Abstract
Background
Due to the increasing emergence of antibiotic resistance in Enterococcus faecalis (E. faecalis), it indicated as potentially opportunistic pathogen causing various healthcare-associated and life-threatening diseases around the world.
Objective
The aim of this meta-analysis was to evaluate the weighted pooled resistance rates in clinical E. faecalis isolates based on over time, areas, antimicrobial susceptibility testing (AST), and infection source.
Methods
We searched the studies in PubMed, Scopus, and Web of Science (November 30, 2022). All statistical analyses were carried out using the statistical package R.
Results
The analysis encompassed a total of 74 studies conducted in 28 countries. According to the meta-regression, the chloramphenicol, fosfomycin, imipenem, linezolid, minocycline, norfloxacin, quinupristin-dalfopristin, and tetracycline resistance rate increased over time. Analysis revealed statistically significant differences in antibiotic resistance rates for ampicillin, chloramphenicol, erythromycin, gentamicin, penicillin, rifampicin, teicoplanin, tetracycline, and vancomycin across various countries.
Conclusions
Globally, the prevalence of drug resistant E. faecalis strains are on the increase over time. Daptomycin and tigecycline can be an effective agent for the treatment of clinical E. faecalis infections. Considering the low prevalence of antibiotic resistance in continents of Europe and Australia, it is suggested to take advantage of their preventive strategies in order to obtain efficient results in other places with high prevalence of resistance.
Similar content being viewed by others
Introduction
Enterococcus faecalis, a Gram-positive bacterium found in humans, animals, and various environments, is primarily a commensal microorganism but can act as an opportunistic pathogen causing various healthcare-associated and life-threatening diseases [1,2,3]. It is responsible for 80–90% of enterococcal infections, including life-threatening diseases, and is a leading cause of nosocomial infections worldwide [1, 4, 5]. Hospital-acquired infections are a global threat due to the rise of drug-resistant E. faecalis strains, which are resistant to most antimicrobial agents, including vancomycin [1, 2, 5,6,7]. This poses challenges for healthcare systems and requires new targeted therapies. Multidrug-resistant (MDR) E. faecalis strains can transfer resistant genes to other pathogens, further complicating treatment and increasing the risk to patients and healthcare professionals [5, 8, 9]. Understanding the global status of antimicrobial resistance (AMR) in E. faecalis isolates is crucial for effective infection control and treatment strategies. The rise of AMR is influenced by factors like antibiotic misuse, genetic factors, and the persistence of resistant strains in healthcare settings [10, 11]. E. faecalis has intrinsic resistance mechanisms, such as the absence of antibiotic targets, low-affinity targets, impermeability to certain antibiotics, presence of efflux pumps, and lack of uptake mechanisms for various antimicrobials [12, 13].
Intrinsic resistance in E. faecalis is typically encoded in the chromosome and not easily transferable between bacteria. MDR in E. faecalis can arise from the acquisition of resistance genes through mobile genetic elements like transposons, integrons, and plasmids, as well as chromosomal mutations and antibiotic-modifying enzymes [1, 11, 14,15,16]. Resistance genes can be transferred horizontally between closely related bacteria. The ability of E. faecalis to form biofilms enhances its resistance to antibiotics, posing challenges in treatment [2, 17].
E. faecalis exhibits resistance to vancomycin and beta-lactam antibiotics through various mechanisms [18,19,20,21]. Vancomycin resistance is primarily due to the acquisition of vancomycin-resistant genes (vanA, vanB, vanC, vanD, vanE, vanG, vanL, and vanM), which alter the peptidoglycan synthesis pathway [18,19,20,21]. Beta-lactam resistance results from producing beta-lactamases, such as penicillin-binding proteins (PBPs) that inactivate the antibiotics [22, 23]. Additionally, mutations in regulatory genes (e.g., pbp4, pbp5) contribute to beta-lactam resistance by modifying antibiotic targets [22, 23].
AMR in E. faecalis infections leads to increased morbidity, mortality, and healthcare costs. Limited treatment options for resistant strains highlight the need for alternative therapeutic strategies and the development of new antimicrobial agents [24, 25]. A comprehensive understanding of the global prevalence and trends of antimicrobial resistance in E. faecalis is essential to effectively address this challenge [1, 11, 14,15,16].
To address this challenge effectively, a comprehensive understanding of the global prevalence and trends of AMR in E. faecalis is crucial. We reviewed global E. faecalis resistance patterns, identifying high resistance rates to multiple antibiotics. Our findings guide clinical practice, policies, and research to combat multidrug-resistant strains and improve patient outcomes.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines are followed in the reporting of this review [26].
Search strategy and study selection
We conducted a thorough and methodical exploration of various databases, including PubMed, Scopus, and Embase, until November 30, 2022. Our search approach involved the use of specific keywords such as Enterococci, Enterococcus faecalis, E. faecalis, antimicrobial resistance, antibiotic resistance, clinical samples, and human samples, with a focus on the Title/Abstract/Keywords fields. We did not impose any limitations on our database search. The study investigators were responsible for designing and executing the search strategy. Additionally, we meticulously scrutinized the reference lists of all relevant studies to identify any other noteworthy publications. To ensure data accuracy, we combined the records obtained from the database search and eliminated any duplicated entries using EndNote X8 (Thomson Reuters, New York, NY, USA). To verify the search results, one team researcher randomly assessed them and confirmed that no pertinent studies had been overlooked.
Eligibility criteria
The meta-analysis incorporated articles that fulfilled specific inclusion criteria. These criteria encompassed studies that examined antibiotic resistance in E. faecalis isolates derived from human sources. Furthermore, the articles had to undergo peer review and be published in the English language within the timeframe of 2000–2022. Additionally, the studies were required to specify the total number of E. faecalis clinical isolates and provide information on the antibiotic resistance rate observed in these isolates. Conversely, certain exclusion criteria were applied. These criteria encompassed studies that contained duplicate data or were overlapping articles. Non-clinical isolates and reports on antibiotic resistance of Enterococcus spp other than E. faecalis were also excluded. Moreover, reviews, cohort studies, pharmacokinetic studies, and conference abstracts were not considered. Lastly, articles that did not clearly present or report antibiotic resistance rates were also excluded from the meta-analysis.
Data extraction
Each included study provided the following information: the first author’s name, publication year, continent, WHO regions, country, number of E. faecalis clinical isolates, number of antibiotic resistance rate in E. faecalis isolates, infection source (bloodstream, gastrointestinal tract, urinary tract, mixed), and antimicrobial susceptibility testing (MIC-based methods and disk diffusion agar). Two independent examiners collected the data, and another researcher verified its accuracy.
Quality assessment
Two reviewers independently evaluated the quality of the studies included in the analysis. They employed a modified version of the Newcastle–Ottawa assessment scale, which was adapted for cross-sectional studies [27]. Each study was assigned a score ranging from 0 to 8 points, with a score of 6 or higher indicating high quality, while a score of 5 or lower indicated low quality. In cases where there was a disagreement between the two reviewers, a third reviewer was consulted to resolve the discrepancy.
Statistical analysis
The meta-analysis included studies that provided raw data on antibiotic resistance in clinical isolates of E. faecalis. The analysis was conducted using the meta-prop [28] command in the R statistical software, considering prevalence statistics based on antibiotic, region (continents/countries), year, infection source, and antimicrobial susceptibility testing (AST). The results of the meta-analysis consisted of a prevalence statistic accompanied by 95% confidence intervals, which were calculated using weighted prevalence statistics from all the studies within the specified sub-group. Meta-regression models were employed to examine any changes in antibiotic resistance over time. To assess publication bias, Egger and Begg tests were utilized. All statistical interpretations were reported with a 95% confidence interval (CI). The statistical package R 3.6.0, developed by the R Foundation for Statistical Computing in Vienna, Austria, was used for all the statistical analyses [29].
Study outcomes
The primary focus of this study was to determine the prevalence of antibiotic resistance in clinical isolates of E. faecalis, using the guidelines provided by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). To further analyse the data, subgroup analyses were conducted based on the following factors: (1) year of publication (2000–2013, 2014–2022), (2) geographical location (continent/country), (3) source of infection, and (4) antimicrobial susceptibility testing (AST).
Results
Systematic literature search
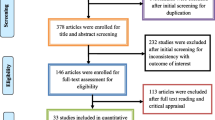
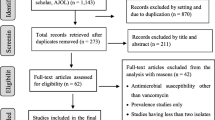
The initial search yielded a total of 4580 records. After an initial screening of the title and abstract, 4430 articles were deemed irrelevant or duplicates and were subsequently excluded. The full texts of the remaining 150 articles were thoroughly reviewed (Fig. 1). Among these 150 articles, 79 were excluded for the same reasons mentioned earlier. Ultimately, 74 studies [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] published between 2000 and 2022 (Supplementary Table) were included in the analysis. The screening and selection process was visually summarized in the PRISMA flow chart (Fig. 1).
