Abstract
Background
Proteus mirabilis is a significant nosocomial pathogen that is frequently associated with a wide range of infections, necessitating heightened attention to mitigate potential health risks. Hence, this study was performed to investigate the impact of sub-minimum inhibitory concentrations (MICs) of ciprofloxacin (CIP) on Proteus mirabilis clinical isolates.
Methods
The sub-MICs of CIP were selected using the growth curve approach. The untreated and treated isolates with sub-MICs of CIP were assessed for their biofilm development, motilities on agar, and other virulence factors. The cell morphology of untreated and treated isolates with sub-MIC of CIP was explored using electron microscope. Moreover, the expression levels of the virulence genes in isolates were measured using quantitative real-time PCR.
Results
Data revealed that sub-MICs of CIP significantly (p < 0.05), in a concentration-dependent manner, inhibited biofilm formation and other virulence factors in the selected isolates. Electron microscope analysis showed cell enlargement and various abnormalities in the cell wall and membrane integrity.
Conclusion
Sub-MICs of CIP exhibited inhibition of virulence and alterations in morphological integrity against P. mirabilis isolates.
Similar content being viewed by others
Background
Proteus mirabilis is a Gram-negative bacterium that belongs to the family Enterobacteriaceae. It is well-known for its urease production and distinctive ability to swarm, which looks like a bull's-eye on agar plates [1]. It is an opportunistic pathogen that can cause a wide range of infections in humans, including those of the wounds, the eyes, neonatal meningoencephalitis, empyema, osteomyelitis, respiratory system, and gastrointestinal tract, though it is most commonly associated with urinary tract infections. [2]. The pathogenicity of P. mirabilis is mainly related to virulence factors such as fimbriae, flagella, toxins like hemolysin, and extracellular enzymes like urease and protease, in addition to crystalline biofilm formation [3].
Ciprofloxacin (CIP) is a renowned second-generation, broad-spectrum fluoroquinolone antibiotic with potent bactericidal activity against clinically important bacteria that cause a range of diseases, particularly UTIs [4]. Moreover, CIP has better bioavailability as well as enhanced pharmacokinetic and pharmacodynamic properties [5]. Indeed, the World Health Organization has designated ciprofloxacin as a critically important antibiotic [6].
Antibiotics are still the most effective means of controlling bacterial pathogens. To exert their effect successfully, antibiotics should be taken in successive doses at a concentration above the minimum inhibitory concentration (MIC) [7]. However, they only exceed the MIC during treatment for a certain period, after which it transiently falls below the MIC because of the pharmacokinetics of the antibiotics until the subsequent administration restarts the cycle [8]. Additionally, bacteria may encounter sub-MICs due to the use of antibiotics in livestock farming and agriculture, drug-drug interactions, an unlikely dose regimen, and certain clinical or health conditions of the patient that may decrease the bioavailability of antibiotics [9].
Antibiotics at these concentrations (sub-MICs) are not lethal, but they can affect bacteria in a variety of ways, including affecting biofilm formation, inducing morphological changes, influencing the cell surface structure, impacting motility as well as enzyme and toxin production, and altering bacterial adhesion to host cells [10]. Therefore, it is of clinical significance to expand our current understanding of the effects of sub-MICs of antibiotics. The aim of this study was to explore how sub-MICs of CIP affect P. mirabilis virulence, fine and ultrastructure, motility, and biofilm formation.
Materials and methods
Isolation and preservation of P. mirabilis
Clinical isolates were collected from patients admitted to different departments of Tanta University Hospitals over a period from November 2020 to September 2021. The identification of the isolates was conducted through microscopical and routine biochemical techniques [11]. Proteus mirabilis ATCC 35659 was used as a reference strain. The isolates with confirmed identity were preserved at – 70 °C in a Luria–Bertani (LB) broth with 25% v/v glycerol [11].
Antimicrobial susceptibility testing
Clinical isolates susceptibility pattern to various antibiotics was determined using the Kirby-Bauer disc diffusion method [12], and the results were interpreted in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [13]. Escherichia coli ATCC 25922 was used as the control strain. The antibiotic disks used (Oxoid, UK) were gentamicin, tobramycin, piperacillin/tazobactam, imipenem, cephazolin, cefoxitin, cefotaxime, cefepime, ciprofloxacin, trimethoprim/sulfamethoxazole, aztreonam, ampicillin, amoxycillin/clavulanic acid, and doxycycline.
Phenotypic screening of virulence factors
Virulence determinants of isolates were screened for the identification of the most virulent strains showing strong biofilm formation and positive for urease, protease, and hemolysin production as follows:
Biofilm production
The isolates were cultured in 96-well plates containing tryptic soy broth (TSB) and 1% glucose for 24 h at 37 °C. A negative control containing TSB only was also performed. The plates were washed twice with phosphate-buffered saline (PBS) after discarding the media. The resulting biofilms were fixed with 150 μl of methanol for 20 min, then stained for 15 min with 0.1% crystal violet, followed by rinsing with PBS. The residual biofilms were solubilized using 33% (v/v) glacial acetic acid. The optical density (OD) was measured at 570 nm by a microplate reader (Sunrise Tecan, Grodig, Austria) [14]. Isolates were then classified as negative, weak, moderate, or strong biofilm producers [15].
