Abstract
Backgrounds
Observable emergence of Vancomycin-Non susceptible Coagulase-negative Staphylococci (VNS-CoNS) associated with skin and soft tissue infections spreading among the urban and rural populace is gradually intensifying severe complications. The isolated VNS-CoNS were evaluated with Matrix-assisted Laser Desorption/ionization Time of Flight Mass Spectrometry (MALDI ToF MS) for species characterization and pan-antimicrobial resistance pattern.
Methods
Out of 256 clinical samples collected including pus, abscess, ear swabs, eye swabs, and aspirates, 91 CoNS isolates were biotyped and further characterized with MALDI-TOF MS. Staphylococci marker genes, Vancomycin susceptibility, and biofilm assays were performed.
Results
Of 91 CoNS isolates, S.cohnii (2.3%), S.condimentii (3.4%), S. saprophyticus (6.7%), and S.scuri (21.1%) were characterized with MALDI-TOF with significant detection rate (99.4%; CI 95, 0.775–0.997, positive predictive values, 90.2%) compared to lower biotyping detection rate (p = 0.001). Hemolytic VNS-CoNS lacked nuc, pvl and spa genes from wound, ear, and aspirates of more 0.83 MARI clustered into a separate phylo-diverse group and were widely distributed in urban and peri-urban locations. MALDI TOF–MS yielded a high discriminatory potential of AUC-ROC score of 0.963 with true-positivity prediction. VNS-CoNS of MIC ≥ 16 µg/mL were observed among all the ages with significant resistance at 25th and 75th quartiles. More than 10.5% of CoNS expressed multi-antibiotic resistance with more than 8 µg/mL vancomycin cut-off values (p < 0.05).
Conclusion
Antibiotic resistant CoNS should be considered significant pathogens rather than contaminant. Biofilm producing VNS-S. sciuri and S. condimentii are potential strains with high pathological tropism for skin, soft tissues and wound infections, and these strains require urgent surveillance in peri-urban and rural communities.
Similar content being viewed by others
Introduction
Coagulase-negative staphylococci (CoNS) are now gaining significant importance with much emphasis on the vancomycin-non susceptible Coagulase-negative staphylococci (VNS-CoNS) that were observed to be substantially associated with colonization of the skin, soft tissue, and mucous membranes in different clinical infections [1]. These strains were implicated in severe wound, abscess, skin and soft tissue related infections [2], and it is now gradually emerging to intensify severe complications. The persistence and high tropism of CoNS (particularly Staphylococcus sciuri and S. condimentii strains), predisposed the populace to unwanted morbidities [3]. Previously, these CoNS were referred to as non-pathogenic commensals, but malnutrition, genetic re-assortment via human and animal interaction [4, 5], and misuse of antimicrobial agents were key driven factors that propel their virulence and resistance to commonly used antibiotics [6].
Vancomycin was an earlier known antibiotic as the first-line drug for staphylococci infection, but the increasing rate of CoNS resistance to this antibiotic is becoming a serious concern in several therapeutics approaches [7]. However, VNS-CoNS are now considered important strains as recent observations have suggested that the prolonged infections with these strains among the hospitalized patients with indwelling devices and in immunocompromised patients [8] were complicated by the expression of multidrug resistance with a resultant treatment failure [3, 9].
Skin and soft tissue infections associated with CoNS are now considered a major pathological issue. The increasing prevalence of S. saprophyticus and S. sciuri were usually identified as contaminants in infectious disease diagnosis, particularly in low-income settings [10]. Furthermore, poor detection of VNS-CoNS kept enhancing its prevalence and dissemination [11], particularly in low-income countries. The paucity of data on these strains prevalence in peri-urban and rural communities of southwest Nigeria increases the morbidity rate, therapeutic failure, and hospital-acquired infections [12]. Usually, these strains are considered non-significant contaminants but are now emerging and becoming prevalent with very low-level susceptibility to vancomycin, with increasing association with nosocomial infections. The hydrophobicity phenomenon observed among these strains plays a major role in biofilm production and tissue adherence, facilitating infectivity potential and enhancing antimicrobial resistance [13]. The VNS-CoNS strains are now increasing their spread and intensity among the populace. This study aims to characterize the emerging VNS-CoNS strains associated with skin and soft tissue infections and further analyse the strains antimicrobial resistance pattern.