Characteristics of included studies
Data on antibiotic resistance were collected from 28 countries spanning 5 continents, including Poland, Egypt, Iran, Sweden, Turkey, China, US, Brazil, Uganda, India, Cuba, South Korea, Hungary, Kuwait, Italy, Israel, Australia, Germany, Taiwan, Lebanon, Romania, Hong Kong, Czechia, Algeria, Indonesia, Nigeria, Pakistan, and Japan. The forest plot in Fig. 2 presented the proportion of resistance isolates to selected antibiotics, with subgroup analyses by time, area, source of samples, and AST shown in the Supplementary File. The Supplementary File also included meta-regression results for changes in antibiotic resistance over time. Figure 3 displayed changes in resistance proportions based on time, continent, WHO regions, AST, and source of samples for the selected antibiotics, while Fig. 4 showed changes in resistance proportions based on countries. The summary of resistance rate trends and the results of publication bias tests using Egger and Begg tests were presented in the Supplementary File.
Subgroup analysis
Overview of antibiotic resistance prevalence
Proportion of vancomycin resistance through 30 reports, with 1533 resistant isolates among 11912 investigated isolates was 4.3% (95% CI 0.022, 0.082), and heterogeneity between report was significant (I2 = 98.01%, p = 0.001). Proportion of gentamicin resistance through 41 reports, with 4775 resistant isolates among 9198 investigated isolates was 49.7% (95% CI 0.421, 0.574), and heterogeneity between report was significant (I2 = 97.03%, p = 0.001). Proportion of amoxicillin-clavulanic acid resistance through 4 reports, with 39 resistant isolates among 132 investigated isolates was 29.4% (95% CI 0.158, 0.482), and heterogeneity between report was not significant (I2 = 0.00%, p = 0.662). Proportion of ampicillin resistance through 56 reports, with 1703 resistant isolates among 12065 investigated isolates was 9.5% (95% CI 0.058, 0.151), and heterogeneity between report was significant (I2 = 97.30%, p = 0.001). Proportion of chloramphenicol resistance through 24 reports, with 494 resistant isolates among 3483 investigated isolates was 18.4% (95% CI 0.126, 0.261), and heterogeneity between report was significant (I2 = 92.12%, p = 0.001). Proportion of ciprofloxacin resistance through 45 reports, with 2505 resistant isolates among 5647 investigated isolates was 44.2% (95% CI 0.364, 0.523), and heterogeneity between report was significant (I2 = 94.82%, p = 0.001). Proportion of daptomycin resistance through 6 reports, with 2 resistant isolates among 1103 investigated isolates was 0.7% (95% CI 0.002, 0.021), and heterogeneity between report was not significant (I2 = 21.18%, p = 0.274). Proportion of doxycycline resistance through 7 reports, with 186 resistant isolates among 404 investigated isolates was 31.4% (95% CI 0.126, 0.593), and heterogeneity between report was significant (I2 = 93.96%, p = 0.001). Proportion of erythromycin resistance through 31 reports, with 3348 resistant isolates among 5591 investigated isolates was 60.3% (95% CI 0.523, 0.677), and heterogeneity between report was significant (I2 = 96.23%, p = 0.001). Proportion of fosfomycin resistance through 9 reports, with 53 resistant isolates among 888 investigated isolates was 6.5% (95% CI 0.02, 0.187), and heterogeneity between report was significant (I2 = 91.65%, p = 0.001). Proportion of imipenem resistance through 11 reports, with 96 resistant isolates among 3578 investigated isolates was 2.6% (95% CI 0.008, 0.081), and heterogeneity between report was significant (I2 = 94.58%, p = 0.001). Proportion of levofloxacin resistance through 21 reports, with 2361 resistant isolates among 4981 investigated isolates was 36.7% (95% CI 0.283, 0.46), and heterogeneity between report was significant (I2 = 95.28%, p = 0.001). Proportion of linezolid resistance through 32 reports, with 1074 resistant isolates among 9601 investigated isolates was 1.3% (95% CI 0.004, 0.043), and heterogeneity between report was significant (I2 = 77.24%, p = 0.001). Proportion of minocycline resistance through 3 reports, with 150 resistant isolates among 1273 investigated isolates was 44.9% (95% CI 0.051, 0.925), and heterogeneity between report was significant (I2 = 88.10%, p = 0.001). Proportion of moxifloxacin resistance through 3 reports, with 100 resistant isolates among 281 investigated isolates was 26.4% (95% CI 0.046, 0.725), and heterogeneity between report was significant (I2 = 79.79%, p = 0.007). Proportion of nitrofurantoin resistance through 20 reports, with 718 resistant isolates among 3932 investigated isolates was 6.2% (95% CI 0.027, 0.138), and heterogeneity between report was significant (I2 = 96.61%, p = 0.001). Proportion of norfloxacin resistance through 4 reports, with 24 resistant isolates among 124 investigated isolates was 31.3% (95% CI 0.062, 0.759), and heterogeneity between report was significant (I2 = 90.47%, p = 0.001). Proportion of penicillin resistance through 24 reports, with 1532 resistant isolates among 4245 investigated isolates was 33.6% (0.2, 0.506), and heterogeneity between report was significant (I2 = 97.44%, p = 0.001). Proportion of quinupristin-dalfopristin resistance through 13 reports, with 1609 resistant isolates among 3025 investigated isolates was 34.3% (95% CI 0.137, 0.632), and heterogeneity between report was significant (I2 = 98.49%, p = 0.001). Proportion of rifampicin resistance through 10 reports, with 988 resistant isolates among 1664 investigated isolates was 61.3% (95% CI 0.442, 0.759), and heterogeneity between report was significant (I2 = 95.66%, p = 0.001). Proportion of streptomycin resistance through 22 reports, with 2864 resistant isolates among 5430 investigated isolates was 48.2% (95% CI 0.391, 0.573), and heterogeneity between report was significant (I2 = 96.46%, p = 0.001). Proportion of teicoplanin resistance through 35 reports, with 1493 resistant isolates among 9966 investigated isolates was 5.3% (95% CI 0.027, 0.099), and heterogeneity between report was significant (I2 = 96.98%, p = 0.001). Proportion of tetracycline resistance through 27 reports, with 3085 resistant isolates among 4659 investigated isolates was 66.6% (0.568, 0.751), and heterogeneity between report was significant (I2 = 95.70%, p = 0.001). Proportion of tigecycline resistance through 11 reports, with 18 resistant isolates among 3691 investigated isolates was 0.7% (95% CI 0.005, 0.011), and heterogeneity between report was not significant (I2 = 0.00%, p = 0.533). Proportion of cotrimoxazole resistance through 12 reports, with 826 resistant isolates among 1827 investigated isolates was 49.7% (95% CI 0.33, 0.665), and heterogeneity between report was significant (I2 = 96.44%, p = 0.001).
Subgroup analysis based on year group
The subgroup analysis provided valuable insights into the trends of antibiotic resistance across different year groups. It identified significant disparities in the prevalence rates of resistance to three antibiotics ciprofloxacin, penicillin, and rifampicin. For ciprofloxacin, a notable decrease in resistance was observed from the 2000–2013 year group, which had a prevalence rate of 54.3%, to the 2014–2022 year group, where the rate dropped to 35.2%. This decline suggests a possible change in the susceptibility patterns of the targeted bacterial strains during this period. Similarly, penicillin resistance also exhibited a downward trend when comparing the 2000–2013 year group to the 2014–2022 year group. The resistance rate fell from 54.2 to 16.1%, indicating a substantial decrease in the prevalence of penicillin-resistant strains. In the case of rifampicin, the analysis revealed a significant drop in resistance between the same two year groups. The resistance rate decreased from a high of 73% in 2000–2013 to 36.2% in 2014–2022, demonstrating an appreciable change in susceptibility patterns over time.
To analyze the trends for changes in the rate of antibiotics resistance in different years, we performed a meta-regression analysis for changes in the proportions of antibiotics resistance over time (Supplementary File). According to the meta-regression, the chloramphenicol, fosfomycin, imipenem, linezolid, minocycline, norfloxacin, quinupristin-dalfopristin, and tetracycline resistance rate increased over time; although, was not statistically significant.
