Abstract
Background
Epidemiological and clinical evidence suggests that high-dose intake of omega 3 fatty acids (n-3 FA) have a favorable role in altering serum triglycerides (TG) and non-high density lipoprotein cholesterol (non-HDL-C) when combined with statins in hyperlipidemic patients. Their efficacy in altering low-density lipoprotein cholesterol (LDL-C) particle size is yet to be established.
Aim
This study evaluated the effects of supplementing 4 g/day Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) on serum blood lipids, including small, dense LDL-C particle concentration, in hyperlipidemic patients receiving stable statin therapy.
Methods
In this randomized, placebo-controlled, double-blind parallel group study, 44 patients on statin therapy for > 8 weeks with non-HDL-C concentrations above 130 mg/dL were randomized into two groups. For 8 weeks, together with their prescribed statin, the intervention group received 4 g/day EPA + DHA (3000 mg EPA + 1000 mg DHA in ethyl ester form) and the placebo group received 4 g/day olive oil (OO). Measurements of serum non-HDL-C, TG, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), LDL-C (including large - LDL I; intermediate - LDL II; and small - LDL III subclasses), very-low-density lipoprotein cholesterol (VLDL-C) concentration, were taken at baseline and post-intervention. Dietary intake was assessed with a weighed intake, 3-day food diary at week 4. Primary outcome measures were percent change in LDL III, non-HDL-C and LDL particle number.
Results
At the end of treatment, the median percent change in serum LDL III concentration was significantly greater in the n-3 FA group plus atorvastatin compared to placebo (− 67.5% vs − 0%, respectively; P < 0.001). Supplementation with n-3 FA plus atorvastatin led to significant reductions in serum non-HDL-C (− 9.5% vs 4.7%, P < 0.01), TG (− 21.5% vs 6.2%, P < 0.001) and VLDL-C (− 36.9% vs 4.0%, P < 0.001) and TC (− 6.6% vs 2.1%, P < 0.001). Between the groups, no significant difference in percent change in the serum concentration of LDL-C, HDL-C, as well as in the LDL I and LDL II subclasses was observed.
Conclusion
In this group of hyperlipidemic patients on a stable statin prescription, OM3 plus atorvastatin improved small dense LDL concentrations, non-HDL-C, VLDL-C and TG to a greater extent than atorvastatin alone. Further studies are warranted in this area.
Trial registration
This trial was retrospectively registered on 23 May 2019 on ClinicalTrials.gov with ID: NCT03961763.
Similar content being viewed by others
Background
Hyperlipidemia is one of the known etiological causes of atherosclerosis. Epidemiological studies have shown that elevated serum concentrations of TG and LDL-C as well as low concentrations of HDL-C are independently and partially linked to the development of atherosclerosis [1]. Although much focus has been on LDL-C as the primary therapeutic target of atherosclerosis, The National Lipid Association suggest that non-HDL-C might be a superior indicator of cardiovascular disease (CVD) risk than LDL-C is, based on clinical evidence [2]. More importantly, LDL-C particle size is also suggested to be an important determinant of atherosclerotic process. Small and dense LDL-C particles have an increased capacity to penetrate the arterial wall and are more readily oxidized compared to the large and buoyant ones [3]. Consistent with this, clinical studies collectively show a positive relation between serum concentration of small, dense LDL-C particles and CVD risk [4,5,6,7,8,9,10]..