Enzymes and toxin production
The isolates were inoculated into urea broth for urease detection and incubated at 37 °C for 24 h until a color change to magenta (pink) could be observed [16]. Protease was detected on Mueller–Hinton agar containing 3% skimmed milk. After 48 h of incubation at 37 °C, plates were examined to evaluate the formation of lysis zones around the inoculated bacteria [16]. Hemolysin was detected on Columbia blood agar plates. After incubation at 37 °C for 48 h, the zone of hemolysis could be observed [16].
Determination of minimum inhibitory concentration (MIC)
The agar dilution assay based on CLSI guidelines [12, 13] was used to determine the MIC values of ciprofloxacin (CIP) against P. mirabilis isolates. E. coli ATCC 25922 was used as the control strain.
Growth curve assay
The sub-MICs of CIP for all further experiments were selected by plotting the growth curve using a spectrophotometric method [17]. Briefly, isolates were cultured in the absence or presence of CIP (at 1/2, 1/4, or 1/8 MICs) at 37 °C. At specific time intervals, an aliquot was collected from each culture, and the absorbance was read at 600 nm.
Biofilm assay
The impact of CIP was evaluated against biofilm-producing P. mirabilis isolates using a crystal violet assay, as described previously [14]. CIP was introduced to TSB containing bacteria of 106 CFU/ml at sub-MICs (1/4 or 1/8 MICs), and culture without adding any antibiotics was used as a control [18]. The formula used to determine the percent reduction in biofilm formation was:
Motility assay
For the swarming assay, a 0.5 McFarland solution of P. mirabilis overnight culture was centrally inoculated on dried LB agar (1.5%) plates in the absence or presence of CIP (at 1/4 or 1/8 MICs). The plates were incubated overnight at 37 °C, and the swarming zones were measured in mm [19]. Similarily, the isolates were stabbed into the center of the dried LB agar (0.4%) plates with or without CIP (at 1/4 or 1/8 MICs). The plates were incubated overnight at 37 °C, and the swimming zones were measured in mm [19].
Urease assay
The isolates were cultured in LB broth overnight at 37 °C with or without CIP (at 1/4 or 1/8 MICs). Following that, the isolates were centrifuged, re-suspended in PBS, and adjusted to 1.0 at OD600. Then, 100 µl of each bacterial suspension was inoculated into a test tube containing 9.5 ml of Christensen’s medium in liquid form and 0.5 ml of urea (at 40%) and incubated at 37 °C for 3 h. After incubation, bacterial cells were centrifuged, and the change in the color of the supernatant was measured at 570 nm [20]. The percent reduction in enzyme production was calculated as previously mentioned.
Protease assay
The isolates were cultured overnight at 37 °C in LB broth with or without CIP (at 1/4 or 1/8 MICs). The culture supernatant (1 ml) was mixed with an equal volume of 1% (w/v) casein in 0.1 M sodium phosphate buffer (pH 7.0). The mixture was then incubated for 10 min at 30 °C before being terminated with the addition of 2 ml of 0.4 M trichloroacetic (TCA) acid. The mixture was then incubated at room temperature for 30 min before being centrifuged at 10,000 rpm for 5 min. The supernatant (1 ml) was then mixed with 5 ml of 0.4 M Na2CO3 for 10 min, and then 1 ml of Folin’s reagent mixed with 3 ml of distilled water (1:3 v/v) was added. The mixture was left to stand at 30 °C for 30 min before the OD at 660 nm was measured [21]. The percent reduction in enzyme production was calculated as previously mentioned.
Hemolysin assay
Isolates were grown overnight at 37 °C in LB broth with or without CIP (at 1/4 or 1/8 MICs). Following centrifugation and filtration of the supernatant, 600 µl was combined with 600 µl of a 2% suspension of red blood cells (RBCs) in saline and incubated at 37 °C for 2 h. The suspension was then centrifuged for 8 min at 4 °C at 10,000 rpm, and the released hemoglobin was determined by measuring absorbance at 540 nm. In the same conditions, a negative control of erythrocytes in LB broth and a positive control of totally hemolyzed erythrocytes by adding sodium dodecyl sulfate (0.1%) were employed [22]. The percentage of hemolysis inhibition was calculated as follows:
Fine and ultrastructure investigation
A representative isolate (code P17) was cultured overnight at 37 °C in LB broth with and without a sub-MIC of CIP. Then, the solutions (treated and control) were washed with PBS three times, and the final pellets were processed for further examination. The final pellets were resuspended, fixed with 2.5% glutaraldehyde in PBS buffer (pH 7.4) for 2 h at room temperature, and then postfixed with 1% OsO4 in PBS buffer (pH 7.4) for 1 h at 4 °C. A droplet of each bacterial suspension was placed on the microscope slide, then dehydrated with ethanol, and air-dried. The slides were then mounted on metal stubs and sputter-coated with gold, then examined using a scanning electron microscope (SEM) (Akashi Seisakusho, Japan) [23]. The final pellets were fixed for 24 h in 2.5% glutaraldehyde in PBS (pH 7.4), rinsed with the same buffer, and then postfixed for 2 h in 1% OsO4 in PBS (pH 7.4). The samples were then dehydrated with ethanol and embedded in Epon 812 epoxy resin. Ultrathin sections were cut on an ultramicrotome, which were then double stained with uranyl acetate and Reynolds lead citrate, then examined using a transmission electron microscope (TEM) (Jeol-1200 ECII, Japan) [23].
