Abstract
Background
Heterozygous familial hypercholesterolemia (HeFH) due to low-density lipoprotein receptor (LDLR) mutations predisposes patients to highly elevated levels of cholesterol, and patients are at increased risk of adverse cardiovascular events and other morbidities. Whether the LDLR mutation and high cholesterol levels affect the risk of cancer remains unknown. The purpose of the present study was to assess the long-term cancer risk in HeFH relatives.
Methods
Study participants were identified by cascade screening during 1992–1994. A comparison cohort was matched 10:1 to the relatives from the Danish general population based on birth year, gender and address. All participants were followed until a cancer diagnosis, migration, death, or end of follow-up as of December 31, 2019. The primary endpoint was any incident cancer diagnosis.
Results
In total, we included 221 relatives with a median age of 37 years (interquartile range: 27–53 years). A total of 117 (53%) of the relatives carried a LDLR gene mutation. The crude hazard ratio of our primary endpoint did not reveal any differences in cancer incidence in mutation-carrying relatives compared with the general population cohort (1.18; 95% CI, 0.81–1.71). Nonmutation-carrying relatives however had a lower cancer incidence than the general population (0.45: 95% CI, 0.26–0.80). Thus, the risk among mutation-carrying HeFH relatives compared with nonmutation-carrying HeFH relatives was increased (HR: 2.39; 95% CI, 1.24–4.61).
Conclusion
In Denmark, LDLR mutation-carrying HeFH relatives did not have a different cancer risk than the general population. In contrast, nonmutation-carrying relatives had a lower risk of cancer.
Similar content being viewed by others
Introduction
Heterozygous familial hypercholesterolemia (HeFH) is an autosomal dominant genetic disorder affecting lipoprotein metabolism in at least 1 in 250 people worldwide [1, 2]. The disease is caused by a mutation in the low-density lipoprotein receptor (LDLR) and leads to impaired clearance of low-density lipoprotein cholesterol (LDL-C) from the circulation and subsequently elevated levels of LDL-C [3]. Untreated HeFH will result in highly elevated levels of plasma LDL-C, which are associated with a substantially increased risk of cardiovascular disease [4,5,6].
In addition to the risk of cardiovascular diseases, elevated LDL-C levels have further been associated with an increased risk of several types of cancers and cancer progression. [7,8,9,10,11,12] Given the increased need for cholesterol in proliferating cancer cells, HeFH may be associated with cancer development [7]. The association between HeFH and cancer might be explained by the reported regulatory mechanisms of LDLR levels in plasma e.g. by receptor-mediated endocytosis of serum LDL which enhances the cholesterol level [13]. This is demonstrated in the most common cancers for men and women (prostate cancer and breast cancer, respectively) and in the cancer type with highest mortality rates (lung cancer) [12, 14, 15].
A combined in vivo study on mouse models and publicly available human datasets regarding tumor LDLR expression concluded that high levels of circulating LDL-C and high cellular LDLR expression in cancer cells were associated with high proliferation of estrogen receptor-positive breast cancer cells [15]. Furthermore, low tumor cell LDLR expression was associated with apoptosis and reduced tumor growth in a high-cholesterol setting [15]. Therefore, we hypothesized that elevated LDL-C levels in mutation-carrying HeFH relatives was associated with an increased incidence of cancer.
We aimed to investigate the association of genetically confirmed HeFH among relatives with a predisposed high LDL-C level on the risk of cancer.
Methods and materials
Study design and cohorts
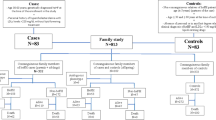
During 1992–1994, patients with prevalent clinical familial hypercholesterolemia (FH) or newly diagnosed FH (probands) were screened and included in a cohort at the “Lipid Clinic” at Aarhus University Hospital, Denmark. HeFH was clinically defined as a plasma level of total cholesterol > 8.0 mmol/L (308.9 mg/dL), LDL-C > 6.0 mmol/L (231.7 mg/dL; if available), presence of tendinous xanthomata in the patient or a first-degree relative, and a family history of hypercholesterolemia. All probands identified were offered genetic testing [16]. Subsequently, cascade screening including genetic testing was performed on all family members if LDLR mutation was detected in the proband, and the mutation was followed as far as possible in the respective family pedigree. Both mutation-carrying and nonmutation-carrying HeFH relatives who thus comprised our study population, were identified (Fig. 1).