Marine-derived n-3 FA EPA and DHA are known to play a role in altering lipid metabolism by upregulating FA catabolism through the nuclear receptor peroxisome proliferator–activated receptor-α (PPAR- α) activation and decreasing lipogenesis by downregulating sterol responsive element binding protein-1c (SREBP1-c) [11]. Although statins are the first drug of choice to alter blood lipids, accumulating evidence suggests that marine-derived n-3 FA EPA and DHA supplementation effectively decreased serum triglycerides and non-HDL-C in several clinical studies. In two large clinical trials EVOLVE (n = 399) and MARINE (n = 229), 4 g/day EPA and DHA supplementation alone resulted in 31%(p < 0.001) and 33% (p < 0.0001) reduction in serum TG concentrations as well as 10% (p < 0.01) and 18% (p < 0.0001) reduction in non-HDL-C concentrations, respectively [12, 13].. LDL-C, however, is usually reported to rise on EPA and DHA treatment, possibly due to increased production of LDL-C from VLDL-C [14]. In a systematic review, a 6 mg/dL (+ 3, + 8, P = 0.0006) increase in serum LDL-C concentrations was reported [15]. In the EVOLVE trial, LDL-C increased 19% (p < 0.001) with 4 g/day EPA and DHA intake [12]. However, the increase in LDL-C with EPA and DHA monotherapy is usually counter balanced when these fatty acids are combined with statins [16]. Indeed, clinical trials that compare statin monotherapy with n-3 combination therapies collectively show added benefits on blood lipid profile and conclude that EPA and DHA enhance statin-mediated improvements, with 30-55% reductions in TG, as well as 10-20% reductions in non-HDL-C without a concomitant increase in LDL-C [17,18,19,20]. In the COMBOS trial (n = 254), 4 g/day EPA and DHA supplementation combined with simvastatin resulted in 10% reduction in non-HDL-C (p < .001) with no accompanying increase in LDL-C [17], while the same study design in ESPRIT trial (n = 647) showed a 7% decrease in non-HDL-C (p < 0.01) with no significant change in LDL-C [18].
As opposed to the great number of clinical studies that investigate the role of n-3 FA in altering blood lipids, their effect on LDL-C particle size and concentration has not been widely investigated. A decrease in LDL III concentration from baseline (− 1.23 mmol/L ± 2.99, p < .05) was reported when 1.68 g/d EPA and DHA is combined with statin for 5 weeks, despite the relatively low-dose of n-3 FA supplements and short duration [21]. Similar results are reported with 4 g/day EPA and DHA combination therapy with statin [22]. Subsequent studies that tested the efficacy of EPA and DHA with statin and reported positive results, did not measure LDL-C particle size and concentration in their studies despite these earlier findings [18,19,20, 23, 24]. In another study, coadministration of n-3 FA with statin increased LDL particle size and decreased TG level in 51 dyslipidemic patients [25]. More recently, an improvement in atherogenic lipoprotein particle size and concentration when statin-prescribed patients were administered with 4 g/day EPA and DHA was reported, while TC, TG and LDL-C concentrations also significantly decreased (from 4.43 ± 0.55 to 3.89 ± 0.42 p < 0.001, from 5.06 ± 1.29 to 3.32 ± 1.52 p < 0.001, from 2.34 ± 0.44 to 2.08 ± 0.29 p = 0.003, respectively) [26]. In the present intervention, the effect of 4 g/day EPA and DHA supplements on the lipid profile of dyslipidemic patients on stable statin therapy was explored.
Methods
Participants
Eligible participants were Caucasian men or women aged between 50 and 79 years who had been receiving a stable dose of atorvastatin for the control of serum LDL-C concentration for at least for 8 weeks before initial screening. Inclusion criteria was current combined hyperlipidemia with a non-HDL-C level above 130 mg/dL as per the National Lipid Association treatment goals [2].
Exclusion criteria included current use of n-3 FA supplements, patients that had any type of heart surgery, patients that were diagnosed with any type of cancer and/or have had any kind of cancer therapy, patients that have had kidney failure and patients that have had liver failure in the past 6 months, as well as ongoing pregnancy or lactation. All patients were asked to provide signed informed consent before study-protocol was conducted.
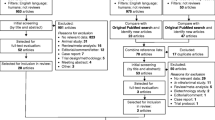
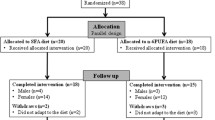
100 patients who had been prescribed atorvastatin for more than 8 weeks were invited for the initial screening, during which their serum LDL-C was measured. Thirty-eight patients did not meet the inclusion criteria, and 12 eligible patients did not give consent to take part in the intervention. 44 patients, out of the remaining 50, were randomly recruited for the study. The random sampling was carried out using randomizer.com.
Study design
This was a randomized, double-blind, placebo controlled, parallel groups study. Figure 1 summarizes the trial design. Before entry into the intervention phase of the study, 8-weeks of dietary lead in was done in accordance with NICE guidelines (2014) and patients were instructed to maintain this diet throughout the study. Adherence to dietary advice was measured by a 3-day weighed intake food diary at Week 4. Nutrient content of the diet was estimated using Nutritics software (Nutritics Ltd., Ireland) using UK: SACN 2015 food composition tables. This study was approved by the University of Chester Faculty of Clinical Sciences and Medicine Research Ethics Committee (REF: 1288/17/GD/CSN).
