research protocol. Thus, misclassification and under-ascertainment of CAD cases were minimized.
Limitations
When interpreting the present research findings, a number of limitations should be considered. First, the single-center, retrospective, and cross-sectional nature of the study might have biased the results toward confirming a causal association between CAD risk and the candidate indices. Since no participant follow-up data were available, associations between the novel cardiometabolic indices, and incidence of future acute CV events and related morbidity and mortality could not have been explored. Moreover, due to the lack of data on a number of relevant cardiometabolic variables, including dietary intake, physical activity, and waist circumference, the potentially confounding effect of these variables on the study biomarkers and outcome could not have been investigated. Furthermore, since the control subjects were identified using noninvasive methods and were free from cardiac symptoms, we cannot fully ensure that all were free from coronary atherosclerosis, considering the fact that they did not undergo angiography.
Conclusion
In conclusion, novel atherogenicity indices and insulin resistance surrogate markers, in particular, AIP, CRI-I and II, CHOLINDEX, LCI, and TyG, might be useful in predicting CAD risk. As these indices have the isolated dyslipidemia and/or hyperglycemia biomarkers built in their formulas, they could possibly yield more robust and comprehensive estimates of CAD risk than the traditional lipid and/or glycemia biomarkers by themselves. However, designing long-term prospective cohort surveys is essential to particularly explain the diagnostic value of such novel indices in the early detection of atherosclerotic CVDs and CAD incidence.
Availability of data and materials
The datasets of the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AIP:
-
Atherogenic index of plasma
- BMI:
-
Body mass index
- CRI-I, CRI-II:
-
Castelli’s risk indices-I,II
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CAG:
-
Coronary angiography
- CHOLINDEX:
-
Cholesterol index
- CVDs:
-
Cardiovascular disorders
- FBS:
-
Fasting blood sugar
- HDL-C:
-
High-density lipoprotein cholesterol
- GUMS:
-
Guilan University of Medical Science
- HOMA-IR:
-
Homeostatic Model Assessment for Insulin Resistance
- sdLDL-C:
-
Small dense low-density lipoprotein cholesterol
- LCI:
-
Lipoprotein combine index
- LDL-C:
-
Low-density lipoprotein cholesterol
- VLDL-C:
-
Very low density lipoprotein cholesterol
- METS − IR:
-
Metabolic score for insulin resistance index
- BMI:
-
Body mass index
- OR:
-
Odds ratio
- aOR:
-
Adjusted odds ratio
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- PCI:
-
Percutaneous coronary intervention
- TyG:
-
Triglyceride-glucose index
- TyG-BMI:
-
Triglyceride-glucose body mass index
References
World Health Organization (WHO). Cardiovascular diseases (CVDs). Available at: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Cited date May 10, 2022.
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234:16812–23.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–596.
Tardif J-C. Coronary artery disease in 2010. Eur Heart J Suppl. 2010;12:C2–10.
Mendis S, Puska P, Norrving Be, Organization WH: Global atlas on cardiovascular disease prevention and control. World Health Organization; 2011.
Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–26.
Pahwa R, Jialal I. Atherosclerosis. [Updated 2020 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507799/.
Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41.
Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5:927–46.
Mahdavi-Roshan M, Shilan A, kasmaei p, farmanbar r, Shakiba M, Zareban I, Mohamadkhah F: Factors predicting nutritional behaviors related to gastric cancer: A model-guided study. Drug Invention Today 2018, 10.
Virchow R. Phlogose und thrombose in gefassystem. Gesammelte abhandlungen zur wissenschaftlichen medicin. 1856;458–63.
Wilkins LW. Classics in arteriosclerosis research: On experimental cholesterin steatosis and its significance in the origin of some pathological processes by N. Anitschkow and S. Chalatow, translated by Mary Z. Pelias, 1913. Arteriosclerosis. 1983;3(2):178–82.
Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD. Lusis AJ: Atherosclerosis: Basic Mechanisms. Circulation. 1995;91:2488–96.
von Rokitansky KF: A manual of pathological anatomy. Blanchard & Lea; 1855.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. The Lancet. 2014;384:626–35.
Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. 2004;26:637–44.
Austin MA. Plasma triglyceride and coronary heart disease. Arterioscler thromb. 1991;11:2–14.
Wang L, Chen F, Xiaoqi C, Yujun C, Zijie L. Atherogenic Index of Plasma Is an Independent Risk Factor for Coronary Artery Disease and a Higher SYNTAX Score. Angiology. 2021;72:181–6.
Álvarez-Álvarez MM, Zanetti D, Carreras-Torres R, Moral P, Athanasiadis G. A survey of sub-Saharan gene flow into the Mediterranean at risk loci for coronary artery disease. Eur J Hum Genet. 2017;25:472–6.
Bauersachs R, Zannad F. Rivaroxaban: a new treatment paradigm in the setting of vascular protection? Thromb Haemost. 2018;118:S12–22.
Hanson MA, Fareed MT, Argenio SL, Agunwamba AO, Hanson TR. Coronary artery disease. Prim Care. 2013;40:1–16.