Subgroup analysis based on country
The subgroup analysis conducted on antibiotic resistance rates across various countries revealed significant disparities in the prevalence of resistance to different antibiotics. Among the countries studied, Australia consistently demonstrated the lowest rates of resistance, while Taiwan consistently exhibited the highest rates. In terms of specific antibiotics, Australia exhibited the lowest rates for erythromycin (31.5%), penicillin (0.4%), teicoplanin (0.1%), ampicillin (0.1%), and vancomycin (0.1%). Sweden also reported minimal resistance to chloramphenicol (0.4%) and gentamicin (5.1%). Poland, Turkey, and Taiwan each displayed low resistance levels to ciprofloxacin (0.9%), rifampicin (5.1%), and tetracycline (12.8%), respectively. In contrast, South Korea experienced the highest resistance rates for erythromycin (91.2%), gentamicin (91.8%), and rifampicin (96.4%). Taiwan had the highest resistance rates for penicillin (95%), teicoplanin (81%), ciprofloxacin (83%), and vancomycin (99%). Algeria demonstrated the highest resistance to tetracycline at 92.5%. Additionally, India and Italy showed the highest resistance rates to ampicillin (74.1%) and chloramphenicol (72.9%), respectively.
Subgroup analysis based on WHO regional offices
The subgroup analysis identified significant differences in antibiotic resistance rates among various WHO regional offices. The Region of the Americas had the lowest ampicillin resistance rate at 0.4%, while the South-East Asia Region had the highest ampicillin resistance at 77.3%. The Eastern Mediterranean Region had the lowest erythromycin resistance rate at 46%, with the South-East Asia Region having the highest at 79%. For gentamicin, the Eastern Mediterranean Region/Islamic Republic of Iran region had the lowest resistance rate at 29.5%, while the South-East Asian Region had the highest at 85.5%. The lowest levofloxacin resistance rate was in the Eastern Mediterranean Region/Islamic Republic of Iran region at 10.3%, and the European Region had the highest at 96%. Penicillin resistance was lowest in the European Region at 1.4% and highest in the South-East Asia Region at 97.5%. Rifampicin resistance was lowest in the European Region at 38.9% and highest in the Western Pacific Region at 91.8%. Teicoplanin resistance was lowest in the African Region at 0.6% and highest in the Western Pacific Region at 34.6%.
Subgroup analysis based on AST methods
The subgroup analysis revealed a statistically significant disparity in the prevalence of antibiotic resistance, including that of ciprofloxacin, gentamicin, penicillin, quinupristin-dalfopristin, among various AST classification. For the antibiotic ciprofloxacin, the AST classification with the lowest rate of resistance was Multiple Standards, exhibiting a prevalence rate of 8.9%, while conversely, the AST classification with the highest resistance rate was observed in CLSI, with a prevalence rate reaching 46.9%. For the antibiotic gentamicin, the AST classification with the lowest rate of resistance was EUCAST, exhibiting a prevalence rate of 11.8%, while conversely, the AST classification with the highest resistance rate was observed in Unknown, with a prevalence rate reaching 53.7%. For the antibiotic penicillin, the AST classification with the lowest rate of resistance was Multiple Standards, exhibiting a prevalence rate of 0.4%, while conversely, the AST classification with the highest resistance rate was observed in CLSI, with a prevalence rate reaching 38.9%. For the antibiotic quinupristin dalfopristin, the AST classification with the lowest rate of resistance was CLSI, exhibiting a prevalence rate of 25.1%.
Discussion
Initially considered a benign member of the gut microbiota in both humans and animals, E. faecalis has emerged as an opportunistic pathogen and ranks among the most prevalent bacteria in nosocomial-acquired infections worldwide, trailing only Escherichia coli and Staphylococcus. Numerous global studies have highlighted the predominance of E. faecalis in a broad spectrum of life-threatening infections, especially among hospitalized patients [1]. The ability of E. faecalis to cause widespread and difficult-to-treat diseases is due to its resistance to various antimicrobial substances, which has now become one of the most significant global public health threats. This bacterium has extensive inherent and acquired resistance to different types of antibiotics [104]. Several risk factors, including immunosuppression, comorbid diseases, prolonged hospital stays, heightened occupancy rates particularly patients hospitalized in intensive care units, insufficient exposure to antibiotics or disinfectants, and irrational/extensive antibiotic usage, are associated with the development of resistance among E. faecalis strains [1, 5]. Significantly, Higher morbidity and mortality rates, extended length of hospital stay, higher treatment costs, and the spread of resistance determinants to other infectious agents are the consequences of infections caused by resistant pathogens including E. faecalis [5, 105, 106].
To our knowledge, our study represents the first systematic review and meta-analysis research to comprehensively assess the pooled prevalence of AMR profile of E. faecalis around the World. In the present study, the prevalence of resistance of E. faecalis strains to 25 different antibiotics has been evaluated from 2000 to 2022 in the world.
The most studies have been conducted on vancomycin, followed by ampicillin, and the least studies are related to minocycline, moxifloxacin and norfloxacin antibiotics. Considering that E. faecalis is one of the most common causes of MDR hospital infections, it is necessary to study and monitor their antimicrobial resistance to prevent and control the spread of resistant strains [107].
The findings of our study indicate that the highest resistance was observed against tetracycline, with a prevalence rate of 66.6%. The high proportion of tetracycline resistance among clinical strains of E. faecalis considered as a serious public health warning. Subsequently, the high frequency of resistance in E. faecalis strains was associated with rifampicin, erythromycin, gentamicin, trimethoprim-sulfamethaxazole (cotrimoxazole), streptomycin, minocycline and ciprofloxacin with rates of 61.3, 60.3, 49.7, 49.7, 48.2, 44.9, and 44.2% respectively. Correspondingly, numerous studies have revealed significant resistance of Enterococcus faecium strains to tetracycline and erythromycin, which indicates that they are not recommended for the treatment of infections caused by this bacterium. Both antibiotics possess a broad-spectrum effect against various pathogens, potentially contributing to the development of resistance in enterococci during the treatment of other infections [6, 108,109,110]. These findings underline the importance of enhanced monitoring of the prescription and use of these antimicrobial agents in the treatment of enterococci infections.
The lowest frequency of antibiotic resistance in E. faecalis clinical strains is related to daptomycin and tigecycline with a prevalence of 0.7%. These findings suggest that daptomycin and tigecycline are the most effective drugs for treating E. faecalis infections. Consistent with our discoveries, other systematic review studies focusing on Enterococcus faecium strains demonstrate a considerable sensitivity of these strains to daptomycin and tigecycline, underscoring their viability as appropriate treatment choices for combating resistant infections attributed to this strain [5, 6]. Our study also revealed that linezolid, imipenem, vancomycin, teicoplanin, nitrofurantoin, fosfomycin, and ampicillin displayed significantly the lowest resistance rates at 1.3, 2.6, 4.3, 5.3, 6.2, 6.5, and 9.5%, respectively. Although the considerable low level of resistance of E. faecalis strains to these antibiotics has made them appropriate options for the treatment of clinical infections, their recent increase in resistance rate necessitates improved executive management to prevent the overgrowth and dissemination of resistant bacteria [1, 6, 107, 111, 112]. This underscores the need for a more vigilant approach to their use in clinical settings to effectively address the challenge of resistance.
The healthcare systems have faced significant challenges due to the COVID-19 pandemic, resulting in constrained roles of infectious diseases services, irrational and excessive utilization of disinfectants and antimicrobial agents, and a rise in the incidence of infections caused by MDR microorganisms [113]. Numerous studies conducted globally have demonstrated an augmentation in the resistance of gram-negative and gram-positive bacteria, including enterococci, to a range of antibiotics during the COVID-19 period in comparison to pre-pandemic times [113,114,115,116,117,118]. Our study reveals notable alterations in the resistance rates of E. faecalis strains to certain antibiotics when comparing the pre- and post-Covid-19 pandemic periods. Specifically, the prevalence of resistance to ampicillin and penicillin during the period spanning 2000–2019 (prior to the Covid-19 pandemic) was significantly higher than the period of 2020–2022 (post the Covid-19 pandemic). On the other hand, the resistance of E. faecalis to amoxicillin-clavulanic acid, chloramphenicol, fosfomycin, imipenem, minocycline, moxifloxacin, norfloxacin, quinupristin/dalfopristin, and tetracycline has displayed a relatively increased level in the period 2020–2022 compared to 2000–2019. Although the difference in the outcomes of these antibiotics between the pre- and post-Covid-19 pandemic periods does not exhibit statistical significance, it elucidates the importance of escalating resistance during the covid-19 pandemic [117, 119, 120]. The irrational and excessive utilization/prescription of antibiotics, self-antibiotics medication and non-prescription drug sales, empirical administration of antimicrobials, physician prescribed antibacterials for in-patients admitted for the viral infection, and antibiotics prescribed by general practitioners have been identified as the main risk factors contributing to the elevated levels of antibiotic resistance during the COVID-19 pandemic, particularly in underdeveloped or developing countries [115, 117, 119, 120]. Consequently, it is essential to enforce antibiotic resistance surveillance and adhere strictly to antibiotic prescriptions in accordance with antimicrobial stewardship programs (ASP) and the guidelines outlined by the WHO. This approach is crucial not only for bolstering resistant-infections prevention or control but also for ensuring robust and consistent AMR surveillance as an integral component of the COVID-19 pandemic response and recovery [114].