After dietary lead-in, baseline measurements of fasting blood TG, TC, HDL-C, LDL-C, and LDL-C subgroups (LDL I (large buoyant LDL), LDL II (intermediate density LDL), and LDL III (small dense LDL) were carried out for all patients at 2 visits separated by 1 week, and the means were used as baseline values. Non-HDL-C was calculated by subtracting HDL-C from TC. After baseline measurements, all 44 patients were randomized by www.randomizer.com in equal numbers to receive either EPA (75%, 3000 mg) and DHA (25%, 1000 mg) 4 g/day ethyl esters (Wiley’s Finest, USA) or OO (placebo, Ali Raif Pharma, Turkey) 4 g/day for 8 weeks in combination with the same dose of atorvastatin they have been prescribed to. The total EPA and DHA dose recommended by American Heart Association for lipid lowering is approximately 4 g/day [27] which is the common therapeutic dose used in several major clinical trials including COMBOS [17], MARINE [13], ESPIRIT [18], EVOLVE [12], ANCHOR [20]. For all patients, atorvastatin dosage has been kept constant throughout the trial.
Both groups took four 1000 mg capsules orally 4 times daily and compliance was measured by the number of capsules consumed relative to the number estimated to be consumed. n-3 FA tablets included EPA and DHA ethyl esters (1000 g), other n-3 FA (60 mg), fish gelatin, glycerine, purified water and alpha tocopherol, while placebo tablets contained extra virgin olive oil.
Baseline measurements were repeated at the end of week 8. Study participants and investigators remained blinded to all laboratory results until the last subject completed the 8-week intervention period. During the treatment phase, patients attended clinic visits at weeks 4 and 8.
Biochemical measurements
Laboratory analyses were performed on the serum or serum of 12 hours fasting blood samples. LDL-C, VLDL-C and HDL-C were measured with homogeneous enzymatic colorimetric assay of Roche Diagnostics (USA). TG and TC was measured with enzymatic colorimetric assay of Roche Diagnostics (USA). LDL-C subgroups were analyzed using electrophoresis by Lipoprint (Quantimetrix, USA).
Statistical analysis
Power analysis was performed by using the G*Power software (v3.1.9) program to determine the sample size. At the baseline, a pilot study was performed with 10 people in each group. When the percentage change in non-HDL values of the groups was investigated, this was found to be 3.25 ± 17.02 in the control group and − 8.86 ± 3.81 in n-3 FA group. According to evaluation performed by using these data, effect size was calculated to be d = 0.982 and each group should include 18 people to obtain 80% power at a level of α = 0.05. Sample size was assigned as 44 to allow for subject attrition and other potential causes of study withdrawal up to 20%. Demographic and baseline analyses were performed for all study participants, whilst efficacy analysis was performed only on patients that successfully completed 8-weeks study protocol. The primary efficacy end point was non-HDL and LDL III particle concentration percentage change from baseline; secondary efficacy end points were changes in TG, TC, LDL-C, VLDL-C and HDL-C.
Normal distribution of the sample was tested with Shapiro-Wilk’s test as sample size was less than 100 [28]. Independent samples t-test was used for the intergroup comparisons of change in quantitative variables from baseline to post-intervention with normal distribution and Mann Whitney U test was used for the intergroup comparisons of quantitative variables without normal distribution. Paired Samples t-test was used for the in-group comparisons of quantitative variables with normal distribution and Wilcoxon Signed Ranks test was used for the in-group comparisons of quantitative variables without normal distribution. Pearson’s chi-square test was used for comparison of qualitative data. Levene’s test was conducted to check homogeneity of variance between groups. P < 0.05 was the threshold for statistical significance.
Results
Subjects
Of the 44 patients randomly assigned to the trial, 41 have completed it. In the treatment arm, one subject reported adverse effect (nausea) and one was lost to follow-up. In the placebo group one subject was lost to follow-up.