Relative gene expression analysis
The total RNA was extracted from untreated and ciprofloxacin-subMIC-treated P. mirabilis isolates using the PureLink® RNA Mini Kit (Thermo Scientific, USA). The yield and purity of the RNA extracted were measured using a NanoDrop spectrophotometer (ThermoFisher Scientific, USA). Then, RNA was reverse transcribed into cDNA using power cDNA synthesis kit (iNtRON Biotechnology, Korea). To analyze the expression pattern of selected genes in the absence and presence of sub-MIC of ciprofloxacin, qPCR was carried out using Power SYBR® Green PCR Master Mix (ThermoFisher Scientific, USA) in Rotor-Gene Q (Qiagen, USA). The expression of selected genes using specific primers (Table 1) was normalized by the housekeeping 16s rRNA gene, and the relative gene expression was determined by the 2−ΔΔCt method [24]. A PCR mixture without a template was used as a negative control. Only genes with a relative 2−ΔΔCt value above 1.0 or below 1.0 were considered significant [25].
In-vivo wound infection model
The Research Ethics Committee (Faculty of Pharmacy, Tanta University, Egypt) approved the following protocols while they followed the standard rules for handling and caring for laboratory animals (TP/RE/2/24 p-01). The procedures were established as previously performed [30]. Male BALB/c mice (n = 30) were obtained from the Cairo University College of Veterinary Medicine's animal house (Cairo, Egypt). Mice weighed 120–150 g and were 6–8 weeks old at the time of the study. Following the creation of the wound infection, all mice were housed as individuals in a ventilated cage to prevent fighting and cross-contamination. They were supplied with free access to food and filtered water and were kept on a 12 h light/dark cycle at room temperature. Three groups of mice (10 mice each) were assigned at random. The control group, Group I, was not treated with any materials. Groups II and III were given bacteria and treated with 1/4 or 1/8 MICs of CIP, respectively. Under xylazine (5 mg/kg) and ketamine (40 mg/kg) anaesthesia, the backs of all mice were shaved and sterilized with 10% povidone-iodine. A nearly 10-mm circular incision was made on the dorsal inter-scapular region of each animal. The wounds were infected with 10 μl of the bacterial suspension in PBS (106 CFU). Following 30 min of inoculation, 20 μl of the vehicle (PBS) was injected subcutaneously in the control group I, and CIP (at 1/4 or 1/8 MICs) was injected subcutaneously in the treated groups II and III, respectively.
On the sixth day of the experiment, mice were anesthetized with isoflurane and were euthanized by cervical dislocation (CD) according to the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020 Edition). Hematoxylin–eosin (H&E) staining was employed for histopathological examination of the skin lesions.
Statistical analysis
A one-way ANOVA was employed for comparison. A p-value < 0.05 was considered statistically significant. GraphPad Prism version 5 software was used to create graphs and run statistical tests. All experiments were done in triplicate, and the results were expressed as mean ± SD.
Results
Identification of P. mirabilis isolates
A total of 60 isolates were recovered from urine (35/60, 58.3%), followed by wounds (13/60, 21.6%), blood (7/60, 11.6%), and sputum (5/60, 8.3%).They were identified as Gram-negative rods. They produced non-lactose-fermenting colonies on MacConkey’s agar, swarming on nutrient agar, and hydrogen sulfide from Kligler’s iron agar. They were positive for urease and phenylalanine deaminase production and negative for indole production.
Antimicrobial susceptibility patterns
P. mirabilis isolates (n = 60) showed variable degrees of resistance pattern to the representative antibiotics used. The percentages of antimicrobial resistance were as follows: gentamycin (40%), tobramycin (13.3%), piperacillin + taobzactam (28.3%), imipenem (0%), cefazolin (83.3%), cefotaxime (93.3%), cefepime (11.7%), cefoxitin (4%), ciprofloxacin (76.7%), trimethoprim-sulfamethoxazole (93.3%), Aztreonam (93.3%), ampicillin (85%), amoxicillin + lavulanic acid (55%), doxycycline (78.3%).