It is noteworthy that the trend of drug resistance rate of E. faecalis strains has demonstrated significant changes over the years (from 2000 to 2022) with regard to certain antibiotics. The trend of resistance rate to ciprofloxacin, penicillin, and rifampicin have shown a significant decrease during this period. Meanwhile, the resistance trends for doxycycline, erythromycin, moxifloxacin, ampicillin, levofloxacin, and vancomycin have displayed a non-significant decrease over time. The downward trend of resistance rate to these antibiotics in the world may be attributed to effective management and monitoring policies governing the rational prescription and consumption of antibiotics, alongside the implementation of appropriate strategies to curb the spread of resistant strains in developed countries [121, 122]. Conversely, the trend of resistance rate to minocycline, fosfomycin, norfloxacin, quinupristin/dalfopristin, chloramphenicol, tetracycline, nitrofurantoin, and teicoplanin have shown a non-significant increase over the same period. The comparative analysis conducted in this study reveals that resistance levels to specific antibiotics were notably elevated in certain countries when compared globally. Taiwan exhibited significantly higher resistance to vancomycin, penicillin, teicoplanin, and ciprofloxacin. South Korea showed meaningfully increased resistance to gentamicin, erythromycin, and rifampicin. India and Italy had significantly higher resistance to ampicillin and chloramphenicol, respectively. Algeria demonstrated significantly higher resistance level to tetracycline in comparison to other countries. Conversely, Australia had significantly lower resistance levels to erythromycin, penicillin, teicoplanin, ampicillin, and vancomycin. Sweden exhibited significant decreased resistance rates to chloramphenicol and gentamicin. Turkey showed a significant lower resistance to rifampicin, while Poland had a lower resistance to ciprofloxacin in comparison to other countries. These findings highlight the disparities in antibiotic resistance patterns across different regions. Our data clearly demonstrates that clinical E. faecalis strains in Asian countries exhibit significantly higher levels of antibiotic resistance compared to strains from other global regions. The considerable increase in resistance observed in Asia, especially in developing countries, underscores the absence of effective AMR national action plans and strict monitoring programs aimed at mitigating antibiotic resistance [122, 123]. In contrast, the considerable decrease in the prevalence of drug-resistant strains in Australia and subsequently in Sweden highlights the pivotal role of well-implemented healthcare policies and strategies in preventing the emergence and dissemination of AMR within these countries.
Global findings reveal that the greatest number of studies pertaining to the prevalence of antibiotic resistance in E. faecalis strains has been conducted in Asia, surpassing research conducted in other continents. Subsequently, Europe represents a higher number of studies in comparison to other continents, while the continent of Oceania has the least studies in this domain. The prevalence of resistance to ciprofloxacin and norfloxacin among E. faecalis strains in Asia is significantly higher than in other continents. Africa has also shown a statistically significant increase in resistance to ampicillin and penicillin relative to other continents. Conversely, Oceania has experienced a significant decrease in resistance to gentamicin, ampicillin, ciprofloxacin, and penicillin. Europe and the Americas follow, with notably lower resistance to chloramphenicol and norfloxacin, respectively. Based on our findings, it is evident that Asian and African countries are significantly impacted by the escalating of AMR in clinical strains. Economically, the scarcity and elevated cost of broad-spectrum antibiotics in these continents have likely resulted in heightened usage of narrow-spectrum antibiotics, consequently contributing to an increase in resistance against them [6]. In general, the proportions of antibiotic resistance in the studied pathogens were lower in high-income countries compared to low- and lower-middle-income nations. This observation aligns with data from the Global Burden of Disease study 2019, indicating that the burden of infections caused by antibiotic-resistant bacteria is notably higher in areas with limited resources, such as African countries, in contrast to high-income regions such as Europe and North America [7]. In underdeveloped or developing countries, several factors contribute to the emergence of MDR microorganisms. These include limited access to effective antibiotics, unregulated administration and production of antibacterials, and abbreviated antibiotic treatment due to financial constraints [115, 123]. Additionally, the complexities underlying AMR in developing countries may be attributed to healthcare professionals’ practices, patient behaviors regarding antimicrobial use, and the incomplete antibiotic supply chain. These factors encompass inadequate hygiene practices, suboptimal prescribing practices, insufficient patient education, constrained diagnostic facilities, unauthorized antimicrobial sales, the absence of comprehensive ASP, and non-medical use of antimicrobials [124, 125].
The subgroup analysis based on WHO regional offices indicates a significantly elevated resistance of clinical E. faecalis strains to ampicillin, gentamicin, erythromycin, and penicillin within the South-East Asia region. In contrast, the Americas, Eastern Mediterranean/Islamic Republic of Iran, Eastern Mediterranean, and European regions exhibit significantly lower resistance to these antibiotics, respectively. Our study indicates a significantly higher frequency of resistance to rifampicin and teicoplanin in the Western Pacific region compared with other regions. Conversely, the European and African regions show significantly lower resistance to rifampicin and teicoplanin, respectively. The Eastern Mediterranean/Islamic Republic of Iran region demonstrates a significantly lower resistance rate to levofloxacin compared with other regions. Given that South-East Asian countries are substantial importers of poultry and livestock, the excessive use of antibiotics in these products contributes to the spread of MDR enterococci, which can be transmitted to humans through consumption of these products. This, in turn, leads to increased antibiotic resistance in these countries [6, 126]. Moreover, inappropriate consumption/prescription of antibiotics by patients/physicians, as well as in industries, are likely the primary factors lead to the enhancement of antibiotic-resistance in developing countries such as South-East Asian countries. In contrast, access to antibiotics is restricted in other regions [123, 125].
Enterococci infections are experiencing a rapid escalation in hospitals globally, primarily attributable to their ability to survive under harsh conditions for long periods and remarkable adaptability to environmental conditions. This capability positions enterococci as a crucial reservoir for the transmission and spread of drug resistance determinants [5]. Furthermore, the emergence and spreading of AMR-E. faecalis are influenced by other factors, encompassing environmental, societal, and economic effects, alongside local and regional idiosyncrasies [127,128,129]. Addressing AMR-E. faecalis requires a comprehensive approach that integrates multiple strategies for prevention, control, and treatment. We suggest the implementation of the following measures to curtail the further escalation of AMR among E. faecalis, particularly in countries with a high prevalence of resistance. First, establish a national AMR policy is imperative to comprehensively comprehend the emergence, spread, and aspects influencing antibiotic resistance. Second, stringent management policies should be enforced to prevent the transmission of resistant infections within hospital environments. Third, measures should be taken to prohibit antibiotic self-medication and deter irrational and unconventional antibiotic consumption/prescription practices. Fourth, efforts are needed to educate healthcare professionals and patients about the appropriate use of antimicrobials, alongside training healthcare workers for the nosocomial infections control methods. Fifth, the development of rapid point-of-care infectious agent detection should be employed to support the accurate use of antimicrobial drugs. Sixth, regular surveillance programs are essential to ascertain the precise prevalence of antibiotic resistance. Strengthening surveillance and monitoring systems is essential for tracking resistance patterns, detecting new resistant strains early, and enabling timely interventions. Seventh, implement strict infection control and prevention strategies, including hygiene practices, hand hygiene, and environmental cleaning protocols, to prevent the spread of resistant strains. As well, regular enforcing ASP in healthcare settings is vital to guide appropriate treatment strategies and minimize resistance development in clinical isolates. Lastly, there is a need to foster the expansion of new antimicrobial agents. Encouraging research and development efforts contributes to the development of new antibiotics, diagnostic tools, and alternative therapies, ultimately enhancing the fight against AMR-E. faecalis isolates. Integrating these strategies into clinical practice and healthcare policies is essential in mitigating the challenges posed by resistant strains of E. faecalis and improving patient outcomes. A coordinated global response involving healthcare providers, researchers, policymakers, and the pharmaceutical industry is necessary to achieving the goals effectively and defeat this pressing global health threat.
The heterogeneous results in this study can be attributed to varying resistance patterns based on the geographical area under study, studied population, source and size of the samples, and variations in bacterial identification and AST methodologies.