The baseline demographics of the patients are listed in Table 1. The patients were predominantly men (63%) with a mean (±SD) age of 60.62 ± 9.56 years and body mass index of 23.8 ± 3.0 kg/m2. There was no statistically significant difference between groups regarding age, BMI values and gender ratios; as well as baseline TG, TC, HDL-C, non-HDL-C, LDL-C, VLDL-C, LDL-C I, LDL II and LDL III concentrations (Tables 1 and 2).
Diet
The analysis of total energy derived from dietary components showed no significant difference between the groups (Table 2). No significant change in body weight was observed throughout the intervention.
Laboratory measurements
Baseline and week 8 values as well as percentage change from baseline to the end of treatment for the primary and secondary endpoints are shown in Table 3.
Primary endpoints
For LDL III concentration, the percent change over 8 weeks, in serum LDL III concentration in n-3 FA group was determined to be significantly greater compared to the change in the placebo group (− 67.5% vs − 0%, respectively; P < 0.001). This was also the case for serum non-HDL-C. The non-HDL value observed in n-3 FA group was determined to be statistically significantly different compared to the placebo group (− 9.47% vs + 4.65%, respectively; P = 0.007).
Secondary endpoints
Percent change over 8 weeks, in serum TG (− 21.5% vs 6.2%, P < 0.001) and VLDL-C (− 36.9% vs 4.0%, P < 0.001) and TC (− 6.6% vs 2.1%, P < 0.001) were significantly greater in the n-3 FA group compared to the placebo group respectively (Fig. 2).
Discussion
In this randomized, parallel-group, double-blind, placebo-controlled trial of men and women with hyperlipidemia on statins, 4 g/d EPA + DHA supplementation produced significant reductions in the primary endpoints of non-HDL-C and LDL-III particle concentration (− 9.47 ± 4.58, p < 0.001 and − 67.5 (− 100, − 31.25), P < 0.01, respectively) compared with OO.
There are relatively few studies investigating the role of marine n-3 FA in altering LDL-C particle phenotype. In a recent randomized controlled trial (n = 53), 4 g/day prescription n-3 FA supplementation for 8 weeks resulted in significant increases in LDL particle sizes and significant decreases in blood lipid, lipoprotein and apolipoprotein concentrations in the intervention arm [29]. An earlier single-blind placebo controlled study that randomized hyperlipidemic subjects (n = 33) into three groups (pravastatin, 6 g/day EPA and DHA, placebo) initially for 6 weeks and then provided combined pravastatin and 6 g/day EPA and DHA therapy to all subjects for 12 weeks, reported a significant increase in LDL stokes’ diameter from 25.0 to 25.9 nm (P < 0.05) in the n-3 FA group, although LDL III particle concentration was not measured in this study [30]. In another randomized controlled trial (n = 42), in which subjects were randomized to atorvastatin alone or atorvastatin and 1.68 g/day EPA and DHA for 5 weeks after dietary run in, a significant reduction in LDL III particle concentration (− 1.23 mmol/L, P < 0.05) in the treatment arm was reported [21]. However, this change was not significantly different when compared to the change in the placebo group. Maki et al. found a significant increase in LDL-C particle size from 19.9 (19.2, 22.0) nm to 20.4 (19.3, 21.7) nm (P = 0.024) in their cross-over study (n = 39) when 4 g/day EPA and DHA supplementation was combined with simvastatin [22]. Different from a previous study [30], Maki et al. also measured LDL III particle concentration and reported no significant change between control and intervention arms. However, in more recent studies, n-3 FA together with statins partially improved both lipoprotein particle size and concentration [25, 26, 29]. It is of particular importance that this present study showed a difference in LDL III particle concentration, given the fact that LDL III and IV particle concentration is suggested to be a stronger predictor of CVD than LDL-C particle size [31, 32].