At baseline, pretreatment plasma concentrations of total cholesterol, high-density lipoprotein cholesterol and triglycerides were measured by standard enzymatic assays. LDL-C was calculated using the Friedewald Eq [17]. All study participants with confirmed LDLR mutations and thus elevated LDL-C levels were recommended lipid-lowering treatment with statins at study inclusion. Since early 1990s, statins have been available in Denmark and, thus, all subjects with hypercholesterolemia participating in the study were offered drug treatment. At the time of initiation, the most common statins used were lovastatin, pravastatin and simvastatin. Whether a prescription was redeemed was the individual patient’s choice.
Based on the HeFH study cohort, we generated a population-based comparison cohort based on 10:1 matching from the general Danish population. Participants were matched based on birth year, gender, and address using the Danish Civil Registration System, [18] and controls had to be alive when the corresponding relative entered the study. Study participants were excluded if they had a previous or prevalent cancer diagnosis.
Registries
Using a wide range of nationwide population-based registries, we retrieved all necessary health care information to complete this study. The Danish National Health Service provides tax-supported health care with free access to all public hospitals to all Danish residents. Furthermore, all Danish citizens are assigned a civil personal registration number, a unique identification number that can be used to link registry data [18]. The Danish National Patient Registry (DNPR) has collected data from all nonpsychiatric Danish hospitals since 1977 and from emergency rooms and outpatient clinics since 1995 [19]. The DNPR provides a full overview of all discharges, and diagnoses are included and classified according to the International Classification of Diseases (eighth revision until 1994 and 10th revision hereafter). To identify all cancer diagnoses in the study population, we extracted individual medical records on the HeFH families and control cohort from the DNPR. Furthermore, to ensure compliance and adherence to the recommended treatment with lipid-lowering drugs among the mutation-carrying HeFH relatives, we extracted data and information on medication use from the Danish National Prescription Registry (NPR) [20]. The NPR contains nationwide coverage on all prescription drugs redeemed in Denmark since 1994. The Danish Civil Registration System provided information on vital and migration status for each individual in the study cohort during the follow-up period [18].
Study outcome
In this study, the primary endpoint was cancer incidence, which was defined as the time from inclusion until the earliest occurrence of cancer regardless of type and location. In situ cancers were not included in the primary outcome. As a secondary outcome, we assessed all-cause mortality. Information regarding vital status was retrieved using the Danish Civil Registration and defined as the time from inclusion until death.
Follow-up and statistical analysis
Follow-up began at an individual’s date of inclusion and was continued until the first event of cancer diagnosis, migration, death, or December 31, 2019, whichever came first. Median follow-up was calculated. In Table 1, baseline data are presented as absolute numbers (percentages) and medians (interquartile ranges [IQRs]) whenever appropriate. Cancer incidence was graphically illustrated by cumulative incidence using the Kaplan–Meier estimator.
We estimated the incidence rates of cancer and used Cox proportional hazard regression models to estimate crude hazard ratios (HRs) with associated 95% confidence intervals (CIs), comparing the rate of incident cancer in mutation-carrying and nonmutation-carrying HeFH relatives with a reference to a general population cohort. A 95% CI was used to determine statistical uncertainty as consistent with best practice in observational epidemiology [21]. To account for possible clustering effects within families, we used robust variance estimation. No other adjustments was made. Using log–log plots, we graphically assessed the proportional hazard assumptions that were not violated. Furthermore, we calculated the prevalence of primary statin prevention across both groups of HeFH relatives.
In sensitivity analyses, non-melanoma skin cancers were excluded from the analysis as to the low risk of spread and mortality of the disease [22].
All statistical analyses were performed using Stata software (Stata v16.1). The follow-up did not involve any contact with study participants or any interventions. Thus, patient consent and approval from the ethics committee were not required according to Danish regulations. The study was approved by the Danish Data Protection Agency and the Central Denmark Region (record no. 1–16-02–381-19).