The main strength of our study is that, for the first time, we have comprehensively investigated the prevalence of antibiotic resistance in E. faecalis strains isolated from clinical samples all over the world during 22 years. Furthermore, we conducted comparative analyses between different points, examining the resistance trend over the time and comparing its fluctuations in long-term time periods, including in the periods before and after the COVID-19 pandemic. Nonetheless, it should be noted that there are several limitations to our study. First, only published full-text research articles were evaluated in our study. Second, only the studies on clinical strains of E. faecalis were assessed and other studies on environmental samples were excluded. Third, the lack of differentiation of clinical samples, which ultimately did not conclude the prevalence of E. faecalis in various infections. Forth, microbiological diagnostics is not routinely performed for typical infections, such as uncomplicated UTIs in outpatient care, consequently AMR patterns may not be sufficiently reflected in the dataset. Fifth, the lack of unit definition of resistance in the analysis of the literature that was used. In 2019, EUCAST redefined susceptibility testing categories as: susceptible (S), susceptible increased exposure (I), and resistant (R). EUCAST changes in category (I) may have led to changes in pooled resistance ratios compared to pre-2019 data. Sixth, considering that this is a global systematic review study, we might have missed some relevant studies. Finally, the lack of information on resistance mechanisms and associated genetic factors restricts our understanding of the primary drivers of resistance.
Conclusions
Our study highlights the alarming global prevalence of antibiotic resistance in E. faecalis, particularly affecting developing countries. This urgent issue calls for comprehensive strategies to address antibiotic resistance. Understanding regional resistance patterns is crucial for informing research, guiding antimicrobial stewardship programs, and curbing the spread of resistant strains. Daptomycin and tigecycline show potential as treatment options due to lower resistance rates, but careful management is needed to prevent drug-resistant strains. Learning from successful preventive strategies in low-resistance regions like Europe and Australia can help combat resistance. A multipronged approach involving surveillance, research, and stewardship programs is vital to preserve existing antibiotics' efficacy and protect public health.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- MDR:
-
Multidrug resistance
- AST:
-
Antimicrobial susceptibility testing
- AMR:
-
Antimicrobial resistance
- ASP:
-
Antimicrobial stewardship program
- WHO:
-
World Health Organization
References
Orababa OQ, Soriwei JD, Akinsuyi SO, Essiet UU, Solesi OM. A systematic review and meta-analysis on the prevalence of vancomycin-resistant enterococci (VRE) among Nigerians. Porto Biomed J. 2021;6(1):e125. https://doi.org/10.1097/j.pbj.0000000000000125.
Mwikuma G, Kainga H, Kallu SA, Nakajima C, Suzuki Y, Hang’ombe BM. Determination of the prevalence and antimicrobial resistance of Enterococcus faecalis and Enterococcus faecium associated with poultry in four districts in Zambia. Antibiotics. 2023;12(4):657.
Grudlewska-Buda K, Skowron K, Bauza-Kaszewska J, Budzyńska A, Wiktorczyk-Kapischke N, Wilk M, et al. Assessment of antibiotic resistance and biofilm formation of Enterococcus species isolated from different pig farm environments in Poland. BMC Microbiol. 2023;23(1):89. https://doi.org/10.1186/s12866-023-02834-9.
El Zowalaty ME, Lamichhane B, Falgenhauer L, Mowlaboccus S, Zishiri OT, Forsythe S, et al. Antimicrobial resistance and whole genome sequencing of novel sequence types of Enterococcus faecalis, Enterococcus faecium, and enterococcus durans isolated from livestock. Sci Rep. 2023;13(1):18609. https://doi.org/10.1038/s41598-023-42838-z.
Dadashi M, Sharifian P, Bostanshirin N, Hajikhani B, Bostanghadiri N, Khosravi-Dehaghi N, et al. The global prevalence of daptomycin, tigecycline, and linezolid-resistant Enterococcus faecalis and Enterococcus faecium strains from human clinical samples: a systematic review and meta-analysis. Front Med. 2021;8:720647.
Jabbari Shiadeh SM, Pormohammad A, Hashemi A, Lak P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: a systematic review and meta-analysis. Infect Drug Resist. 2019. https://doi.org/10.2147/IDR.S206084.
Rödenbeck M, Ayobami O, Eckmanns T, Pletz MW, Bleidorn J, Markwart R. Clinical epidemiology and case fatality due to antimicrobial resistance in Germany: a systematic review and meta-analysis, 1 January 2010 to 31 December 2021. Eurosurveillance. 2023;28(20):2200672.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27.
Farsi S, Salama I, Escalante-Alderete E, Cervantes J. Multidrug-resistant enterococcal infection in surgical patients, what surgeons need to know. Microorganisms. 2023. https://doi.org/10.3390/microorganisms11020238.
Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021. https://doi.org/10.3390/pathogens10101310.
Hollenbeck BL, Rice LB. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2012;3(5):421–33. https://doi.org/10.4161/viru.21282.
Sirichoat A, Flórez AB, Vázquez L, Buppasiri P, Panya M, Lulitanond V, et al. Antibiotic resistance-susceptibility profiles of Enterococcus faecalis and Streptococcus spp. from the human vagina, and genome analysis of the genetic basis of intrinsic and acquired resistances. Front Microbiol. 2020;11:1438.
Kristich CJ, Rice LB, Arias CA. Enterococcal infection—treatment and antibiotic resistance. Enterococci: from commensals to leading causes of drug resistant infection. 2014.
Cirrincione S, Neumann B, Zühlke D, Riedel K, Pessione E. Detailed soluble proteome analyses of a dairy-isolated Enterococcus faecalis: a possible approach to assess food safety and potential probiotic value. Front Nutr. 2019;6:71.
Adeniji OO, Nontongana N, Okoh AI. Prevalence of class 1 integron and in vitro effect of antibiotic combinations of multidrug-resistant enterococcus species recovered from the aquatic environment in the eastern cape province, South Africa. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24032993.
Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther. 2014;12(10):1221–36.
Reinseth IS, Ovchinnikov KV, Tønnesen HH, Carlsen H, Diep DB. The increasing issue of vancomycin-resistant enterococci and the bacteriocin solution. Probiotics Antimicrob Proteins. 2020;12(3):1203–17. https://doi.org/10.1007/s12602-019-09618-6.
Ahmed MO, Baptiste KE. Vancomycin-resistant enterococci: a review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb Drug Resist. 2018;24(5):590–606.
Faron ML, Ledeboer NA, Buchan BW. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J Clin Microbiol. 2016;54(10):2436–47.
Boneca IG, Chiosis G. Vancomycin resistance: occurrence, mechanisms and strategies to combat it. Expert Opin Ther Targets. 2003;7(3):311–28.
Arthur M, Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993;37(8):1563–71.
Zango UU, Ibrahim M, Shawai SAA, Shamsuddin IM. A review on β-lactam antibiotic drug resistance. MOJ Drug Des Develop Ther. 2019;3(2):52–8.
Fontana R, Canepari P, Lleo M, Satta G. Mechanisms of resistance of enterococci to beta-lactam antibiotics. Eur J Clin Microbiol Infect Dis. 1990;9:103–5.
Khalil MA, Alorabi JA, Al-Otaibi LM, Ali SS, Elsilk SE. Antibiotic resistance and biofilm formation in Enterococcus spp. isolated from urinary tract infections. Pathogens. 2022. https://doi.org/10.3390/pathogens12010034.
Cui P, Feng L, Zhang L, He J, An T, Fu X, et al. Antimicrobial resistance, virulence genes, and biofilm formation capacity among Enterococcus species from Yaks in Aba Tibetan autonomous prefecture, China. Front Microbiol. 2020;11:1250.
Moher D, Liberati A, Tetzlaff J, Altman DG, med PGJP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;6(7):e1000097
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11(1):e0147601.
Schwarzer GJRn. meta: An R package for meta-analysis. 2007;7(3):40–5.
Team R. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013.
Arbune M, Gurau G, Niculet E, Iancu AV, Lupasteanu G, Fotea S, et al. Prevalence of antibiotic resistance of ESKAPE pathogens over five years in an infectious diseases hospital from South-East of Romania. Infect Drug Resist. 2021;14:2369–78. https://doi.org/10.2147/idr.S312231.
Batistão DWdF, Gontijo-Filho PP, Conceição N, Oliveira AGd, Ribas RM. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz. 2012;107:57–63.
Bhatt P, Patel A, Sahni A, Praharaj A, Grover N, Chaudhari C, et al. Emergence of multidrug resistant enterococci at a tertiary care centre. Med J Arm Forces India. 2015;71(2):139–44.
Boccella M, Santella B, Pagliano P, De Filippis A, Casolaro V, Galdiero M, et al. Prevalence and antimicrobial resistance of Enterococcus Species: a retrospective cohort study in Italy. Antibiotics. 2021. https://doi.org/10.3390/antibiotics10121552.