Mahdavi-Roshan M, Salari A, Vakilpour A, Savar Rakhsh A, Ghorbani Z. Rice bran oil could favorably ameliorate atherogenicity and insulin resistance indices among men with coronary artery disease: post hoc analysis of a randomized controlled trial. Lipids Health Dis. 2021;20:153.
Cai G, Shi G, Xue S, Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine (Baltimore). 2017;96:e8058.
Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:137.
Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20:76.
Si Y, Fan W, Han C, Liu J, Sun L. Atherogenic Index of Plasma, Triglyceride-Glucose Index and Monocyte-to-Lymphocyte Ratio for Predicting Subclinical Coronary Artery Disease. Am J Med Sci. 2021;362:285–90.
Won KB, Jang MH, Park EJ, Park HB, Heo R, Han D, Chang HJ. Atherogenic index of plasma and the risk of advanced subclinical coronary artery disease beyond traditional risk factors: An observational cohort study. Clin Cardiol. 2020;43:1398–404.
Kalelioglu T, Genc A, Karamustafalioglu N, Emul M. Assessment of cardiovascular risk via atherogenic indices in patients with bipolar disorder manic episode and alterations with treatment. Diabetes Metab Syndr. 2017;11(Suppl 1):S473-s475.
Castelli WP, Abbott RD, McNamara PM. Summary estimates of cholesterol used to predict coronary heart disease. Circulation. 1983;67:730–4.
Hanak V, Munoz J, Teague J, Stanley A Jr, Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219–22.
Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch Med Res. 2019;50:285–94.
Sachdeva A, Cannon CP, Deedwania PC, LaBresh KA, Smith SC Jr, Dai D, Hernandez A, Fonarow GC. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(111–117):e112.
Soares LP, Fabbro ALD, Silva AS, Sartorelli DS, Franco LF, Kuhn PC, Moises RS, Vieira-Filho JPB, Franco LJ. Cardiovascular Risk in Xavante Indigenous Population. Arq Bras Cardiol. 2018;110:542–50.
Koca TT, Tugan CB, Seyithanoglu M, Kocyigit BF. The Clinical Importance of the Plasma Atherogenic Index, Other Lipid Indexes, and Urinary Sodium and Potassium Excretion in Patients with Stroke. Eurasian J Med. 2019;51:172–6.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:407–77.
Braunwald E, Bonow R: Cardiovascular Medicine. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 9 [sup] th ed. Philadelphia: Elsevier Saunders; 2012.
Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34:583–8.
Oguntola SO, Hassan MO, Duarte R, Dix-Peek T, Dickens C, Olorunfemi G, Vachiat A, Paget G, Manga P, Naicker S. Atherosclerotic vascular disease and its correlates in stable black South African kidney transplant recipients. Int J Nephrol Renovasc Dis. 2018;11:187–93.
Tecer D, Sunar I, Ozdemirel AE, Tural R, Kucuksahin O, Sepici Dincel A, Ataman S. Usefullnes of atherogenic indices and Ca-LDL level to predict subclinical atherosclerosis in patients with psoriatic arthritis? Advances in Rheumatology. 2019;59:49.
Akpınar O, Bozkurt A, Acartürk E, Seydaoğlu G. A new index (CHOLINDEX) in detecting coronary artery disease risk. Anadolu Kardiyol Derg. 2013;13:315–9.
Olamoyegun MA, Oluyombo R, Asaolu SO. Evaluation of dyslipidemia, lipid ratios, and atherogenic index as cardiovascular risk factors among semi-urban dwellers in Nigeria. Ann Afr Med. 2016;15:194–9.
Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, Sánchez-Lázaro D, Meza-Oviedo D, Vargas-Vázquez A, Campos OA, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178:533–44.
Yu X, Wang L, Zhang W, Ming J, Jia A, Xu S, Li Q, Ji Q. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: A nationwide study. J Diabetes Investig. 2019;10:1050–8.
Er L-K, Wu S, Chou H-H, Hsu L-A, Teng M-S, Sun Y-C, Ko Y-L. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS ONE. 2016;11:e0149731–e0149731.
Guo Q, Zhou S, Feng X, Yang J, Qiao J, Zhao Y, Shi D, Zhou Y. The sensibility of the new blood lipid indicator–atherogenic index of plasma (AIP) in menopausal women with coronary artery disease. Lipids Health Dis. 2020;19:27.
Wu TT, Gao Y, Zheng YY, Ma YT, Xie X. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17:197.
Qin Z, Zhou K, Li Y, Cheng W, Wang Z, Wang J, Gao F, Yang L, Xu Y, Wu Y, et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19:23.
Li Y-W, Kao T-W, Chang P-K, Chen W-L, Wu L-W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. 2021;11:9900–9900.
Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, Hu J, Xu W, Yi N, Lei S. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. 2018;17:37.
Tecer D, Sunar I, Ozdemirel AE, Tural R, Kucuksahin O, Sepici Dincel A, Ataman S. Usefullnes of atherogenic indices and Ca-LDL level to predict subclinical atherosclerosis in patients with psoriatic arthritis? Adv Rheumatol. 2019;59:49.
