Abstract
Background
For treatment of ventriculitis, vancomycin and meropenem are frequently used as empiric treatment but cerebrospinal fluid (CSF) penetration is highly variable and may result in subtherapeutic concentrations. Fosfomycin has been suggested for combination antibiotic therapy, but data are sparse, so far. Therefore, we studied CSF penetration of fosfomycin in ventriculitis.
Methods
Adult patients receiving a continuous infusion of fosfomycin (1 g/h) for the treatment of ventriculitis were included. Routine therapeutic drug monitoring (TDM) of fosfomycin in serum and CSF was performed with subsequent dose adaptions. Demographic and routine laboratory data including serum and CSF concentrations for fosfomycin were collected. Antibiotic CSF penetration ratio as well as basic pharmacokinetic parameters were investigated.
Results
Seventeen patients with 43 CSF/serum pairs were included. Median fosfomycin serum concentration was 200 [159–289] mg/L and the CSF concentration 99 [66–144] mg/L. Considering only the first measurements in each patient before a possible dose adaption, serum and CSF concentrations were 209 [163–438] mg/L and 104 [65–269] mg/L. Median CSF penetration was 46 [36–59]% resulting in 98% of CSF levels above the susceptibility breakpoint of 32 mg/L.
Conclusion
Penetration of fosfomycin into the CSF is high, reliably leading to appropriate concentrations for the treatment of gram positive and negative bacteria. Moreover, continuous administration of fosfomycin appears to be a reasonable approach for antibiotic combination therapy in patients suffering from ventriculitis. Further studies are needed to evaluate the impact on outcome parameters.
Similar content being viewed by others
Background
In acute hydrocephalus, external ventricular drains (EVD) are often used as temporary devices for the therapeutic diversion of cerebrospinal fluid (CSF). As a percutaneously placed catheter, EVDs are prone to bacterial colonization that can lead to ventriculitis, requiring antibiotic therapy [1]. As opposed to meningitis, little meningeal inflammatory exists in ventriculitis, resulting in only little antibiotic penetration into the CSF [2]. Therefore, despite high-dose antibiotic therapy subtherapeutic CSF levels may be present. To prevent from subtherapeutic concentrations a routine therapeutic drug monitoring (TDM) from the cerebrospinal fluid (CSF) has been established in our institution to adjust antibiotic therapy accordingly [3]. This is only possible within certain limits as toxicity may occur at high serum levels, mandating the measurement of serum concentrations, as well.
As initial empirical therapy, meropenem and vancomycin are indicated [4] but current data shows variable and often low penetration into the CSF [5]. Therefore, combination antibiotic therapy has been suggested to overcome this problem [4]. Fosfomycin exerts a broad-spectrum bactericidal activity covering both gram positive and negative bacteria including Staphylococcus spp. and Pseudomonas spp. As recently discussed [6] fosfomycin also shows biofilm activity against Staphylococcus spp. which makes it suitable for the treatment of device associated central nervous system (CNS) infections. Therefore, the local antibiotic therapy standard for EVD associated ventriculitis includes adding fosfomycin where timely removal or replacement of EVD is not possible. Fosfomycin is eliminated renally and shows a half-life of approximately 2 h in adults with normal renal function. With a small volume of distribution (Vd), small molecular size and almost no protein-binding fosfomycin is distributing widely into all body compartments. Preliminary results have shown that fosfomycin penetrates well into the CSF even in the absence of meningeal inflammation [2, 7, 8]. Even though, current data show that fosfomycin exhibits time-dependent bactericidal activity it is most often used as intermittent infusion. Assuming that fosfomycin pharmacokinetics are relatively similar to meropenem, where continuous infusion lead to more sufficient CSF levels [3, 9], this administration type was adopted for fosfomycin. Moreover, this approach has been used previously in CNS infections with fosfomycin being administered by continuous infusion (24 g/24 h) [10, 11]. As this approach has not been systematically studied, we aimed to investigate fosfomycin CSF concentrations attained in neurocritical care patients with device (EVD) associated ventriculitis.
Methods
Study design and population
This analysis was reported to the Ethics Committee of the Hamburg Chamber of Physicians (Reference: WF-028/20, February 11, 2020). Due to the non-interventional nature of this study and anonymous recording of data, written informed consent was waived.