Previous studies have shown similar results regarding the effect of n-3 FA supplementation on serum non-HDL-C concentrations. The COMBOS trial reported a 9.1% reduction (P < 0.001) in non-HDL-C with dietary supplementation of 4 g/day EPA and DHA during 40 mg/day simvastatin therapy compared to 2.2% (P < .001) with corn oil [17]. The same dose of n-3 FA and statin caused 6.9% reduction (P < 0.01) in ESPIRIT trial, compared to 0.9% (P > 0.05) with olive oil [18]. Similarly, in respective studies of combination therapy, approximately 10% significant reduction in non-HDL-C was reported [16, 21, 24, 33]. Higher reductions in have also reported in the past. Maki et al. for instance, showed 40% reduction (P < 0.001) in their study of a similar design with 39 patients, however, the control group also showed a large significant reduction in serum non-HDL-C concentrations (34%, P < 0.001) [22]. Furthermore, in the ANCHOR trial, a 15% reduction (P < 0.0001) in non-HDL-C was also reported [20]. This could be due to the use of EPA ethyl esters alone in this study, given that EPA might lead to greater average reductions in non-HDL-C than DHA [34]. However, little is known about the individual effects of these fatty acids on distinct lipids, and studies suggest they have complementary roles to each other, therefore their combined use is more widely preferred, as in the present study.
In the present study, the decrease in non-HDL-C cholesterol in the n-3 FA group was likely achieved mainly due to lowering of VLDL concentrations (− 36.88 ± 11.75%, P < 0.0001), as well as other triglyceride-rich lipoproteins such as chylomicron remnants. The significant reduction in VLDL-C concentration is consistent with the previous evidence [17,18,19,20, 22]. In human physiology, increased concentrations of VLDL-C particles positively correlate with increased TG concentrations. TG-reducing effects of n-3 FAs are mediated by transcription of several nuclear receptors that play a key role in lipid metabolism, including PPAR-α, which increases fatty acid oxidation in the liver, adipose, heart and skeletal muscle, as well as sterol regulatory element binding proteins (SREBP), especially SREBP-1c, the major activator of hepatic lipogenesis [11]. PUFA metabolites, such as eicosanoids and oxylipins, are potent activators of PPARs [35]. Through these mechanisms, marine n-3 FA may downregulate VLDL metabolism through decreasing TG synthesis and increasing triglyceride clearance.
The significant 21.51 ± 12.15% (P < 0.001) reduction in TG in the present study is slightly lower in magnitude than the findings of some o previous studies. Kastelein et al. reported a 31% reduction (P < 0.001) and Bays et al. reported 33% (P < 0.0001) reduction in serum TG concentrations with 4 g/day EPA and DHA monotherapy [12, 13]. When the same n-3 FA dose was combined with a statin, a 28% reduction was reported [17]. Similarly, in a randomized controlled trial of hyperlipidemic patients (n = 56), co-administration of 4 g/day n-3 FA with statin treatment for 16 weeks reduced serum TG concentrations more effectively than statin monotherapy (− 34.8% vs. -15.2%, P = 0.0176) [36]. The relatively smaller reduction in TG achieved in the present study may be due to the difference in the baseline characteristics of the patients when compared to the aforementioned studies. Mean (±SD) baseline serum TG concentrations of the intervention group in the present study (145.05 ± 52.67 mg/dL, 1.62 ± 0.60 mmol/l) were much lower than the EVOLVE and MARINE trials (655 and 679 mg/dL, 7.40 and 7.67 mmol/l, median values, respectively) and the percent reduction in TG concentrations highly depends on the baseline values [37, 38]. This is supported by the mean (±SD) baseline TG concentration of the n-3 FA groups in ESPIRIT trial which was 287 ± 82.8 mg/dL (3.25 ± 0/94 mmol/l) and the study achieved a reduction in serum TG concentration of − 20%, (P < 0.01), similar to the present study [18].
Reducing serum TG concentration is important for two reasons. Firstly, TG, as a substrate of LDL-C synthesis, is deemed a target in clinical management of dyslipidemia and each 1 mmol/l reduction in TG concentrations is believed to reduce CVD risk by 14% in men and 37% in women [39]. More importantly, it has been long known that clinically significant reductions in TG concentrations are typically accompanied by a shift in LDL particle size from smaller and denser particles (LDL III, IV) to larger and more buoyant particles (LDL-C I, II) [40]. Serum TG concentration are inversely related to the LDL-C particle size and it can been hypothesized that reducing TG would result in an increase in LDL-C particle size [41]. In the present study, the significant decrease in serum concentration of LDL III (− 67%, P < 0.01) from 3.5 mg/dL to 1 mg/dL in the n-3 FA group is likely to be linked to the significant decrease in serum TG.