Results
The selection of study participants is shown in Fig. 1. In total, we included 221 relatives from 32 families as well as 2,207 controls from the general population, and the median age at inclusion was 37 years (IQR: 27–53 years) (Table 1). The median time of follow-up was 26.0 years (IQR: 18.7–27.3 years), and 527 incident cancers were observed during 54,507 person-years at risk. Among the 221 relatives, 117 (53%) were carrying an LDLR mutation, of which 56 (48%) were men and 61 (52%) were women.
During follow-up, 108 (92%) mutation-carrying HeFH relatives received treatment with a lipid-lowering drug. The majority were treated with statins, and the most frequently prescribed statins were simvastatin and lovastatin. Among nonmutation-carrying HeFH relatives, only 46 (44%) were on lipid-lowering treatment.
The cumulative incidence of cancer as our primary endpoint is shown in Fig. 2. We observed no difference in cancer risk among mutation-carrying HeFH relatives compared with the general population (HR: 1.18; 95% CI, 0.81–1.71) (Table 2). Among relatives without a mutation, however, we observed a lower cancer incidence compared to controls (HR: 0.45: 95% CI, 0.26–0.80). Thus, the risk among mutation-carrying HeFH relatives compared with nonmutation-carrying HeFH relatives was higher (HR: 2.39; 95% CI, 1.24–4.61).
Examining the outcomes when excluding nonmelanoma skin cancers, the point estimate differed in direction among mutation-carrying HeFH relatives (HR: 0.79; 95% CI 0.45–1.39), whereas the association was similar to the entire cohort among nonmutation-carrying HeFH relatives (HR: 0.39; 95% CI 0.18–0.82) (Table 3). We did not observe any differences in all-cause mortality in any of the groups of HeFH relatives compared with the general population cohort.
Discussion
In HeFH relatives, we assessed the cancer incidence during a long-term follow-up period of greater than 25 years and found no elevated risk of cancer disease among LDLR mutation-carrying HeFH relatives compared with the general population. The risk among HeFH relatives without a genetically confirmed LDLR mutation was significantly lower. Low-malignant cancers, such as nonmelanoma skin cancer, did not drive these results. Despite longer follow-up, all-cause mortality was similar to the previously reported association within this FH cohort [6].
This study is of clinical importance to inform HeFH relatives both with and without an LDLR mutation given that no increased incidence of being diagnosed with cancer has been demonstrated. Furthermore, a lower cancer risk among the relatives without an LDLR mutation was observed. Whether this observation is due to an increased awareness of healthy lifestyle choices or lipid-lowering treatment requires further investigation. The lack of increased cancer incidence is of particular importance, as high levels of LDL-C have been reported to be positively associated with a less favorable prognosis in several major cancer types [23,24,25].
Our results are similar to that noted among Norwegian FH patients with genetically verified FH [26]. Compared with a matched control group randomly drawn from the general Norwegian population, the incidence of total cancers did not differ. The risk of smoking-related cancers however was associated with a reduced incidence of 20% among the FH patients, most likely due to reduced prevalence of smoking. Unfortunately, we were not able to perform such a stratified analysis in our cohort due to small sample size. Age at HeFH diagnosis/inclusion in the groups of mutation-carrying HeFH patients from both studies was similar (33.7 years in the Norwegian cohort versus 33.0 in our cohort). In our study, however, we were able to follow participants for 26 years (median), whereas median follow-up was restricted to 8.7 years in the Norwegian study.
The recent SAFEHEART study from Spain with more than 3,700 participants (74% mutation carriers and 26% noncarriers) observed that mutation-carrying HeFH relatives have a healthier lifestyle with regard to dietary and smoking habits and physical activity compared with their nonmutation-carrying HeFH relatives [27]. In another Spanish study by Perez-Calahorra et al., the healthy lifestyle among mutation-carrying HeFH patient has been evaluated and compared with a general population cohort [28]. They proved a markedly reduced body mass index and smoking habits in the FH cohort. By assessing these lifestyle habits alone, our mutation-carrying HeFH relatives should expectedly have had a lower risk of developing cancer. However, this notion contradicts the findings of the present study, showing that only relatives without LDLR mutations have a lower cancer incidence than the general population cohort. In the SAFEHEART study, the corresponding relatives with an LDLR mutation had a similar cancer incidence as the background population. Unfortunately, no analyses on differences in lifestyle habits could be performed, as access to relevant lifestyle data was not available in this study.