Patients included were diagnosed with ventriculitis and treated with fosfomycin by continuous infusion as combination therapy with meropenem and vancomycin as initial empiric therapy. TDM measurements were performed in regular intervals during EVD diagnostics from serum and CSF. Antibiotic dosages were adjusted according to TDM results from serum and CSF samples. Moreover, antibiotic regimens were deescalated, adjusted, or discontinued after species identification or at the discretion of the treating physician.
Diagnosis of ventriculitis was generally applied according to the CDC/NHSN surveillance definition [12]. Since clinical criteria like meningeal or cranial nerve signs could not be obtained in some patients due to sedation, impaired consciousness or interfering neurological deficits, a suspicion for ventriculitis and subsequent treatment indication was solely based on pathological CSF parameters such as increased leucocytes, elevated protein, decreased absolute glucose or decreased CSF/serum glucose ratio also if no growth was seen in the CSF culture in these cases.
Data collection
Demographic and clinical data were obtained from the patients’ electronic records (Integrated Care Manager ICM, version 10.1, Drägerwerk AG, Lübeck, Germany, and Soarian Clinicals 4.01 SP08, Cerner Health Services, Idstein, Germany). We recorded data on antibiotic serum and CSF concentrations. Renal function was determined by the estimated glomerular filtration rate (eGFR) calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [13]. Moreover, clinical routine data such as CSF parameters as well as microbiological results from CSF and blood cultures were obtained. As fosfomycin is available as a disodium salt, serum sodium levels were collected from the blood gas analysis (BGA), as well. The Simplified Acute Physiology Score II (SAPS II) [14] was recorded as a measure of disease severity. Outcome was assessed at discharge according to the Glasgow Outcome Scale (GOS), which consists of the five categories death (1), persistent vegetative state (2), severe (3), moderate (4) and low disability (5) [15].
Drug administration
Fosfomycin (Infectopharm, Heppenheim, Germany) was reconstituted with water and used with a final concentration of 100 mg/mL. It was administered by continuous intravenous infusion (CI) with an initial dose of 1 g/h. Considering a potential disequilibrium effect in CSF two times the minimal inhibitory concentration (MIC) was targeted. Regarding the general European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint for susceptibility (32 mg/L) 64 mg/L was targeted. For Pseudomonas spp. and Enterococcus spp., a target concentration above 128 mg/L was aimed for. This represented a pragmatic approach adapted from the published wild-type MIC distributions although neither an epidemiological cut-off value (ECOFF) nor a breakpoint has been defined in these cases by the EUCAST, yet [16]. Fosfomycin exposure in serum and CSF was optimized by adapting the infusion rate taking into account a penetration rate of approximately 30% [17].
Bioanalytical method
Fosfomycin serum samples were collected and centrifuged (5000 rpm, 10 min, 20 °C), subsequently. The serum supernatant and CSF samples were stored at dry ice until being further processed. Serum samples were analyzed by LC–MS/MS according to the procedure described by Martens-Lobenhoffer et al. [18]. CSF samples were prepared and analyzed in a similar manner. Calibration ranges were 15–750 mg/L in serum as well as in CSF. Coefficients of variation for serum samples were 6.0% at 15 mg/L and 4.0% at 750 mg/L, respectively, with accuracies of 10.9% to − 0.2%. The corresponding coefficients of variation in CSF were 5.6–3.6%, with accuracies of − 13.9–9.7%. The lower limits of quantification were set to the lower end of the calibration range of 15 mg/L for both matrices. Samples with concentrations above the upper limit of quantification (750 mg/L) were diluted with blank serum (or water in case of CSF samples) and were re-analyzed.
Statistics
Data management, non- compartmental calculations for fosfomycin clearance (CL) and area under the curve (AUC) were performed by using Microsoft Excel 365 (Microsoft Corp., Redmond, WA, USA). CL (Eq. 1) and AUC (Eq. 2) were calculated as follows:
Visualization, statistical evaluation as well as determination of coefficients of correlation (R2) were either calculated by linear or non-linear regression methods included in Prism 9 (GraphPad Software, San Diego, CA, USA).