In the placebo group, serum TC, TG and HDL-C concentrations at week 8 had shown minor significant changes from baseline. One possible explanation for changes in the placebo group in the present study is that late-onset effects of the dietary run-in and continued adherence to the healthy and balanced diet might have improved these variables.
Strengths and limitations
The main strength of the present study is its prospective, randomized, double-blinded and placebo-controlled design. Furthermore, the study population was carefully determined and there were no baseline differences between control and intervention groups. Additionally, small and dense LDL-C was measured with gel electrophoresis that is a strong method for detecting types of lipoprotein particles.
The present study also has certain limitations. First, the small sample size (although powered) and short intervention duration, limits the conclusions that can be drawn. Although the lipid alterations achieved in this study are consistent with the findings of larger studies with longer duration, findings should still be confirmed in a larger population. Furthermore, the capsule load was high (4 capsules per day), and compliance was measured only by capsule count. Ideally, compliance needs to be assessed by plasma and erythrocytes EPA and DHA content. Indeed, the plasma and erythrocyte fatty acid composition should be measured and compared in any further studies.
Like many other studies, this study tested a supplemental form of n-3 FA containing EPA and DHA in ethyl ester (EE) form. However, n-3 FA supplements are also available in TG form and there is ongoing debate about whether different chemical forms of EPA and DHA are absorbed in an identical way by the human body. Some previous findings suggest a comparable bioavailability, whereas others reported a higher bioavailability from TG. An earlier RCT (n = 150) that tested long-term (6 months) moderate consumption (1.68 g/day) of both chemical forms concluded that TG n-FA led to a faster and higher increase in the erythrocyte’s membrane EPA and DHA content [42]. In a more recent trial (n = 22), short term bioavailability of the EE and TG, measured by plasma concentrations of EPA, and DHA, did not differ after 24 hours a single oral dose of ∼1.2 g [43]. Taken together, there is limited evidence to compare bioavailability of EE and TG n-3 FA. However, recent studies point out that EPA and DHA in free fatty acids (FFA) form may have 4-fold greater bioavailability than n-3 FA ethyl esters given that their absorption does not involve pancreatic lipase. Particularly when taken on an empty stomach, the FFA formulation may have provided great flexibility in the dosing schedule. Unfortunately, this prescription n-3 FA was not available to the researchers.
The use of OO as a placebo may have had non-neutral effects on the outcome variables due to its high oleic acid content and the role of oleic acid in CVD prevention [44]. However, 4 g/day was deemed too low a dose to bias the result, especially when compared to the amount used in studies such as PREDIMED, which showed that 50 g/day use of OO reduced CVD risk [45], several studies have used OO as placebo given the lack of a true placebo.
The present study included participants with a normal body weight – which was preserved throughout the intervention. In obese subjects, similar results in lipid profile might be observed [46], also accompanied by a reduction in inflammatory markers [47].
Additionally, physical activity was not monitored throughout the trial. Physical activity is an important factor that effects lipid metabolism and ideally should be evaluated in studies looking into blood lipids.
Lastly, although the present studies and others have demonstrated beneficial effects of n-3 FAs in relation risk, it should be noted that dietary pattern, and more generally, lifestyle factors are stronger determinants of CVD risk compared to the effect of single nutrients alone.
Conclusion
There is a clinical need to effectively reduce serum small, dense LDL-C particle concentration for CVD prevention. Agents that lower serum TG concentrations are usually successful in altering LDL-C particle phenotype. However, the efficacy of n-3 FAs, which are TG lowering agents, in LDL-C particle altering has not been widely investigated. In this double-blind, randomized, controlled trial of combined use of n-3 FA with atorvastatin in patients with hyperlipidemia, supplementation of EPA and DHA at 4 g/d dosage improved the overall lipid profile in 8 weeks, including significant lowering of serum LDL III and non-HDL-C concentrations. Further large-scale studies are required to confirm the results of this trial.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CVD:
-
Cardiovascular disease
- DHA:
-
Docosahexaenoic acid
- EE:
-
Ethyl ester
- EPA:
-
Eicosapentaenoic acid
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- n-3 FA:
-
Omega 3 fatty acids
- non-HDL-C:
-
Non-high density lipoprotein cholesterol
- OO:
-
Olive oil
- PPAR- α:
-
Peroxisome proliferator–activated receptor-α
- PPARs:
-
Peroxisome proliferator–activated receptors
- SREBP1-c:
-
Sterol responsive element binding protein-1c
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- VLDL-C:
-
Very-low-density lipoprotein cholesterol
References
Garg R, Aggarwal S, Kumar R, Sharma G. Association of atherosclerosis with dyslipidemia and co-morbid conditions: a descriptive study. J Nat Sci Biol Med. 2015;6(1):163–8.
Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1--full report. J Clin Lipidol. 2015;9(2):129–69.
Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxidative Med Cell Longev. 2017;2017:1273042.
Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260(13):1917–21.
Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43(9):1363–79.
Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21(4):305–11.
Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20(2):195–203.
Rizzo M, Berneis K. Low-density lipoprotein size and cardiovascular risk assessment. Qjm. 2006;99(1):1–14.
Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–77.
Shen H, Xu L, Lu J, Hao T, Ma C, Yang H, et al. Correlation between small dense low-density lipoprotein cholesterol and carotid artery intima-media thickness in a healthy Chinese population. Lipids Health Dis. 2015;14:137.
Valenzuela R, Ortiz M, Hernández-Rodas MC, Echeverría F, Videla LA. Targeting n-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease. Curr Med Chem. 2020;27(31):5250–72.
Kastelein JJ, Maki KC, Susekov A, Ezhov M, Nordestgaard BG, Machielse BN, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr lowering very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8(1):94–106.
Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the multi-center, plAcebo-controlled, randomized, double-blINd, 12-week study with an open-label extension [MARINE] trial). Am J Cardiol. 2011;108(5):682–90.
Pirillo A, Catapano AL. Omega-3 polyunsaturated fatty acids in the treatment of atherogenic dyslipidemia. Atheroscler Suppl. 2013;14(2):237–42.
Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189(1):19–30.
Nordøy A, Bønaa KH, Nilsen H, Berge RK, Hansen JB, Ingebretsen OC. Effects of simvastatin and omega-3 fatty acids on plasma lipoproteins and lipid peroxidation in patients with combined hyperlipidaemia. J Intern Med. 1998;243(2):163–70.
Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, et al. Efficacy and tolerability of adding prescription Omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29(7):1354–67.
Maki KC, Orloff DG, Nicholls SJ, Dunbar RL, Roth EM, Curcio D, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther. 2013;35(9):1400–11.e1-3.
Bays HE, McKenney J, Maki KC, Doyle RT, Carter RN, Stein E. Effects of prescription omega-3-acid ethyl esters on non--high-density lipoprotein cholesterol when coadministered with escalating doses of atorvastatin. Mayo Clin Proc. 2010;85(2):122–8.
Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110(7):984–92.
Nordøy A, Hansen JB, Brox J, Svensson B. Effects of atorvastatin and omega-3 fatty acids on LDL subfractions and postprandial hyperlipemia in patients with combined hyperlipemia. Nutr Metab Cardiovasc Dis. 2001;11(1):7–16.
Maki KC, McKenney JM, Reeves MS, Lubin BC, Dicklin MR. Effects of adding prescription omega-3 acid ethyl esters to simvastatin (20 mg/day) on lipids and lipoprotein particles in men and women with mixed dyslipidemia. Am J Cardiol. 2008;102(4):429–33.
Vecka M, Dušejovská M, Stankova B, Zeman M, Vavrova L, Kodydkova J, et al. N-3 polyunsaturated fatty acids in the treatment of atherogenic dyslipidemia. Neuro Endocrinol Lett. 2012;33(Suppl 2):87–92.
Chan DC, Pang J, Barrett PH, Sullivan DR, Mori TA, Burnett JR, et al. Effect of omega-3 fatty acid supplementation on arterial elasticity in patients with familial hypercholesterolaemia on statin therapy. Nutr Metab Cardiovasc Dis. 2016;26(12):1140–5.
Lee MW, Park JK, Hong JW, Kim KJ, Shin DY, Ahn CW, et al. Beneficial effects of Omega-3 fatty acids on low density lipoprotein particle size in patients with type 2 diabetes already under statin therapy. Diabetes Metab J. 2013;37(3):207–11.