Bogut A, Mrozik P, Czaja G, Stawecka-Hamerla M. Analysis of the phenotypic and genotypic antimicrobial resistance profiles of clinically significant enterococci isolated in the provincial specialist hospital in Lublin, Poland. Curr Issues Pharm Med Sci. 2021;34(4):174–80. https://doi.org/10.2478/cipms-2021-0032.
Boost M, Lai L, O’Donoghue M. Drug resistance in fecal enterococci in Hong Kong. J Infect Chemother. 2004;10:326–30.
Brauers J, Kresken M, Hafner D, Shah P. Surveillance of linezolid resistance in Germany, 2001–2002. Clin Microbiol Infect. 2005;11(1):39–46.
Conceição N, Oliveira CdCHBd, Silva PRd, Ávila BGM, Oliveira AGD. Trends in antimicrobial resistance among clinical isolates of enterococci in a Brazilian tertiary hospital: a 4-year study. Revista da Soc Brasileira de Med Trop. 2011;44:177–81.
Coombs GW, Daley DA, Yee NWT, Shoby P, Mowlaboccus S. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2020. Commun Dis Intell. 2018;2022:46. https://doi.org/10.33321/cdi.2022.46.17.
Dadfarma N, Fooladi AAI, Oskoui M, Hosseini HM. High level of gentamicin resistance (HLGR) among enterococcus strains isolated from clinical specimens. J Infect Public Health. 2013;6(3):202–8.
Dar SF, Atif MM, Arshad MH, Hayat MF, Dar TF. Urinary tract pathogens and their patterns of resistance to commonly used antibiotics. Rawal Med J. 2020;45(1):22–6.
Dave VP, Pathengay A, Braimah IZ, Panchal B, Sharma S, Pappuru RR, et al. ENTEROCOCCUS ENDOPHTHALMITIS: clinical settings, antimicrobial susceptibility, and management outcomes. Retina. 2020;40(5):898–902. https://doi.org/10.1097/iae.0000000000002462.
De Lorenzis E, Alba AB, Cepeda M, Galan JA, Geavlete P, Giannakopoulos S, et al. Bacterial spectrum and antibiotic resistance of urinary tract infections in patients treated for upper urinary tract calculi: a multicenter analysis. Eur J Clin Microbiol Infect Dis. 2020;39(10):1971–81. https://doi.org/10.1007/s10096-020-03947-z.
Djahmi N, Boutet-Dubois A, Nedjai S, Dekhil M, Sotto A, Lavigne J-P. Molecular epidemiology of Enterococcus sp. isolated in a university hospital in Algeria. Scand J Infect Dis. 2012;44(9):656–62.
Dodson DS, Dominguez SR, MacBrayne CE, Williams MC, Parker SK. Vancomycin-nonsusceptible enterococci mediated by vanC at a large children’s hospital: prevalence, susceptibility, and impact on care of enterococcal bacteremia. Open Forum Infect Dis. 2020;7(5):160. https://doi.org/10.1093/ofid/ofaa160.
Dong D, Zheng Y, Chen Q, Guo Y, Yang Y, Wu S, et al. In vitro activity of omadacycline against pathogens isolated from Mainland China during 2017–2018. Eur J Clin Microbiol Infect Dis. 2020;39(8):1559–72. https://doi.org/10.1007/s10096-020-03877-w.
Dworniczek E, Piwowarczyk J, Bania J, Kowalska-Krochmal B, Wałecka E, Seniuk A, et al. Enterococcus in wound infections: virulence and antimicrobial resistance. Acta Microbiol Immunol Hung. 2012;59(2):263–9.
Emaneini M, Aligholi M, Aminshahi M. Characterization of glycopeptides, aminoglycosides and macrolide resistance among Enterococcus faecalis and Enterococcus faecium isolates from hospitals in Tehran. Pol J Microbiol. 2008;57(2):173–8.
Esfahani S, Ahmadrajabi R, Mollaei H, Saffari F. Co-incidence of type II topoisomerase mutations and efflux expression in high fluoroquinolone resistant Enterococcus faecalis isolated from urinary tract infections. Infect Drug Resist. 2020;13:553–9. https://doi.org/10.2147/idr.S237299.
Fajfr M, Balik M, Cermakova E, Bostik P. Effective treatment for uncomplicated urinary tract infections with oral fosfomycin, single center four year retrospective study. Antibiotics. 2020. https://doi.org/10.3390/antibiotics9080511.
Feizabadi MM, Aliahmadi A, Mobasheri F, Asgharzadeh A, Asadi S, Etemadi G. Phenotypic characteristics and population genetics of Enterococcus faecalis cultured from patients in Tehran during 2000–2001. Can J Microbiol. 2003;49(10):645–9. https://doi.org/10.1139/w03-082/M14663499.
Fereshteh J, Mohammad E, Shadi S, Hossein S, Zohreh A, Marzieh A. Evaluation of antimicrobial susceptibility patterns of Enterococci isolated from patients in Tehran University of Medical Sciences Teaching Hospitals. Acta Med Iran. 1970;47(4):325.
Fernandes SC, Dhanashree B. Drug resistance & virulence determinants in clinical isolatesof Enterococcus species. Indian J Med Res. 2013;137(5):981.
Flamm RK, Duncan LR, Hamed KA, Smart JI, Mendes RE, Pfaller MA. Ceftobiprole activity against bacteria from skin and skin structure infections in the united states from 2016 through 2018. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/aac.02566-19.
Folliero V, Caputo P, Della Rocca MT, Chianese A, Galdiero M, Iovene MR, et al. Prevalence and antimicrobial susceptibility patterns of bacterial pathogens in urinary tract infections in university hospital of campania “luigi vanvitelli” between 2017 and 2018. Antibiotics. 2020. https://doi.org/10.3390/antibiotics9050215.
Franyó D, Kocsi B, Bukta EE, Szabó J, Dombrádi Z. Assessing the intestinal carriage rates of vancomycin-resistant enterococci (VRE) at a tertiary care hospital in Hungary. Folia Microbiol. 2020;65(3):483–90. https://doi.org/10.1007/s12223-019-00751-x.
Friedman G, Stepensky P, Abu Ahmad W, Masarwa R, Temper V, Oster Y, et al. Enterococcal bacteremia in children with malignancies and following hematopoietic stem cell transplantation: a 15-year single-center experience. Pediatr Infect Dis J. 2020;39(4):318–24. https://doi.org/10.1097/inf.0000000000002579.
Gajdács M, Ábrók M, Lázár A, Burián K. Increasing relevance of gram-positive cocci in urinary tract infections: a 10-year analysis of their prevalence and resistance trends. Sci Rep. 2020;10(1):17658. https://doi.org/10.1038/s41598-020-74834-y.
Ghalavand Z, Alebouyeh M, Ghanati K, Azimi L, Rashidan M. Genetic relatedness of the Enterococcus faecalis isolates in stool and urine samples of patients with community-acquired urinary tract infection. Gut Pathog. 2020;12:42. https://doi.org/10.1186/s13099-020-00380-7.
Ghanem G, Hachem R, Jiang Y, Chemaly R, Raad I. Outcomes for and risk factors associated with vancomycin-resistant Enterococcus faecalis and vancomycin-resistant Enterococcus faecium bacteremia in cancer patients. Infect Control Hosp Epidemiol. 2007;28(9):1054–9.
Guo Y, Yang Y, Zheng Y, Wu S, Yin D, Zhu D, et al. Comparative in vitro activities of ceftaroline and tedizolid against clinical strains of staphylococcus aureus and enterococcus: results from the China antimicrobial surveillance network (CHINET) in 2018. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/aac.01461-20.
Haghi F, Lohrasbi V, Zeighami H. High incidence of virulence determinants, aminoglycoside and vancomycin resistance in enterococci isolated from hospitalized patients in Northwest Iran. BMC Infect Dis. 2019;19(1):744. https://doi.org/10.1186/s12879-019-4395-3.
Hällgren A, Abednazari H, Ekdahl C, Hanberger H, Nilsson M, Samuelsson A, et al. Antimicrobial susceptibility patterns of enterococci in intensive care units in Sweden evaluated by different MIC breakpoint systems. J Antimicrob Chemother. 2001;48(1):53–62.
Hasani A, Sharifi Y, Ghotaslou R, Naghili B, Hasani A, Aghazadeh M, et al. Molecular screening of virulence genes in high-level gentamicin-resistant Enterococcus faecalis and Enterococcus faecium isolated from clinical specimens in Northwest Iran. Indian J Med Microbiol. 2012;30(2):175–81. https://doi.org/10.4103/0255-0857.96687.