Results
Between January 2020 and October 2021, 43 pairs of serum and CSF concentrations of fosfomycin were obtained from 17 patients treated for device associated ventriculitis. An overview on patient characteristics is given in Table 1.
The patients showed a regular body composition (body mass index 27 ± 5 kg/m2) and a preserved renal function with a mean estimated GFR of 110 ± 32 ml/min/1.73m2. Fosfomycin dosing was started with 24 g/24 h and adapted to a mean fosfomycin dosing regimen of 20 g/24 h during the course of the therapy.
The mean fosfomycin clearance was 4.2 ± 2.2 L/h and correlated best with the CKD-EPI eGFR values with a coefficient of correlation R2 = 0.63 (see Fig. 1).
The median attained serum concentration of fosfomycin was 200 [159–289] mg/L and the median CSF concentration 99 [66–144] mg/L (Table 2). When considering only the first measurements before a possible dose adaption, the median serum and CSF concentrations were 209 [163–438] mg/L and 104 [65–269] mg/L, respectively. The median area under the curve (AUC) was 4800 [3816–7152] in serum and 2381 [1585–3456] mg*h/L in CSF, respectively. The time course of fosfomycin serum and CSF levels are presented in supplement Fig. 1.
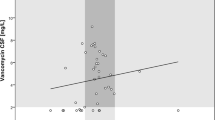
The CSF penetration for fosfomycin was 46 [36–59]% with an R2 of 0.65 (Fig. 2). Penetration ratio per GOS group is presented in supplement Table 1.
All CSF values but one were above the EUCAST breakpoint of 32 mg/L (98%) and 13 CSF values (30%) were above 128 mg/L. In total, 98% of the observed CSF concentrations were above the MIC of 32 mg/L for susceptible isolates (see Fig. 3). When targeting Pseudomonas species with an ECOFF of 128 mg/L 30% of the analyzed CSF specimens achieved this concentration. The corresponding AUC/MIC ratio in CSF for an MIC of 32 mg/L was 74 [50–108].
Most patients received fosfomycin as a part of a combination therapy including meropenem and/or vancomycin. Two patients were deescalated to fosfomycin monotherapy due to infections with Staphylococcus epidermidis. Microbiological results are shown in Table 3.
Within the cohort, serum sodium concentrations ranged between 124 to 167 mmol/L with a mean value of 142 mmol/L. Hypernatremia was already present in 47% (n = 8) of the patients prior to fosfomycin treatment start with a mean sodium value of 153 mmol/L in this subgroup.
Discussion
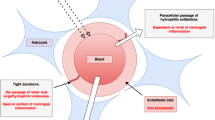
As opposed to meningitis, reaching sufficient bactericidal concentrations in ventriculitis may be difficult. Diffusion of anti-infectives into the CNS is dependent on different factors such as their lipophilicity, protein binding and molecular size [19]. Moreover, meningeal inflammation leads to the opening of tight junctions allowing for the penetration of drugs into the CSF whereas in ventriculitis meningeal inflammation is often not present [19]. Therefore, intermittent infusions might insufficiently attain appropriate plasma-CSF-equilibration resulting in lower penetration ratios. To date, no method allows for a quantification of meningeal inflammation and therefore approximations regarding drug CSF penetration ratios often remain uncertain. This problem was thought to be overcome by administering fosfomycin as a continuous infusion (CI), thereby permanently increasing the diffusion gradient towards the CSF accompanied by routine TDM of serum und CSF samples.
To the best of our knowledge, this is the first study analyzing the penetration whilst using fosfomycin by a CI regimen. Application of fosfomycin by CI or prolonged infusion has been studied by in-vitro and in-vivo analyses [20] and was performed successfully in case studies before [21,22,23]. This is also in concordance with in-vitro data showing pharmacodynamic indexes such as either time-dependent pharmacodynamics for species such as Staphylococcus aureus or Enterococcus spec. [24] or AUC/MIC dependency for Pseudomonas aeruginosa or Escherichia coli [25,26,27]. With the chosen dosing regimen fosfomycin (d1 24 g/d) penetration into the CSF was reliably reaching CSF concentrations well above the defined EUCAST breakpoint of 32 mg/L for susceptible bacteria such as Staphylococcus aureus. The AUC/MIC ratio for an MIC of 32 mg/L was 74 for CSF specimens. This is in concordance with in-vitro data showing bacteriostasis at mean AUC/MIC ratios of 22.7 and a 1-log kill for 83.3 when targeting the group of Enterobacterales [25]. Unfortunately, MICs of the identified isolates were not reported by the local laboratory and therefore, no MIC guided dose adaption could be performed. In general, the achieved mean AUC of 4800 [3816–7152] mg*h/L in serum was higher than in a previous study reporting 4411 mg*h/L in healthy volunteers receiving a CI of 1 g/h [28].