Phenotypic determination of virulence factors
The phenotypic characterization of the virulence factors showed that 45 out of 60 isolates (75%) had the capacity to produce biofilm, including 11 isolates (18.3%), which were strong biofilm producers, 28 isolates (46.6%), which were moderate biofilm producers, and the rest (6 isolates) (10%), which were weak producers. All the isolates (n = 60) showed motility and were positive for urease production, while 10 and 8 isolates (16.6% and 13.3%) showed positivity for protease and hemolysin production, respectively. The positive isolates (n = 8) for all virulence determinants and strong biofilm producers were selected for further investigations.
MIC determination
The MICs of CIP against P. mirabilis isolates (n = 8) were determined according to CLSI guidelines, and the results obtained are presented in Table 2. The MIC values ranged from 2 to 16 μg/ml.
Growth curve assay
The growth of P. mirabilis isolates with and without CIP (1/2, 1/4, or 1/8 MICs) was tested at OD600 (Fig. 1). According to the obtained growth curves, 1/2 MIC of CIP greatly affected the growth rate of the isolates, while 1/4 or 1/8 MICs of CIP had negligible effects on the growth of the isolates, and so they were selected for further experiments.
The effect of sub-MICs of ciprofloxacin on biofilm formation
CIP at sub-MICs (1/4 or 1/8 MICs) significantly (p < 0.05) reduced biofilm development in all P. mirabilis isolates in a concentration-dependent manner. The reduction was higher at 1/4 MIC, with percent ranging from 47.3% to 70.9%, while at 1/8 MIC, it ranged from 16.9% to 47.2% (Fig. 2).
Concentration-dependent inhibition of biofilm formation of P. mirabilis isolates by treatment with sub-MICs of CIP using crystal violet assay. A The scatter plot indicates the percentage of reduction in biofilm formation after treatment with 1/4 and 1/8 MIC of CIP. B A representative image of crystal violet-stained biofilm of P. mirabilis isolate before and after treatment at different concentrations in a microtiter plate. The results were the mean of three experiments. The error bars indicate standard deviations. The asterisks represent statistical significance (p < 0.05)
The effect of sub-MICs of ciprofloxacin on motility
The impact of CIP at 1/4 MIC or 1/8 MIC showed a significant (p < 0.05) inhibition of the swarming and swimming motilities of all P. mirabilis isolates while having no discernible influence on growth (Fig. 3). Swarming motility was suppressed by (81.8–86.3%) at 1/4 MIC and (75.49–84%) at 1/8 MIC, whereas swimming motility was inhibited by (60–72.8%) at 1/4 MIC and (54.3–62.4%) at 1/8 MIC.
Inhibition of P. mirabilis motility by sub-MICs of CIP. A, C The scatter plots indicate the percentage of reduction in swarming and swimming motilities, respectively. B Representative images of the effect of CIP at 1/4 and 1/8 MIC on swarming motility compared to control (no CIP). D Representative images of the effect of CIP at 1/4 and 1/8 MIC on swimming motility compared to control (no CIP). The results were the mean of three experiments. The error bars indicate standard deviations. The asterisks represent statistical significance (p < 0.05)
The effect of sub-MICs of ciprofloxacin on virulence enzymes and toxins
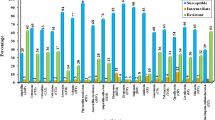
The production of urease, protease, and hemolysin was attenuated in all P. mirabilis isolates in a concentration-dependent way when treated with CIP at sub-MICs (Fig. 4). For urease production, it was significantly (p < 0.05) reduced by (36.7–70.6%) at 1/4 MIC and by (16.9–46.8%) at 1/8 MIC. For protease production, it was suppressed significantly (p < 0.05) by (21.5–69.4%) at 1/4 MIC and by (18.5–43.9%) at 1/8 MIC. For hemolysin production, it exhibited a significant (p < 0.05) reduction ranging from 31.8 to 69.9% at 1/4 MIC. However, it did not exhibit a statistically significant reduction at 1/8 MIC.
Concentration-dependent inhibition of P. mirabilis virulence. The scatter plots indicate the percentage of reduction in (A) urease, (B) protease, and (C) hemolysin production after treatment with 1/4 and 1/8 MIC of CIP. The results were the mean of three experiments. The error bars indicate standard deviations. The asterisks represent statistical significance (p < 0.05)
The impact of sub-MICs of ciprofloxacin on cell morphology
By exposing the representative isolate (code P17) to 1/4 MIC of CIP, there was enlargement in some cells with irregular morphology showed by SEM compared to untreated cells. Also, the TEM investigation showed that nuclear morphology changes appear as some mesosome-like structures and non-membrane-enclosed bodies are formed with changes in the cell wall and cell membrane integrity (Figs. 5).
The impact of sub-MIC of ciprofloxacin on gene expression
Quantitative real-time PCR was used to study the effect of ciprofloxacin (at 1/4 MIC) on the three representative isolates (code P17, P18, and P38) that were most affected in the previous assays. The mRNA expression levels of treated samples were calibrated relative to the control group (in the absence of CIP). The expression of genes was significantly (p < 0.05) affected in the three isolates (Fig. 6). The data showed the downregulation of flhDC, mrpA, ureC, zapA, and hmpA genes. It also revealed the upregulation of rsmA.