Methods
Bacteria strains
Total of 256 clinical samples, including pus (n = 58), abscess (n = 84), ear swabs (n = 62), eye swabs (n = 18), aspirates (n = 34) were collected from out- and in-patients attending the Federal Medical Centre in Abeokuta, Nigeria between June 2018 and March 2019. Inadequate patients’ bio-data is a limitation to have complete demographic status, but they majorly dwell in urban and peri-urban communities in southwest Nigeria. The selection of patients with staphylococcal infection was based on the assessment of the physicians and the Ethical approval for the study was obtained (FMCA/470/HREC/09/2017). Suspected staphylococci colonies from 7% sheep blood agar culture, were phenotypically characterized on Baird-Parker medium, Mannitol salt agar (MSA) and MSA supplemented with Cefoxitin (4 µl/g) as previously described [14]. Typical colonies were further tested for hemolytic activity and coagulase production [15] and further biotyped with Analytical Profile Index (API).
Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) analysis
Identified CoNS strains were selected for species confirmation using MALDI-TOF MS [16]. Each strain was sub-cultured on sheep blood agar and incubated overnight at 37 °C to obtain a single pure colony for MALDI-TOF MS analysis. Briefly, a single pure colony of each strain was spotted on a ground steel MALDI target plate in duplicate and allowed to dry. To each spot, 1 μL of the matrix (Bruker Daltonik GmbH, Bremen, Germany), containing a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile (Sigma-Aldrich) and 2.5% trifluoro-acetic acid (Sigma-Aldrich) was added to the spot and was allowed to dry. Thereafter, the spotted plate was placed for analysis in Microflex LT MALDI-TOF MS instrument (Bruker Daltonik GmbH) with the following parameter settings; Ion Source (IS) 1 20 kV, IS2 18.5 kV, lens 8.5 kV, pulsed ion extraction 250 nucleotides with no gating. Spectra calibration and the measurements were carried out for species identification with reference strains, and the highest score for each species was selected according to the manufacturer’s instructions.
Strain genotyping
Multiplex real-time PCR was adopted for the simultaneous detection of staphylococci marker genes; tuf (Staphylococcus spp.), nuc (S. aureus), mecA (methicillin resistance) and pvl (Panton-Valentine leukocidin) [17] using the primer sets shown in Additional file 2: Table S1. Briefly, each reaction volume of 20 µl contained 0.8 µl of 10uM primers of tuf-P1, tuf-P2, nuc-P1, nuc-p2, mecA-P1,mecA-P2, pvltaqF and pvltaqR. Probe analyte of 0.2 µl of 10uM each of nuc, mecA, and pvlTAQT, DNA template (1 µl) and water (18 µl) were also added. The amplification cycle consisted of an initial denaturation step at 95 °C for 15 min, followed by 30 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 60 s programmed and extension 72 °C for 60 s and final extension 72 °C for 5 min. Data evaluation was done with consideration of the negative and positive controls.
Antibiogram
Susceptibility of the strains to different antimicrobial agents, including gentamicin (GEN), kanamycin (KAN), streptomycin (STR), chloramphenicol (CHL), ciprofloxacin (CIP), tetracycline (TET), clindamycin (CLI), erythromycin (ERY), mupirocin (MUP), linezolid (LZD), vancomycin (VAN), penicillin (PEN), fusidic acid (FUS), cefoxitin (FOX), trimethoprim (TMP), sulfamethoxazole (SMX), vancomycin (VAN), amoxicillin (AMX), ofloxacin (OFX), ceftazidime (CAZ), cefuroxime (CFX), rifampicin (RIF), tiamulin (TIA), and Quinupristin-dalfopristin (SYN) were evaluated for minimum inhibitory concentration (MIC) at dilution ranges of 0.5–128 µg/mL using semi-automated broth micro-dilution assay in a commercially prepared microtiter plate [18]. The MICs were interpreted according to the epidemiological cut-off values (ECOFFs) described by the European Committee for antimicrobial susceptibility guidelines (www.eucast.org) [19]. The multi-antibiotic resistance index (MARI) of each strain was also determined [20, 21]. Commonly used antibiotics including TET, CAZ, CIP, GENT, AMX, CFX, OFX, SMX, ERY, FOX; were assayed for susceptibility using disc diffusion method [22] on Mueller–Hinton agar plates. Zones of inhibitions were measured and interpreted according to CLSI guidelines [23].