Because our cohort included one patient with renal insufficiency (eGFR < 50 mL/min/1.73m2), increased AUC and serum concentrations (> 400 mg/L) using the initial standard fosfomycin dose were seen.
The penetration ratio of 46% in our cohort is within the range of 30–60% as reported earlier by Silica et al. [11] who used intermittent infusion regimens with up to 16 g/d in patients with meningitis. Pfausler et al. conducted lower CSF concentrations of 62 ± 32 mg/L when using 8 g fosfomycin 8 hourly in patients with ventriculitis [17]. This translated into a penetration ratio of approximately 25% when compared to serum. However, higher CSF concentrations as required for MIC > 64 mg/L which are often present in Pseudomonales or other non-fermenting bacteria could not be attained in all specimens of our cohort, even though 24 g/d by continuous infusion was used empirically.
More recently, CSF protein levels could be associated with an improved CSF penetration ratio of meropenem in patients with ventriculitis [3], whereas in our cohort no such association with variables such as GOS or other laboratory parameters could be identified for fosfomycin.
Fosfomycin CI was generally well tolerated as it was infused via a central line. In cases where increased serum sodium levels occurred during treatment, hypernatremia was already present prior to fosfomycin start. This is in concordance with Putensen et al. who also found a moderate incidence for hypernatremia of 14.3% [29]. Besides, none of the treated patients had to discontinue fosfomycin treatment due to adverse events.
Our study has certain limitations. We performed an analysis from clinically obtained samples that were collected at irregular time intervals from patients with ventriculitis presenting different underlying pathologies. However, our data therefore represents “real-world” conditions from a mixed cohort of patients. Moreover, there is currently no guideline regarding required antibiotic CSF concentrations for the treatment of ventriculitis. Thus, we suggest that concentrations one to two times the MIC of the targeted pathogen are sufficient as inflammation occurs within the CSF and the surrounding tissue. On the other hand, tissue concentrations obtained by microdialysis as reported for meropenem can exceed the CSF concentrations by a factor of three [30]. Hence, if targeting either CSF concentrations representing a multiple of the MIC or if lower targets could also be sufficient for successful treatment remains a matter of debate. To clarify, further studies are needed to understand the pathophysiology of ventriculitis and to determine the antibiotic targets to optimize patient outcome.
Conclusion
Fosfomycin administration by continuous infusion is a feasible way in patients with ventriculitis resulting in appropriate drug concentrations in CSF. Our patient cohort showed a high fosfomycin CSF penetration rate of 46%, making it a potential treatment option for susceptible gram negative and positive pathogens. Future studies are needed to systematically investigate fosfomycin treatment in infections of the central nervous system regarding efficacy and patient outcome.
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- CRP:
-
C-reactive protein
- CSF:
-
Cerebrospinal fluid
- ECOFF:
-
Epidemiological cut-off values
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- EVD:
-
External ventricular drain
- GFR:
-
Glomerular filtration rate
- GOS:
-
Glasgow outcome scale
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- MIC:
-
Minimal inhibitory concentration
- SAPS II:
-
Simplified acute physiology score II
- TDM:
-
Therapeutic drug monitoring
References
Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255(11):1617–24. https://doi.org/10.1007/s00415-008-0059-8.
Nau R, Seele J, Djukic M, Eiffert H. Pharmacokinetics and pharmacodynamics of antibiotics in central nervous system infections. Curr Opin Infect Dis. 2018;31(1):57–68. https://doi.org/10.1097/QCO.0000000000000418.