Histopathological examination of wound infection
The wound-healing potential of sub-MICs of CIP was also evaluated using H&E stains, as shown in Fig. 7. The histological examination of tissues in the control group, which was infected with P. mirabilis and untreated, revealed extensive tissue damage, skin ulceration accompanied by intense inflammation (acute and chronic inflammatory cells), and the presence of necrotic debris without any epithelization. The histopathological assessment of the treated groups, infected with P. mirabilis and treated with sub-MICs of CIP, revealed moderate to complete epithelization accompanied by underlying granulation tissue and few inflammatory cells.
Representative photomicrograph of different treatment groups. A, B Control untreated group showing replacement of ulcertaed skin area with large esinophilic scab (stars) invaded with many inflammatory cells including many neutrophils (thin arrows). Many inflammatory cells admixed with many necrotic epidermal cells filling the wound gap. C Control untreated group showing closure of wound area with reepithelized epidermal cells and angiogenesis (thin arrow). Many epidermal cells showing vacuolation (arrowhead) with acanthosis (thick arrow) and epidermal layer covered with thick esinophilic crust invaded with inflammatory cells (star). D 1/4 MIC-treated group showing complete regeneration of epidermis and dermis with normal hair follicle and sebaceous gland. E, F 1/8 MIC-treated group showing restoration of epidermal layer with either mild acanthosis (thick arrow) or epidermal vacuolation (arrowhead) with mild dermal inflammatory cells (thin arrow), see inset image. Image magnification = 100×, inset = 400×
The scoring of wounds demonstrated that the application of 1/4 MIC or 1/8 MIC of CIP resulted in enhanced wound angiogenesis, proliferation of fibroblasts, activation of collagen deposition-activated hair follicles, epidermal regeneration, and a reduction in the infiltration of inflammatory cells and scab formation, as indicated in Table 3.
Discussion
Proteus mirabilis is one of the most prevalent causes of nosocomial infections. It plays a crucial role in urinary tract infections (UTIs). It is third in causing complicated UTIs, following E. coli and Klebsiella pneumoniae, and second in causing catheter-associated bacteriuria, following Providencia stuartii, in long-term catheterized patients [31]. It is also a significant contributor to the development of wound infections and was reported as a prominent Gram-negative isolate in wound infections [32, 33]. Its dissemination is due to the presence of a variety of virulence factors, including peritrichous flagella, fimbriae, urease, protease, hemolysin, and a substantial capacity to form biofilms [31]. We focused on the fluoroquinolone ciprofloxacin which is used worldwide in both human and animal sectors, and therefore has both clinical and environmental effects.
Biofilm formation enables organisms to survive harsh environments and renders them more resistant (10–1,000 times higher) to drugs as well as the immune system of the host [34]. The process of biofilm development in P. mirabilis encompasses a series of interconnected processes that collectively facilitate the formation of biofilms, involving the expression of adhesive proteins (in particular MR/P fimbriae), swarming motility, and urease production [1]. In addition, the seriousness of P. Mirabilis infection depends on flagellar motility and other virulence determinants that could affect the pathogenesis through adherence to epithelial and catheter surfaces, stone formation, cell invasion and cytotoxicity, and histological damage, as well as immune evasion [1]. In this work, we employed CIP at sub-MIC levels that had negligible influence on growth. According to our findings, sub-MIC of CIP significantly reduced biofilm formation, and other virulence determinants in a concentration-dependent way.
These findings are consistent with the relative gene expression levels determined by quantitative real-time PCR. Sub-MIC of CIP-treated isolates demonstrated considerable down-regulation of examined genes when compared to untreated control isolates. P. mirabilis motility is mediated through the class 1 flagellar master regulator gene, flhDC (flagellar transcriptional activator) [35]. CIP at sub-MIC down-regulated the relative expression of flhDC. The MR/P (mannose-resistant Proteus-like) fimbria is one of the most well-studied and important adherence structures produced by P. mirabilis [36]. Our findings showed the down-regulation of mrpA encoding the main pilin subunit. In addition, our findings showed down-regulation of the genes encoding other virulence, as follows: ureC (encoding major urease structural subunit), zapA (encoding protease enzyme), and hmpA (encoding hemolysin toxin) when exposed to CIP at sub-MIC.
Furthermore, our molecular data showed the up-regulation of the gene rsmA, a repressor of secondary metabolites, which is an important part of a global regulatory system controlling the expression of many genes in the stationary phase. Increased expression of rsmA in P. mirabilis inhibits swarming, differentiation of swarmer cells, and the expression of virulence factors, including hemolysin, protease, urease, and flagellin [37], as well as biofilm formation [26, 37].
Considering the observed down-regulation of flhDC, mrpA, ureC, zapA, and hmpA, along with the up-regulation of rsmA, besides the conducted phenotypic studies, it can be understood why the sub-MICs of ciprofloxacin attenuated virulence factors and biofilm development. A noteworthy previous study [38] has established a correlation between swarming behaviour and the expression of virulence characteristics, specifically invasion and the production of hemolysin, urease, and protease. Hence, the inhibition of swarming motility and the down-regulation of flagellar transcriptional activator could be associated with the inhibition and down-regulation of virulence factors.