Heidari H, Emaneini M, Dabiri H, Jabalameli F. Virulence factors, antimicrobial resistance pattern and molecular analysis of enterococcal strains isolated from burn patients. Microb Pathog. 2016;90:93–7. https://doi.org/10.1016/j.micpath.2015.11.017.
Hope D, Ampaire L, Oyet C, Muwanguzi E, Twizerimana H, Apecu RO. Antimicrobial resistance in pathogenic aerobic bacteria causing surgical site infections in Mbarara regional referral hospital, Southwestern Uganda. Sci Rep. 2019;9(1):17299. https://doi.org/10.1038/s41598-019-53712-2.
Hsueh P-R, Chen W-H, Teng L-J, Luh K-T. Nosocomial infections due to methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci at a university hospital in Taiwan from 1991 to 2003: resistance trends, antibiotic usage and in vitro activities of newer antimicrobial agents. Int J Antimicrob Agents. 2005;26(1):43–9.
Inamdar D, Basavaraju A, Undi M. Epidemiological profile, speciation and antibiogram of enterococcus species in the era of resistance. Acta Microbiol Bulg. 2021;37:149–56.
Iseppi R, Di Cerbo A, Messi P, Sabia C. Antibiotic resistance and virulence traits in vancomycin-resistant enterococci (VRE) and extended-spectrum β-lactamase/AmpC-producing (ESBL/AmpC) enterobacteriaceae from humans and pets. Antibiotics. 2020. https://doi.org/10.3390/antibiotics9040152.
Jahansepas A, Sharifi Y, Aghazadeh M, Ahangarzadeh RM. Comparative analysis of Enterococcus faecalis and Enterococcus faecium strains isolated from clinical samples and traditional cheese types in the Northwest of Iran: antimicrobial susceptibility and virulence traits. Arch Microbiol. 2020;202(4):765–72. https://doi.org/10.1007/s00203-019-01792-z.
Jain S, Kumar A, Kashyap B, Kaur IR. Clinico-epidemiological profile and high-level aminoglycoside resistance in enterococcal septicemia from a tertiary care hospital in east Delhi. Int J Appl Basic Med Res. 2011;1(2):80.
Jannati E, Amirmozaffari N, Saadatmand S, Arzanlou M. Faecal carriage of high-level aminoglycoside-resistant and ampicillin-resistant Enterococcus species in healthy Iranian children. J Glob Antimicrob Resist. 2020;20:135–44. https://doi.org/10.1016/j.jgar.2019.06.022.
Jia W, Li G, Wang W. Prevalence and antimicrobial resistance of Enterococcus species: a hospital-based study in China. Int J Environ Res Public Health. 2014;11(3):3424–42.
Jiang G, Li J, Long H, Qiulin C, Jin R, Yaodong Y, et al. Study on risk factors, bacterial species, and drug resistance of acute pyelonephritis associated with ureteral stent after percutaneous nephrolithotomy. Eur J Clin Microbiol Infect Dis. 2021;40(4):707–13. https://doi.org/10.1007/s10096-020-04050-z.
Kaçmaz B, Aksoy A. Antimicrobial resistance of enterococci in Turkey. Int J Antimicrob Agents. 2005;25(6):535–8.
Kafil HS, Mobarez AM, Moghadam MF. Adhesion and virulence factor properties of Enterococci isolated from clinical samples in Iran. Indian J Pathol Microbiol. 2013;56(3):238.
Kapoor L, Randhawa V, Deb M. Antimicrobial resistance of enterococcal blood isolates at a pediatric care hospital in India. Jpn J Infect Dis. 2005;58(2):101–3.
Karmarkar M, Gershom ES, Mehta P. Enterococcal infections with special reference to phenotypic characterization & drug resistance. Indian J Med Res. 2004;119:22–5.
Kelesidis T, Humphries R, Uslan DZ, Pegues D. De novo daptomycin-nonsusceptible enterococcal infections. Emerg Infect Dis. 2012;18(4):674.
Kim JK, Nam KY, Chung IY, Jeung WJ, Kwon YH, Park JM, et al. Emerging Enterococcus isolates in postoperative endophthalmitis by selection pressure of fluoroquinolones: an 11-year multicenter and experimental study. Emerg Microb Infect. 2020;9(1):1892–9. https://doi.org/10.1080/22221751.2020.1810134.
Lee DS, Choe H-S, Lee SJ, Bae WJ, Cho HJ, Yoon BI, et al. Antimicrobial susceptibility pattern and epidemiology of female urinary tract infections in South Korea, 2010–2011. Antimicrob Agents Chemother. 2013;57(11):5384–93.
Li G, Hou S, Li Y, Liu S, Teng D, Hou D. Surveillance of gram-positive cocci infections and drug resistance. Cell Mol Biol. 2015;61(4):90–3.
Lin Z, Pu Z, Xu G, Bai B, Chen Z, Sun X, et al. Omadacycline efficacy against Enterococcus faecalis Isolated in China: in vitro activity, heteroresistance, and resistance mechanisms. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/aac.02097-19.
Liu Y, Cao B, Gu L, Wang H. Molecular characterization of vancomycin-resistant enterococci in a Chinese hospital between 2003 and 2009. Microb Drug Resist. 2011;17(3):449–55.
Luh K-T, Hsueh P-R, Teng L-J, Pan H-J, Chen Y-C, Lu J-J, et al. Quinupristin-dalfopristin resistance among gram-positive bacteria in Taiwan. Antimicrob Agents Chemother. 2000;44(12):3374–80.
Marzieh A, Mohammad E, Fereshteh J, Shadi S, Zohreh A, Hossein S, et al. Antibiotic susceptibility pattern of gram-positive cocci cultured from patients in three University Hospitals in Tehran, Iran during 2001–2005. Acta Med Iran. 1970;47(4):329.
Nicoletti G, Schito G, Fadda G, Boros S, Nicolosi D, Marchese A, et al. Bacterial isolates from severe infections and their antibiotic susceptibility patterns in Italy: a nationwide study in the hospital setting. J Chemother. 2006;18(6):589–602.
Norafika AN, Prihatiningsih S, Indriani DW, Indriati DW. A retrospective cross-sectional study of urinary tract infections and prevalence of antibiotic resistant pathogens in patients with diabetes mellitus from a public hospital in Surabaya, Indonesia. Germs. 2020;10(4):157–66. https://doi.org/10.1868/germs.2020.1201.
Oh WS, Ko KS, Song J-H, Lee MY, Park S, Peck KR, et al. High rate of resistance to quinupristin-dalfopristin in Enterococcus faecium clinical isolates from Korea. Antimicrob Agents Chemother. 2005;49(12):5176–8.
Olawale KO, Fadiora SO, Taiwo SS. Prevalence of hospital acquired enterococci infections in two primary-care hospitals in Osogbo, Southwestern Nigeria. Afr J Infect Dis. 2011. https://doi.org/10.4314/ajid.v5i2.66513.
Quiñones D, Goñi P, Rubio MC, Duran E, Gómez-Lus R. Enterococci spp. isolated from cuba: species frequency of occurrence and antimicrobial susceptibility profile. Diagn Microbiol Infect Dis. 2005;51(1):63–7.
Richter SS, Kealey DE, Murray CT, Heilmann KP, Coffman SL, Doern GV. The in vitro activity of daptomycin against Staphylococcus aureus and Enterococcus species. J Antimicrob Chemother. 2003;52(1):123–7. https://doi.org/10.1093/jac/dkg288.
Rostkowska OM, Kuthan R, Burban A, Salińska J, Ciebiera M, Młynarczyk G, et al. Analysis of susceptibility to selected antibiotics in klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis and Enterococcus faecium causing urinary tract infections in kidney transplant recipients over 8 years: single-center study. Antibiotics. 2020. https://doi.org/10.3390/antibiotics9060284.
Sader HS, Jones RN. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin surveillance program (2007–2008). Diagn Microbiol Infect Dis. 2009;65(2):158–62.
Sader HS, Watters AA, Fritsche TR, Jones RN. Daptomycin antimicrobial activity tested against methicillin-resistant staphylococci and vancomycin-resistant enterococci isolated in European medical centers (2005). BMC Infect Dis. 2007;7:1–9.
Saifi M, Dallal MS, Pourshafie M, Eshraghian M, Pourmand M, Salari M, et al. High level resistance of Enterococcus faecium and E. faecalis isolates from municipal sewage treatment plants to gentamicin. Iran J Public Health. 2008;37(1):103–7.
Salah AN, Elleboudy NS, El-Housseiny GS, Yassien MA. Cloning and sequencing of lsaE efflux pump gene from MDR Enterococci and its role in erythromycin resistance. Infect Genet Evol. 2021;94:105010. https://doi.org/10.1016/j.meegid.2021.105010.