Konig C, Grensemann J, Czorlich P, Schlemm E, Kluge S, Wicha SG. A dosing nomograph for cerebrospinal fluid penetration of meropenem applied by continuous infusion in patients with nosocomial ventriculitis. Clin Microbiol Infect. 2022. https://doi.org/10.1016/j.cmi.2022.02.017.
Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Michael Scheld W, et al. Infectious diseases society of america’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017. https://doi.org/10.1093/cid/ciw861.
Mader MM, Czorlich P, Konig C, Fuhrmann V, Kluge S, Westphal M, et al. Intrathecal penetration of meropenem and vancomycin administered by continuous infusion in patients suffering from ventriculitis-a retrospective analysis. Acta Neurochir. 2018;160(11):2099–105. https://doi.org/10.1007/s00701-018-3680-z.
Karvouniaris M, Brotis A, Tsiakos K, Palli E, Koulenti D. Current perspectives on the diagnosis and management of healthcare-associated ventriculitis and meningitis. Infection Drug Resist. 2022;15:697.
Kuhnen E, Pfeifer G, Frenkel C. Penetration of fosfomycin into cerebrospinal fluid across non-inflamed and inflamed meninges. Infection. 1987;15(6):422–4. https://doi.org/10.1007/bf01647220.
Kumta N, Roberts JA, Lipman J, Wong WT, Joynt GM, Cotta MO. A systematic review of studies reporting antibiotic pharmacokinetic data in the cerebrospinal fluid of critically Ill Patients with uninflamed meninges. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/AAC.01998-20.
Blassmann U, Roehr AC, Frey OR, Vetter-Kerkhoff C, Thon N, Hope W, et al. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Critical Care. 2016. https://doi.org/10.1186/s13054-016-1523-y.
Portier H, Armengaud M, Becq-Giraudon B, Bousser J, Desbordes JM, Duez JM, et al. Treatment with a cefotaxime-fosfomycin combination of staphylococcal or enterobacterial meningitis in adults. Presse Med. 1987;16(43):2161–6.
Sicilia T, Fadon A, Rodríguez A, Soto J. Fosfomycin in pneumococcal meningitis. Chemotherapy. 1977;23(Suppl 1):429–40. https://doi.org/10.1159/000222087.
CDC/NHSN. CDC/NHSN Surveillance definitions for specific types of infections. https://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf (2018). Accessed 3 Aug 2018.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–4. https://doi.org/10.1016/s0140-6736(75)92830-5.
European Committee on Antimicrobial Susceptibility Testing: fosfomycin ECOFF. https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=100&search%5Bspecies%5D=-1&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50 (2021). Accessed 2021 02 19.
Pfausler B, Spiss H, Dittrich P, Zeitlinger M, Schmutzhard E, Joukhadar C. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomy-associated ventriculitis. J Antimicrob Chemother. 2004;53(5):848–52. https://doi.org/10.1093/jac/dkh158.
Martens-Lobenhoffer J, Bode-Boger SM. A validated method for the quantification of fosfomycin in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr, B. 2015;990:164–8. https://doi.org/10.1016/j.jchromb.2015.03.029.
Tattevin P, Solomon T, Brouwer MC. Understanding central nervous system efficacy of antimicrobials. Intensive Care Med. 2019;45(1):93–6. https://doi.org/10.1007/s00134-018-5270-1.
Antonello RM, Di Bella S, Maraolo AE, Luzzati R. Fosfomycin in continuous or prolonged infusion for systemic bacterial infections: a systematic review of its dosing regimen proposal from in vitro, in vivo and clinical studies. Eur J Clin Microbiol Infect Dis. 2021;40(6):1117–26. https://doi.org/10.1007/s10096-021-04181-x.
Cree M, Stacey S, Graham N, Wainwright C. Fosfomycin-Investigation of a possible new route of administration of an old drug: a case study. J Cyst Fibros. 2007;6(3):244–6.
Stahl JP, Croize J, Bru JP, Girard-Blanc MF, François P, Gaillat J, et al. Diffusion of fosfomycin into the cerebrospinal fluid in purulent meningitis. Presse Med. 1984;13(44):2693–5.
Portier H, Tremeaux JC, Chavanet P, Gouyon JB, Duez JM, Kazmierczak A. Treatment of severe staphylococcal infections with cefotaxime and fosfomycin in combination. J Antimicrob Chemother. 1984;14:277–84.