In agreement with our study, Wojnicz et al. revealed that sub-MICs of ciprofloxacin showed inhibition of adherence and attachment capabilities to epithelial cells [39]. Additionally, Drago et al. documented that quinolones at sub-MICs exert anti-adherence activity [40]. Also, Abdullah et al. reported that ciprofloxacin at sub-MICs exerts anti-urease activity on P. mirabilis, which is a cornerstone in pathogenicity and biofilm formation [41]. Furthermore, Gupta et al. proved that exposure to a sub-MIC of ciprofloxacin inhibits the production and expression of virulence like protease and hemolysin [42]. In addition, Dong et al. and Gümüş et al. demonstrated that ciprofloxacin at sub-MICs reduced the relative expression levels of virulence genes [17, 43]. Horii et al. reported that the sub-MIC of mupirocin dose-dependently suppressed bacterial motility and flagella formation in P. mirabilis with reduced flagellin expression [44]. As well, Kawamura-Sato showed, based on molecular analysis, that the inhibition of motility by sub-MICs of macrolides in P. mirabilis was well correlated with reduced expression of flagellin [45]. Besides, Roudashti et al. showed that the sub-MICs of ciprofloxacin significantly reduced motility expression and biofilm formation [46]. Over and above that, there are previous studies that reported a significant reduction in the biofilm formation of P. mirabilis when exposed to sub-MICs of ciprofloxacin [47,48,49].
Moreover, bacteria could adapt to stress in their environment by altering their morphology and ultrastructure. We studied the effect of CIP at sub-MIC using SEM and TEM, which revealed changes in cell size, shape, and cell wall integrity. These findings agree with a previous study by Zhanel et al. [50]. Also, Kwon and Lee showed that exposure to sub-MICs has been shown to induce changes in cell morphology, which may directly interfere with the expression of virulence [51].
Finally, we investigated the impact of CIP at 1/4 or 1/8 MIC on the process of wound healing in mice. The findings of our study revealed that CIP had a positive influence on promoting wound healing. This was determined by histopathological examination, which also demonstrates an increase in epidermal regeneration, collagen deposition, granulation tissue creation, and hair follicles while simultaneously reducing inflammation. Zhanel et al. also observed that subinhibitory concentrations of antimicrobials resulted in reduced bacterial counts, lower histological injury, and longer survival rates compared to the control group [50]. Our findings align with studies that concluded that subinhibitory concentrations of antimicrobials have protective effects in animal models, possibly involving the modulation of virulence factors, a reduction in bacterial adhesion, and decreased infectivity [50].
Conclusion
The application of ciprofloxacin at sub-minimal inhibitory concentrations (sub-MICs), which exhibited noteworthy concentration-dependent inhibitory effects on the formation of biofilms in vitro, as well as the reduction of virulence factors such as motility, enzymes, and toxin production. Our findings illustrated the significance of selecting antibiotics with care and caution, taking their concentrations seriously into consideration, particularly ciprofloxacin, in order to treat P. mirabilis infections and enhance patient outcomes.
Data availability
All data generated or analyzed during this study are included in this article.
References
Armbruster CE, Mobley HL, Pearson MM. Pathogenesis of Proteus mirabilis infection. EcoSal Plus. 2018;8(1):10–128.
Schaffer JN, Pearson MM. Proteus mirabilis and urinary tract infections. Urinary Tract Infections Molecular Pathogenesis and Clinical Management. 2nd ed. Washington, DC. Am Soc Microbiol; 2017: 383–433.
Armbruster CE, Mobley HL. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol. 2012;10(11):743–54.
Castro W, Navarro M, Biot C. Medicinal potential of ciprofloxacin and its derivatives. Future Med Chem. 2013;5(1):81–96.
Bush NG, Diez-Santos I, Abbott LR, Maxwell A. Quinolones: mechanism, lethality, and their contributions to antibiotic resistance. Molecules. 2020;25(23):5662.
WHO. Critically Important Antimicrobials for Human Medicine, World Health Organisation—WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR): Geneva, 2017, 5th edn. p. 48.
Narimisa N, Amraei F, Kalani BS, Mohammadzadeh R, Jazi FM. Effects of sub-inhibitory concentrations of antibiotics and oxidative stress on the expression of type II toxin–antitoxin system genes in Klebsiella pneumoniae. J Global Antimicrob Resist. 2020;21:51–6.
Braga PC, Sasso MD, Sala MT. Sub-MIC concentrations of cefodizime interfere with various factors affecting bacterial virulence. J Antimicrob Chemother. 2000;45(1):15–25.
Chen J, Zhou H, Huang J, Zhang R, Rao X. Virulence alterations in staphylococcus aureus upon treatment with the sub-inhibitory concentrations of antibiotics. J Adv Res. 2021;31:165–75.
Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12(7):465–78.