Ide K, Koshizaka M, Tokuyama H, Tokuyama T, Ishikawa T, Maezawa Y, et al. N-3 polyunsaturated fatty acids improve lipoprotein particle size and concentration in Japanese patients with type 2 diabetes and hypertriglyceridemia: a pilot study. Lipids Health Dis. 2018;17(1):51.
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–333.
Coakes SJ. SPSS version 20.0 for windows : analysis without anguish. Wiley. 2012.
Masuda D, Miyata Y, Matsui S, Yamashita S. Omega-3 fatty acid ethyl esters improve low-density lipoprotein subclasses without increasing low-density lipoprotein-cholesterol levels: a phase 4, randomized study. Atherosclerosis. 2020;292:163–70.
Contacos C, Barter PJ, Sullivan DR. Effect of pravastatin and omega-3 fatty acids on plasma lipids and lipoproteins in patients with combined hyperlipidemia. Arterioscler Thromb. 1993;13(12):1755–62.
Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6(5):381–7.
Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the veterans affairs high-density lipoprotein intervention trial. Circulation. 2006;113(12):1556–63.
Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, et al. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001;85(5):544–8.
Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol. 2012;6(1):5–18.
Shearer GC, Savinova OV, Harris WS. Fish oil -- how does it reduce plasma triglycerides? Biochim Biophys Acta. 2012;1821(5):843–51.
Son JW, Kim CH, Nam MS, Park IB, Yoo SJ. Efficacy and safety of prescription Omega-3 fatty acids added to stable statin therapy in Korean patients with type 2 diabetes and hypertriglyceridemia: a randomized controlled trial. J Lipid Atheroscler. 2019;8(2):221–31.
Harris WS, Ginsberg HN, Arunakul N, Shachter NS, Windsor SL, Adams M, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4(5-6):385–91.
Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad Med. 2014;126(7):7–18.
Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–9.
Krauss RM, Dreon DM. Low-density-lipoprotein subclasses and response to a low-fat diet in healthy men. Am J Clin Nutr. 1995;62(2):478s–87s.
Watson TD, Caslake MJ, Freeman DJ, Griffin BA, Hinnie J, Packard CJ, et al. Determinants of LDL subfraction distribution and concentrations in young normolipidemic subjects. Arterioscler Thromb. 1994;14(6):902–10.
Neubronner J, Schuchardt JP, Kressel G, Merkel M, von Schacky C, Hahn A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur J Clin Nutr. 2011;65(2):247–54.
Chevalier L, Vachon A, Plourde M. Pharmacokinetics of supplemental Omega-3 fatty acids esterified in Monoglycerides, ethyl esters, or triglycerides in adults in a randomized crossover trial. J Nutr. 2021;151(5):1111–8.
Pineo CE, Anderson JJB. Cardiovascular benefits of the Mediterranean diet. Nutr Today. 2008;43(3).
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34.
Zhang YY, Liu W, Zhao TY, Tian HM. Efficacy of omega-3 polyunsaturated fatty acids supplementation in managing overweight and obesity: a meta-analysis of randomized clinical trials. J Nutr Health Aging. 2017;21(2):187–92.
Hernandez JD, Li T, Rau CM, LeSuer WE, Wang P, Coletta DK, et al. ω-3PUFA supplementation ameliorates adipose tissue inflammation and insulin-stimulated glucose disposal in subjects with obesity: a potential role for apolipoprotein E. Int J Obes. 2021;45(6):1331–41.
Acknowledgements
The researchers would like to thank the participants for taking part in this trial.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Blood biomarker analysis were performed at the Pax Clinic laboratory at no cost.
Author information
Authors and Affiliations
Contributions
GD collected, analyzed and interpreted the patient data. SM supervised the preparation and implementation of the trial protocol, reviewed the results and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki Ethical Principles and Good Clinical Practices. It was approved by the University of Chester Faculty of Clinical Sciences and Medicine Research Ethics Committee (REF: 1288/17/GD/CSN).
Consent for publication
All patients were asked to give a signed informed consent before study-protocol was conducted.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dogay Us, G., Mushtaq, S. N-3 fatty acid supplementation mediates lipid profile, including small dense LDL, when combined with statins: a randomized double blind placebo controlled trial. Lipids Health Dis 21, 84 (2022). https://doi.org/10.1186/s12944-022-01686-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01686-y