Gentile M, Iannuzzo G, Simeon V, Mattiello A, Rubba F, Panico C, Panico S, Rubba P. Association between atherogenic index of plasma and carotid intima-media thickness in a cohort of Mediterranean women. Acta Cardiol. 2021;76(9):987–92.
Garg R, Knox N, Prasad S, Zinzuwadia S, Rech MA. The Atherogenic Index of Plasma is Independently Associated with Symptomatic Carotid Artery Stenosis. J Stroke Cerebrovasc Dis. 2020;29:105351.
Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18:361.
Park GM, Cho YR, Won KB, Yang YJ, Park S, Ann SH, Kim YG, Park EJ, Kim SJ, Lee SG, et al. Triglyceride glucose index is a useful marker for predicting subclinical coronary artery disease in the absence of traditional risk factors. Lipids Health Dis. 2020;19:7.
Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The Triglyceride-Glucose Index, an Insulin Resistance Marker, Was Non-linear Associated With All-Cause and Cardiovascular Mortality in the General Population. Front Cardiovasc Med. 2020;7:628109.
Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, Huang PH, Lin SJ. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal Aging Study. Cardiovasc Diabetol. 2021;20:206.
Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Zhang HW, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10:6137–46.
Soltanipour S, Hasandokht T, Soleimani R, Mahdavi-Roshan M, Jalali MM. Systematic Review and Meta-Analysis of the Effects of Soy on Glucose Metabolism in Patients with Type 2 Diabetes. The review of diabetic studies : RDS. 2019;15:60–70.
Zhu L, Lu Z, Zhu L, Ouyang X, Yang Y, He W, Feng Y, Yi F, Song Y. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol Pol. 2015;73:931–8.
Mahdavi-Roshan M, Salari A, Kheirkhah J, Ghorbani Z. The Effects of Probiotics on Inflammation, Endothelial Dysfunction, and Atherosclerosis Progression: A Mechanistic Overview. Heart Lung Circ. 2022;31:e45–71.
Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining Insulin Resistance From Hyperinsulinemic-Euglycemic Clamps. Diabetes Care. 2012;35:1605–10.
Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, Sanchez-Lazaro D, Meza-Oviedo D, Vargas-Vazquez A, Campos OA, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178:533–44.
Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Investig. 2006;116:1813–22.
Jin Y, Wu Y, Zeng Z, Jin C, Wu S, Wang Y, Fu Z. From the Cover: Exposure to Oral Antibiotics Induces Gut Microbiota Dysbiosis Associated with Lipid Metabolism Dysfunction and Low-Grade Inflammation in Mice. Toxicol Sci. 2016;154:140–52.
Elhady M, Elazab AAAM, Bahagat KA, Abdallah NA. Ibrahim GE-S: Fatty pancreas in relation to insulin resistance and metabolic syndrome in children with obesity. J Pediatr Endocrinol Metab. 2019;32:19–26.
Reaven GM. Insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypertension. Parallels between human disease and rodent models. Diabetes Care. 1991;14:195–202.
Peng J, Luo F, Ruan G, Peng R, Li X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017;16:233.
Ghorbani Z, Togha M, Rafiee P, Ahmadi ZS, Rasekh Magham R, Djalali M, Shahemi S, Martami F, Zareei M, Razeghi Jahromi S, et al. Vitamin D3 might improve headache characteristics and protect against inflammation in migraine: a randomized clinical trial. Neurol Sci. 2020;41:1183–92.
Dobiasova M, Frohlich J, Sedova M, Cheung MC, Brown BG. Cholesterol esterification and atherogenic index of plasma correlate with lipoprotein size and findings on coronary angiography. J Lipid Res. 2011;52:566–71.
Acknowledgements
The authors would like to thank the patients whose data were used in this analysis.
Funding
This study was financially supported by the Cardiovascular Diseases Research Center, GUMS (research code = 4258).
Author information
Authors and Affiliations
Contributions
MMR and ZGh: Conceived and designed the research. MMR, ZGh, AS, ASR, AH, MM and NN played an important role in data collection and reviewing the patients' documents. MMR, ZGh, ASR, AH, and MM: Acquired data and performed data analysis. MMR, ZGh, MM, AS, and ASR played an important role in the interpretation of the results. MMR, ZGh, MM, and NN drafted the manuscript. All authors reviewed and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol complied with the guidelines of the 2013 version of the Helsinki Declaration.
The current study protocol was evaluated and confirmed by the Cardiovascular Diseases Research Center institutional review board at GUMS and the Ethics Committee of GUMS (research number = 4258, and ethics code = IR.GUMS.REC.1401.173).
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest concerning this article's research, authorship, and/or publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Comparison of traditional cardio-metabolic factors, atherogenicity indices, and surrogate markers of insulin resistance according to type and severity of atherosclerosis in the studied patients with coronary artery disease.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahdavi-Roshan, M., Mozafarihashjin, M., Shoaibinobarian, N. et al. Evaluating the use of novel atherogenicity indices and insulin resistance surrogate markers in predicting the risk of coronary artery disease: a case‒control investigation with comparison to traditional biomarkers. Lipids Health Dis 21, 126 (2022). https://doi.org/10.1186/s12944-022-01732-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01732-9