Collee JG, Miles RS, Watt B. Tests for identification of bacteria. Mackie McCartney Pract Med Microbiol. 1996;14:131–49.
CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI standard M07. Wayne, PA: Clinical and Laboratory Standards Institute. 11th edn. 2018.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. Clinical and Laboratory Standards Institute. 31st edn. 2021.
Stepanović S, Vuković D, Hola V, Bonaventura GD, Djukić S, Ćirković I, Ruzicka F. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115(8):891–9.
Piechota M, Kot B, Frankowska-Maciejewska A, Grużewska A, Woźniak-Kosek A. Biofilm formation by methicillin-resistant and methicillin-sensitive Staphylococcus aureus strains from hospitalized patients in Poland. BioMed Res Int. 2018;2018:1–7.
Ricci A, Coppo E, Barbieri R, Debbia EA, Marchese A. The effect of sub-inhibitory concentrations of rifaximin on urease production and on other virulence factors expressed by Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus. J Chemother. 2017;29(2):67–73.
Dong G, Li J, Chen L, Bi W, Zhang X, Liu H, Zhi X, Zhou T, Cao J. Effects of sub-minimum inhibitory concentrations of ciprofloxacin on biofilm formation and virulence factors of Escherichia coli. Brazil J Infect Dis. 2019;23:15–21.
Balaji K, Thenmozhi R, Pandian SK. Effect of subinhibitory concentrations of fluoroquinolones on biofilm production by clinical isolates of Streptococcus pyogenes. Indian J Med Res. 2013;137(5):963.
Liaw SJ, Lai HC, Ho SW, Luh KT, Wang WB. Inhibition of virulence factor expression and swarming differentiation in Proteus mirabilis by p-nitrophenylglycerol. J Med Microbiol. 2000;49(8):725–31.
Nicolosi D, Tempera G, Genovese C, Furneri PM. Anti-adhesion activity of A2-type proanthocyanidins (a cranberry major component) on uropathogenic E. coli and P. mirabilis strains. Antibiotics. 2014;3(2):143–54.
Chimbekujwo KI, Ja’afaru MI, Adeyemo OM. Purification, characterization, and optimization conditions of protease produced by Aspergillus brasiliensis strain BCW2. Sci Afr. 2020;8: e00398.
Rossignol G, Merieau A, Guerillon J, Veron W, Lesouhaitier O, Feuilloley MG, Orange N. Involvement of a phospholipase C in the hemolytic activity of a clinical strain of Pseudomonas fluorescens. BMC Microbiol. 2008;8(1):1–4.
Wojnicz D, Kłak M, Adamski R, Jankowski S. Influence of subinhibitory concentrations of amikacin and ciprofloxacin on morphology and adherence ability of uropathogenic strains. Folia Microbiol. 2007;52:429–36.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8.
Wasfi R, Abdellatif GR, Elshishtawy HM, Ashour HM. First-time characterization of viable but non-culturable Proteus mirabilis: induction and resuscitation. J Cell Mol Med. 2020;24(5):2791–801.
Shokouhfard M, Kermanshahi RK, Feizabadi MM, Teimourian S, Safari F. Lactobacillus spp. derived biosurfactants effect on expression of genes involved in Proteus mirabilis biofilm formation. Infect Genet Evol. 2022;100:105264.
Barbour EK, Hajj ZG, Hamadeh S, Shaib HA, Farran MT, Araj G, Faroon O, Barbour KE, Jirjis F, Azhar E, Kumosani T. Comparison of phenotypic and virulence genes characteristics in human and chicken isolates of Proteus mirabilis. Pathogens Global Health. 2012;106(6):352–7.
Filipiak A, Chrapek M, Literacka E, Wawszczak M, Głuszek S, Majchrzak M, Wróbel G, Łysek-Gładysińska M, Gniadkowski M, Adamus-Białek W. Pathogenic factors correlate with antimicrobial resistance among clinical Proteus mirabilis strains. Front Microbiol. 2020;11: 579389.
Cestari SE, Ludovico MS, Martins FH, da Rocha SP, Elias WP, Pelayo JS. Molecular detection of HpmA and HlyA hemolysin of uropathogenic Proteus mirabilis. Curr Microbiol. 2013;67:703–7.
Wang CC, Yang PW, Yang SF, Hsieh KP, Tseng SP, Lin YC. Topical simvastatin promotes healing of Staphylococcus aureus-contaminated cutaneous wounds. Int Wound J. 2016;13(6):1150–7.
Rózalski A, Sidorczyk Z, Kotełko KR. Potential virulence factors of Proteus bacilli. Microbiol Mol Biol Rev. 1997;61(1):65–89.
Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC, Chaudhry R. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care. 2006;29(8):1727–32.
Mohammed A, Adeshina GO, Ibrahim YK. Incidence and antibiotic susceptibility pattern of bacterial isolates from wound infections in a tertiary hospital in Nigeria. Trop J Pharm Res. 2013;12(4):617–21.
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–32.