Biofilm assay
Seventeen CoNS strains showing MARI of more than 0.2 were selected for biofilm assay, which was performed in microtiter plates [24], following the growth of the strains in tryptic soy broth. Pellet of the bacteria was separated and further diluted to standard suspension of 0.5McFarland turbidity in fresh tryptic soy broth and 200 µl was dispensed into the micro-titer plate and incubated at 37 C for 24 h. After incubation, cells were aspirated away from each well and washed with sterile phosphate-buffered saline (PBS). The plates were dried, and 200 μL of 0.2% aqueous Crystal violet solution was added to each well, and the plates were allowed to stand for 15 min. All the wells were washed again with PBS and 50 µl graded ethanol was added for colouration, which was interpreted for biofilm production according to the criteria described by Stepanovic et al. [25].
Vancomycin susceptibility
Vancomycin MICs were graded to evaluate the strain susceptibility using the broth microdilution methods [26]. Briefly, ten serial dilutions of vancomycin (Sigma-Aldrich), were prepared at concentrations of 32 to 0.06 μg/mL and inoculated with standardized bacterial suspension of 0.5McFarlan turbidity. Following the incubation at 37 °C for 24 h, the MIC was determined and interpreted following the CLSI (2018) guidelines [23]. Susceptibility to Vancomycin was classified as Vancomycin susceptible strains (VS-CoNS; MIC ≤ 4 µg/mL); Vancomycin intermediate-CoNS (VI-CoNS; MIC 8–16 µg/mL) and Vancomycin non-susceptible-CoNS (VNS-CoNS; MIC > 16 µg/mL) according to CLSI [23, 27].
Data analysis
The antibiotic resistance relatedness of the VNS-CoNS isolates were analysed using DendroUPGMA construction utility program to extrapolate the dendrogram from a set of variables based on their respective resistance pattern and MARI. At various distance matrices, similar matrices and transformed similar coefficients were calculated into distances making a clustering with the Unweighted Pair Group Method and Arithmetic mean (UPGMA) algorithm [28]. The significance level of the identified VNS-CoNS strains was determined with Chi-square, and comparative evaluation of the detection rate of biochemical typing and MALDI-TOF analytic methods was performed with pair T-test, taking p < 0.05. Evaluation of MALDI-TOF discrimination of CoNS and VNS-CoNS was performed with ROC curve that provides optimal cut-off value reliability at the area under the curve (AUC) closer to 1 and unreliable at 0.50 or less. Age was considered as a variant for risk factor for the Vancomycin intermediate and VNS-CoNS colonization due to various skin and soft tissue infections that impact on adolescents and young adults quality of life. This evaluation was determined with Mann–Whitney U test, taking level of significant difference at p < 0.05. The antibiotic resistance pattern of the CoNS isolates was estimated with Chi-square for comparisons of resistance proportions to various antibiotics (p < 0.05).
Results
Comparative CoNS biotypes detection, biofilm, and resistance pattern
Detection of CoNS strains was significantly higher using MALDI-TOF (99.4%; CI 95, 0.775–0.997) with better sensitivity (84.8%), specificity (100%), and positive predictive values (90.2), compared to biotyping analytical detection (75.2%; CI 95, 0.369–0.717) (Table 1). MALDI-TOF MS provided higher accuracy for detection of VNS-CoNS strains (S. cohnii, S. condimentii, S. scuri and S.saprophyticus) with spectra signature peak characterizing the strains as shown by the Biotyper data processing (Additional file 1: Figure S1). Out of 91 CoNS isolates, S. cohnii (2.3%), S.condimentii (3.4%), S. saprophyticus (6.7%), S. scuri (21.1%) and others (non-staphylococci strains) (66.5%) were identified (Fig. 1A). Significant number of biofilm producing S. scuri (7/59) was observed compared to S. cohnii (1/4), S. condimentii (1/8) and S.saprophyticus (1/20) biofilm producers (Fig. 1B). All the biofilm formers showed resistance to all the antibiotics except CHL compared to non-biofilm formers that resisted only CIP, TMP and SYN (Fig. 1C).
Dendrogram analysis of VNS-CoNS
Vancomycin non-susceptible S. sciuri (wound, ear and pus) and S. saprophyticus (wound) with MARI > 0.83 clustered into phlyo-group A having related multi-antibiotic resistance pattern widely distributed in urban and peri-urban locations. Mixed vancomycin non-susceptible S. condimentii, S. sciuri and S. saprophyticus with lower MARI were grouped in phylo-group B while only one strain of vancomycin non-susceptible S. scuiri with 0.92 MARI singly clustered to group C (Fig. 2). S. sciuri isolated from abscess clustered to group E revealed a lower MARI compared to S. sciuri (wound) belonging to group C. Other CoNS from other sources grouped to B also showed a high resistance pattern to vancomycin but lack nuc, pvl and mecA genes and expressed tuf gene.