Sibel A, Köroglu M, Muharrem A. The evaluation of antimicrobial susceptibility of urine enterococci with the Vitek 2 automated system in eastern Turkey. Southeast Asian J Trop Med Public Health. 2012;43(4):986–91.
Tollu G, Ekin IH. Biotyping and antimicrobial susceptibility of Enterococcus faecalis and E. faecium Isolated from urine and stool samples. Jundishapur J Microbiol. 2020;13(10):e105136. https://doi.org/10.5812/jjm.105136.
Udo EE, Al-Sweih N, Phillips OA, Chugh TD. Species prevalence and antibacterial resistance of enterococci isolated in Kuwait hospitals. J Med Microbiol. 2003;52(2):163–8.
Wang C, Li W, Gao J, Zhang D, Li Y, Li F, et al. Microbial predominance and antimicrobial resistance in a tertiary hospital in northwest china: a six-year retrospective study of outpatients and patients visiting the emergency department. Can J Infect Dis Med Microbiol. 2020;2020:8838447. https://doi.org/10.1155/2020/8838447.
Yasufuku T, Shigemura K, Shirakawa T, Matsumoto M, Nakano Y, Tanaka K, et al. Mechanisms of and risk factors for fluoroquinolone resistance in clinical Enterococcus faecalis isolates from patients with urinary tract infections. J Clin Microbiol. 2011;49(11):3912–6.
Zhang L, Li H, Gao J, Gao J, Wei D, Qi Y. Identification of drug-resistant phenotypes and resistance genes in Enterococcus faecalis isolates from animal feces originating in Xinjiang, People’s Republic of China. Can J Anim Sci. 2020;100(4):674–82. https://doi.org/10.1139/cjas-2018-0161.
Zouain M, Araj G. Antimicrobial resistance of enterococci in Lebanon. Int J Antimicrob Agents. 2001;17(3):209–13.
Smout E, Palanisamy N, Valappil SP. Prevalence of vancomycin-resistant Enterococci in India between 2000 and 2022: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2023;12(1):79. https://doi.org/10.1186/s13756-023-01287-z.
Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca-Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644–52. https://doi.org/10.1093/cid/cix411.
Azzam A, Elkafas H, Khaled H, Ashraf A, Yousef M, Elkashef AA. Prevalence of vancomycin-resistant enterococci (VRE) in Egypt (2010–2022): a systematic review and meta-analysis. J Egypt Public Health Assoc. 2023;98(1):8. https://doi.org/10.1186/s42506-023-00133-9.
Esmail MAM, Abdulghany HM, Khairy RM. Prevalence of multidrug-resistant Enterococcus faecalis in hospital-acquired surgical wound infections and bacteremia: concomitant analysis of antimicrobial resistance genes. Infect Dis. 2019;12:1178633719882929. https://doi.org/10.1177/1178633719882929.
Ahmadpoor N, Ahmadrajabi R, Esfahani S, Hojabri Z, Moshafi MH, Saffari F. High-level resistance to erythromycin and tetracycline and dissemination of resistance determinants among clinical enterococci in Iran. Med Princ Pract. 2021;30(3):272–6. https://doi.org/10.1159/000516216.
Mwikuma G, Kainga H, Kallu SA, Nakajima C, Suzuki Y, Hang’ombe BM. Determination of the prevalence and antimicrobial resistance of Enterococcus faecalis and Enterococcus faecium associated with poultry in four districts in Zambia. Antibiotics. 2023. https://doi.org/10.3390/antibiotics12040657.
Mussa EAM, Alsalahi A, Aljaberi MA, Jasni AS, Desa MNM, Al-Mahdi AYM, et al. Acquired tetracycline resistance genes by transposons and virulence factors in enterococci recovered from overland and aquatic animals: a systematic review. Rev Aquac. 2022;14(1):399–413.
Xin L, Xu X, Shi Q, Han R, Wang J, Guo Y, et al. High prevalence and overexpression of fosfomycin-resistant gene fos X in Enterococcus faecium from China. Front Microbiol. 2022;13:900185.
de Oliveira Araujo G, Huff R, Favarini MO, Mann MB, Peters FB, Frazzon J, et al. Multidrug resistance in enterococci isolated from wild pampas foxes (Lycalopex gymnocercus) and Geoffroy’s Cats (Leopardus geoffroyi) in the Brazilian Pampa Biome. Front Vet Sci. 2020;7:606377.
Petrakis V, Panopoulou M, Rafailidis P, Lemonakis N, Lazaridis G, Terzi I, et al. The impact of the COVID-19 pandemic on antimicrobial resistance and management of bloodstream infections. Pathogens. 2023;12(6):780.
Langford BJ, Soucy J-PR, Leung V, So M, Kwan AT, Portnoff JS, et al. Antibiotic resistance associated with the COVID-19 pandemic: a systematic review and meta-analysis. Clin Microbiol Infect. 2023;29(3):302–9.
Pérez Jorge G, dos Santos R, Goes IC, Gontijo MTP. Les misérables: a parallel between antimicrobial resistance and COVID-19 in underdeveloped and developing countries. Curr Infect Dis Rep. 2022;24(11):175–86. https://doi.org/10.1007/s11908-022-00788-z.
Zhao L, Lv Z, Lin L, Li X, Xu J, Huang S, et al. Impact of COVID-19 pandemic on profiles of antibiotic-resistant genes and bacteria in hospital wastewater. Environ Pollut. 2023;334:122133. https://doi.org/10.1016/j.envpol.2023.122133.
Sulayyim HJA, Ismail R, Hamid AA, Ghafar NA. Antibiotic resistance during COVID-19: a systematic review. Int J Environ Res Public Health. 2022. https://doi.org/10.3390/ijerph191911931.
Toc DA, Botan A, Botescu AMC, Brata VD, Colosi IA, Costache C, et al. A tale of two pandemics: antimicrobial resistance patterns of Enterococcus spp. in COVID-19 era. Antibiotics. 2023. https://doi.org/10.3390/antibiotics12020312.
Mareș C, Petca RC, Petca A, Popescu RI, Jinga V. Does the COVID pandemic modify the antibiotic resistance of uropathogens in female patients? A new storm? Antibiotics. 2022. https://doi.org/10.3390/antibiotics11030376.
Rizvi SG, Ahammad SZ. COVID-19 and antimicrobial resistance: a cross-study. Sci Total Environ. 2022;807(Pt 2):150873. https://doi.org/10.1016/j.scitotenv.2021.150873.
Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6(1):47. https://doi.org/10.1186/s13756-017-0208-x.
Sharma A, Singh A, Dar MA, Kaur RJ, Charan J, Iskandar K, et al. Menace of antimicrobial resistance in LMICs: current surveillance practices and control measures to tackle hostility. J Infect Public Health. 2022;15(2):172–81. https://doi.org/10.1016/j.jiph.2021.12.008.
Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare. 2023. https://doi.org/10.3390/healthcare11131946.
Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6(1):1–8.
Ikhimiukor OO, Odih EE, Donado-Godoy P, Okeke IN. A bottom-up view of antimicrobial resistance transmission in developing countries. Nat Microbiol. 2022;7(6):757–65. https://doi.org/10.1038/s41564-022-01124-w.
Shrestha S, Kharel S, Homagain S, Aryal R, Mishra SK. Prevalence of vancomycin-resistant enterococci in Asia—a systematic review and meta-analysis. J Clin Pharm Ther. 2021;46(5):1226–37.
Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. J Glob Infect Dis. 2019;11(1):36–42. https://doi.org/10.4103/jgid.jgid_110_18.
Hawkins O, Scott AM, Montgomery A, Nicholas B, Mullan J, van Oijen A, et al. Comparing public attitudes, knowledge, beliefs and behaviours towards antibiotics and antimicrobial resistance in Australia, United Kingdom, and Sweden (2010–2021): a systematic review, meta-analysis, and comparative policy analysis. PLoS ONE. 2022;17(1):e0261917.
Rödenbeck M, Ayobami O, Eckmanns T, Pletz MW, Bleidorn J, Markwart R. Clinical epidemiology and case fatality due to antimicrobial resistance in Germany: a systematic review and meta-analysis, 1, January 2010 to 31 December 2021. Euro Surveill. 2023. https://doi.org/10.2807/1560-7917.Es.2023.28.20.2200672.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
LG, MB, LW, TN, contributed to the conception, design, drafting of the work. SM, FMT, ZT, MAM participated in the design of the study, data extraction and performed the statistical analysis. MAM and MS participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable in this section.
Competing interests
Authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guan, L., Beig, M., Wang, L. et al. Global status of antimicrobial resistance in clinical Enterococcus faecalis isolates: systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 23, 80 (2024). https://doi.org/10.1186/s12941-024-00728-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-024-00728-w