Strengths and limitations
This study has some strengths, including the almost complete follow-up of cancer diagnoses during a period of greater than 25 years and the validity of the diagnostic codes used. The HeFH diagnosis is genetically verified. Given that data are obtained from independent medical registries, the results are not biased by self-reporting issues.
In addition, our results should be interpreted within some limitations. Adjustment for lifestyle factors was not possible. Treatment of HeFH includes recommendations toward lowering LDL-C levels by lifestyle interventions, such as a healthy diet, physical activity, discontinuation of smoking, and limitation of alcohol intake; therefore, it would have been valuable to adjust for these factors [29, 30]. However, due to limited data access, this adjustment was not possible. Furthermore, continuous data on LDL-C values would have been of interest to include. Unfortunately, only baseline data were available. Also, the nonmutation-carrying HeFH relatives are expected to be more aware of their own health and lifestyle and thus be more regularly examined by physicians than the general population cohort. This would cause more cancers to be detected and trigger a detection bias, which probably would drive the cancer incidence upward. Thus, this notion cannot explain the lower risk among nonmutation-carrying HeFH relatives. Furthermore, to avoid inducing immortal time bias, we were unable to adjust for the use of lipid-lowering medications. Unfortunately, the small sample size did not allow us to investigate the incidence of individual types of cancers in our HeFH relatives.
Conclusion
LDLR mutation-carrying HeFH relatives in Denmark were not at increased risk of cancer compared with the general population. In this study, being a nonmutation-carrying HeFH relative was associated with a lower risk of cancer in comparison to the general population. However, these relationships are not casual and to better understand these associations, more research including larger cohorts must be performed, particularly in the context of HeFH relatives without LDLR mutations.
Availability of data and materials
The data that support the findings of this study are available from the Danish Health Data Authority (Sundhedsdatastyrelsen). Restrictions apply to the availability of these data, which were used under license for this study and so are not publicly available.
Abbreviations
- CI:
-
Confidence interval
- DNPR:
-
Danish National Patient Registry
- FH:
-
Familial hypercholesterolemia
- HeFH:
-
Heterozygous familial hypercholesterolemia
- HR:
-
Hazard ratio
- IQR:
-
Interquartile ranges
- LDL-C:
-
Low-density lipoprotein cholesterol
- LDLR:
-
Low-density lipoprotein receptor
- NPR:
-
Danish National Prescription Registry
References
Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97(11):3956–64.
Mach F, Baigent C, Catapano AL, Koskina KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;1(290):140–205.
Brown MS, Goldstein JL. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974;185(4145):61–3.
Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet. 1969;294(7635):1380–2.
Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 199;303(6807):893–6.
Kjærgaard KA, Christiansen MK, Schmidt M, Olsen MS, Jensen HK. Long-term cardiovascular risk in heterozygous familial hypercholesterolemia relatives identified by cascade screening. J Am Heart Assoc. 2017;6(6):e005435.
Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019;9(2):219–27.
Kimbung S, Lettiero B, Feldt M, Bosch A, Borgquist S. High expression of cholesterol biosynthesis genes is associated with resistance to statin treatment and inferior survival in breast cancer. Oncotarget. 2016;7(37):59640–51.
Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/ SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1(5):442–56.
Vitols S, Angelin B, Ericsson S, Gahrton G, Juliusson G, Masquelier M, et al. Uptake of low density lipoproteins by human leukemic cells in vivo: Relation to plasma lipoprotein levels and possible relevance for selective chemotherapy. Proc Natl Acad Sci U S A. 1990;87(7):2598–602.
Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2015;112(8):2473–8.