Furness RB, Fraser GM, Hay NA, Hughes C. Negative feedback from a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J Bacteriol. 1997;179(17):5585–8.
Bahrani FK, Mobley HL. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J Bacteriol. 1994;176(11):3412–9.
Liaw SJ, Lai HC, Ho SW, Luh KT, Wang WB. Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. J Med Microbiol. 2003;52(1):19–28.
Ariison C, Lai HC, Hughes C. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992;6(12):1583–91.
Wojnicz D, Jankowski S. Effects of subinhibitory concentrations of amikacin and ciprofloxacin on the hydrophobicity and adherence to epithelial cells of uropathogenic Escherichia coli strains. Int J Antimicrob Agents. 2007;29(6):700–4.
Drago L, De Vecchi E, Mombelli B, Nicola L, Valli M, Gismondo MR. Activity of levofloxacin and ciprofloxacin against urinary pathogens. J Antimicrob Chemother. 2001;48(1):37–45.
Abdullah MA, El-Baky RM, Hassan HA, Abdelhafez ES, Abuo-Rahma GE. Fluoroquinolones as urease inhibitors: anti-Proteus mirabilis activity and molecular docking studies. Am J Microbiol Res. 2016;4(3):81–4.
Gupta P, Chhibber S, Harjai K. Subinhibitory concentration of ciprofloxacin targets quorum sensing system of Pseudomonas aeruginosa causing inhibition of biofilm formation & reduction of virulence. Indian J Med Res. 2016;143(5):643.
Gümüş D, Kalaycı-Yüksek F, Yörük E, Uz G, Çelik E, Arslan C, Aydın EM, Canlı C, Anğ-Küçüker M. Alterations of growth rate and gene expression levels of UPEC by antibiotics at sub-MIC. Folia Microbiol. 2018;63:451–7.
Horii T, Morita M, Muramatsu H, Muranaka Y, Kanno T, Maekawa M. Effects of mupirocin at subinhibitory concentrations on flagella formation in Pseudomonas aeruginosa and Proteus mirabilis. J Antimicrob Chemother. 2003;51(5):1175–9.
Kawamura-Sato K, Iinuma Y, Hasegawa T, Horii T, Yamashino T, Ohta M. Effect of subinhibitory concentrations of macrolides on expression of flagellin in Pseudomonas aeruginosa and Proteus mirabilis. Antimicrob Agents Chemother. 2000;44(10):2869–72.
Roudashti S, Zeighami H, Mirshahabi H, Bahari S, Soltani A, Haghi F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J Microbiol Biotechnol. 2017;33:1–8.
Przekwas J, Gębalski J, Kwiecińska-Piróg J, Wiktorczyk-Kapischke N, Wałecka-Zacharska E, Gospodarek-Komkowska E, Rutkowska D, Skowron K. The effect of fluoroquinolones and antioxidans on biofilm formation by Proteus mirabilis strains. Ann Clin Microbiol Antimicrob. 2022;21(1):1.
Kwiecińska-Piróg J, Skowron K, Zniszczol K, Gospodarek E. The assessment of Proteus mirabilis susceptibility to ceftazidime and ciprofloxacin and the impact of these antibiotics at subinhibitory concentrations on Proteus mirabilis biofilms. BioMed Res Int. 2013;2013:1–8.
Wasfi R, Abd El-Rahman OA, Mansour LE, Hanora AS, Hashem AM, Ashour MS. Antimicrobial activities against biofilm formed by Proteus mirabilis isolates from wound and urinary tract infections. Indian J Med Microbiol. 2012;30(1):76–80.
Zhanel GG, Hoban DJ, Harding GK. Subinhibitory antimicrobial concentrations: a review of in vitro and in vivo data. Can J Infect Diseases Med Microbiol. 1992;3:193–201.
Kwon YW, Lee SY. Effects of antibiotics at sub-minimal inhibitory concentrations on the morphology of Streptococcus mutans and Lactobacillus acidophilus. Oral Biol Res. 2020;44(1):1–7.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, T.E.E, F.I.S, and M.M.E; Data curation, M.A.E and M.M.E; Formal analysis, M.A.E, T.E.E, F.I.S, and M.M.E; Methodology, M.A.E, T.E.E, F.I.S, and M.M.E; Visualization, T.E.E, F.I.S, and M.M.E; Writing—original draft, M.A.E and M.M.E; Writing—review and editing, M.A.E, F.I.S, and M.M.E. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animals’ procedures were performed according to the institutional guidelines for the care and use of laboratory animals. After informed written consent from the participant, the study was conducted following the Declaration of Helsinki, and approved by the Research Ethics Committee of the Faculty of Pharmacy, Tanta University (TP/RE/2/24 p-01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elhosseini, M.A., El-Banna, T.E., Sonbol, F.I. et al. Potential antivirulence activity of sub-inhibitory concentrations of ciprofloxacin against Proteus mirabilis isolates: an in-vitro and in-vivo study. Ann Clin Microbiol Antimicrob 23, 48 (2024). https://doi.org/10.1186/s12941-024-00704-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-024-00704-4