Oliva A, Furustrand Tafin U, Maiolo EM, Jeddari S, Bétrisey B, Trampuz A. Activities of fosfomycin and rifampin on planktonic and adherent Enterococcus faecalis strains in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2014;58(3):1284–93. https://doi.org/10.1128/aac.02583-12.
Lepak AJ, Zhao M, VanScoy B, Taylor DS, Ellis-Grosse E, Ambrose PG, et al. In vivo Pharmacokinetics and Pharmacodynamics of ZTI-01 (Fosfomycin for Injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61(6):e00476-e517.
Asuphon O, Montakantikul P, Houngsaitong J, Kiratisin P, Sonthisombat P. Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int J Infect Dis. 2016;50:23–9. https://doi.org/10.1016/j.ijid.2016.06.017.
Fransen F, Hermans K, Melchers MJB, Lagarde CCM, Meletiadis J, Mouton JW. Pharmacodynamics of fosfomycin against ESBL- and/or carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2017;72(12):3374–81. https://doi.org/10.1093/jac/dkx328.
Al Jalali V, Matzneller P, Wulkersdorfer B, Chou S, Bahmany S, Koch BC, et al. Clinical pharmacokinetics of fosfomycin after continuous infusion compared with intermittent infusion: a randomized crossover study in healthy volunteers. Antimicrob Agents Chemother. 2020;65(1):e01375-e1420.
Putensen C, Ellger B, Sakka SG, Weyland A, Schmidt K, Zoller M, et al. Current clinical use of intravenous fosfomycin in ICU patients in two European countries. Infection. 2019;47(5):827–36. https://doi.org/10.1007/s15010-019-01323-4.
Hosmann A, Ritscher L, Burgmann H, Al Jalali V, Wulkersdorfer B, Wölfl-Duchek M, et al. Meropenem concentrations in brain tissue of neurointensive care patients exceed CSF levels. J Antimicrob Chemother. 2021. https://doi.org/10.1093/jac/dkab286.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was kindly supported by Infectopharm (Heppenheim, Germany).
Author information
Authors and Affiliations
Contributions
CK designed the study, wrote the manuscript, and interpreted the data. JML analyzed the samples and helped to interpret the data. PC helped to acquire the data, helped to write the manuscript, and to interpret the data. SK and MW helped with the interpretation and revised the manuscript. SMBB helped to design the study, to analyze the samples, and to interpret the data. JG designed the study, wrote the manuscript, acquired and interpreted the data, and acquired the funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This analysis was reported to the Ethics Committee of the Hamburg Chamber of Physicians (Reference: WF-028/20, February 11, 2020). Due to the non-interventional nature of this study and anonymous recording of data, written informed consent was waived.
Consent for publication
Not applicable.
Competing interests
CK received a research grant from the DAMP foundation and lecture fees from Shionogi. JML has no conflicts of interest. MW has no conflicts of interest. PC has no conflicts of interest. SK received research support from Ambu, Daiichi Sankyo, ETView Ltd, Fisher & Paykel, Pfizer, and Xenios, lecture fees from Astra, C.R. Bard, Baxter, Biotest, Cytosorbents, Daiichi Sankyo, Fresenius, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Philips, and Zoll, and consultant fees from Bayer, Fresenius, Gilead, MSD and Pfizer. SMBB has no conflicts of interest. JG has received research support from Adroit Surgical, Ambu, ETView, Infectopharm, and Pfizer, and received consultant and lecture fees from Drägerwerk, Fresenius Medical, GE Healthcare, and Smith Medical, and holds shares from AstraZeneca, Bayer, Gilead, and Pfizer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Additional Figure and Table;
Figure 1: Fosfomycin concentration in serum and CSF over time, Table 1: Fosfomycin concentration in CSF and penetration ratio per GOS
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
König, C., Martens-Lobenhoffer, J., Czorlich, P. et al. Cerebrospinal fluid penetration of fosfomycin in patients with ventriculitis: an observational study. Ann Clin Microbiol Antimicrob 22, 29 (2023). https://doi.org/10.1186/s12941-023-00572-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00572-4