Vitols S, Peterson C, Larsson O, Holm P, Åberg B. Elevated uptake of low density lipoproteins by human lung cancer tissue in vivo. Cancer Res. 1992;52(22):6244.
Roslan Z, Muhamad M, Selvaratnam L, Ab-Rahim S. The roles of low-density lipoprotein receptor-related proteins 5, 6, and 8 in cancer: a review. J Oncol. 2019;2019:4536302 Hindawi Limited.
Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115(4):959–68.
Gallagher EJ, Zelenko Z, Neel BA, Antoniou IM, Rajan L, Kase N, et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 2017;36(46):6462–71.
Jensen H, Jensen L, Meinertz H, Hansen P, Gregersen N, Færgeman O. Spectrum of LDL receptor gene mutations in Denmark: implications for molecular diagnostic strategy in heterozygous familial hypercholesterolemia. Atherosclerosis. 1999;146(2):337–44.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–9 Springer.
Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90 Dove Medical Press Ltd.
Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2016;46(3):798.
Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiol. 2007;18(6):805–35. Available from: https://journals.lww.com/epidem/Fulltext/2007/11000/Strengthening_the_Reporting_of_Observational.28.aspx. Cited 2022 Feb 15.
IARC - WHO. Non-melanoma skin cancer; Globocan 2020. 2020. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/17-Non-melanoma-skin-cancer-fact-sheet.pdf.
dos RodriguesSantos C, Fonseca I, Dias S, de Almeida JCM. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer. 2014;14(1):1–10.
Wang C, Li P, Xuan J, Zhu C, Liu J, Shan L, et al. Cholesterol enhances colorectal cancer progression via ROS elevation and MAPK signaling pathway activation. Cell Physiol Biochem. 2017;42(2):729–42.
Hao B, Yu M, Sang C, Bi B, Chen J. Dyslipidemia and non-small cell lung cancer risk in Chinese population: a case-control study. Lipids Health Dis. 2018;17(1):1–7.
Krogh HW, Svendsen K, Igland J, Mundal LJ, Holven KB, Bogsrud MP, et al. Lower risk of smoking-related cancer in individuals with familial hypercholesterolemia compared with controls: a prospective matched cohort study. Sci Rep. 2019;9(1):19273.
Arroyo-Olivares R, Alonso R, Quintana-Navarro G, Fuentes-Jiménez F, Mata N, Muñiz-Grijalvo O, et al. Adults with familial hypercholesterolaemia have healthier dietary and lifestyle habits compared with their non-affected relatives: the SAFEHEART study. Public Health Nutr. 2019;22(8):1433–43.
Perez-Calahorra S, Civeira F, Guallar-Castillón P, Pinto X, Banegas JR, Pedro-Botet J, et al. Behavioural cardiovascular risk factors and prevalence of diabetes in subjects with familial hypercholesterolaemia. Eur J Prev Cardiol. 2020;27(15):1649–60.
Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med. 2000;160(4):459–67.
Fernando CF. The genetic basis of familial hypercholesterolemia: inheritance, linkage, and mutations. Appl Clin Genet. 2010;5(3):53.
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors (KAAK, SH, HKJ, SB) have made substantial contributions to this study in terms of conception, design of the work, acquisition of data and manuscript writing and revisions. KAK and SH have contributed to analysis and interpretation of data. All authors have approved the submitted version, and agrees to be personally accountable for the authors own contributions.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Danish Data Protection Agency and the Central Denmark Region (record no. 1–16-02–381-19).
Consent for publication
Not applicable.
Competing interests
Henrik K. Jensen is supported by a grant from the Novo Nordisk Foundation, Denmark (NNF18OC0031258). Signe Borgquist is supported by a grant from the Novo Nordisk Foundation, Denmark (NNF18OC0034482 & NNF20OC0065928).
The authors declare that they have no other competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kjærgaard, K.A., Harborg, S., Jensen, H.K. et al. Long-term cancer risk in heterozygous familial hypercholesterolemia relatives: a 25-year cohort study. Lipids Health Dis 21, 56 (2022). https://doi.org/10.1186/s12944-022-01666-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01666-2