Dendrogram of characterized vancomycin non-susceptible CoNS clustering related multi-antibiotic resistant phenotypes from different locations (Ru, Rural; Pe, Peri-urban; Ur, Urban; hly, haemolysin; bf, biofilm; vanc, vancomycin; MARI, multi-antibiotic resistance; Pg,Phylo-group; R, Resistance; S, Susceptible; I,Intermediate)
Antibiotic resistance patterns of CoNS strains and evaluation of MALDI-TOF MS detection accuracy with ROC
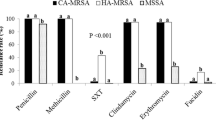
More than 10.5% of all identified CoNS species showed significant resistance to tetracycline (TET), aminoglycoside (GEN and STR), fluoroquinolone (CIP and ofloxacin), cephalosporin (FOX and ceftazidime) and penicillin (Amoxycillin), linezolid, sulphamethoxazole and erythromycin (p = 0.001; Fig. 3a). ROC was applied to determine the level of accuracy (sensitivity and specificity) of detected CoNS and VNS strains based on the generated area under the curve (AUC). Higher AUC (0.963) for MALDI-TOF MS characterization of CoNS strains could predict a higher level of true-positive CoNS compared to the lower classification of VNS strains (AUC = 0.525) (low sensitivity and specificity rates, Fig. 3b).
a Doughnut chart of the overall resistance of isolated CoNS strains to various antibiotics showing multi-antibiotic resistance pattern. b Receiver operating characteristic (ROC) curves for the detection accuracy of the MALDI ToF characterized CoNS strains (AUC = 0.963) and VNS-CoNS isolates with MIC > 16ug/mL (AUC = 0.525)
Vancomycin susceptibility: vancomycin susceptibility
High significant rate of vancomycin non-susceptible S. condimentii (23.85%), S. cohnii (9.54%) and S. saprophyticus (6.36%) were recorded (Fig. 4a).
(Fig. 4a). Figure 4b also show the overall percentage of VNS-CoNS and VI-CoNS found in the study. Identified Vancomycin non-susceptible CoNS with the MIC > 16 µg/mL were distributed among all the ages (median ≈ 34.6 years) with significant resistance at 25th and 75th quartiles (p = 0.001) compared to intermediate vancomycin susceptibility rate (MIC < 8ug/mL; p = 0.012) (Fig. 4b).
Discussion
The present study has shown S. sciuri and a few other CoNS strains to be rapidly emerging strains associated with skin and soft-tissue infections. However, there are scanty reports on this infection in Nigeria and other African countries [29]. Low immunity due to poor nutrition, unhygienic environment, and strain-specific colonization is a major factor that could likely enhance invasion, persistence, and aggression of CoNS infectivity among the studied population [30]. The use of MALDI-TOF MS for characterization of CoNS further enhances the rapid and precise detection required for robust and accurate diagnosis of CoNS skin infections. Its timeliness and high specificity could aid appropriate disregard for false-positive CoNS, leading to unnecessary antimicrobial use and reduction in skin and soft tissue morbidity [31]. MALDI-TOF MS provided high-throughput detection with greater specificity and predictive power to improve CoNS characterization compared to low detection observed in the use of the biochemical method (biotyping) that relies primarily on culture-based methodologies.
Antibiotic resistance relatedness of hemolytic and biofilm-producing VNS-S.sciuri (from wound, ear, and pus) and VNS-S.saprophiticus (wound) with high MARI indicate dissemination of phylo-diverse strains. This study identifies some disseminating VNS-CoNS and highlights clusters of strains characterized with biofilm production that would greatly facilitate and intensify adherence, colonization, persistence, and skin tissue tropism. Production of biofilm allows VNS-CoNS adhesion to epithelial surfaces or medical devices, thereby contributing to its colonization, cell proliferation and accumulation in multilayers. In this process, the accumulation of polysaccharide intercellular adhesin (Pia) and adhesive proteins, such as accumulation-associated protein (Aap) and biofilm-associated protein (Bap), will aid protection against the host immune system facilitating virulence. This mechanism also promotes protection from antibiotic attack, further enhanced the development of resistance to antibiotics, which contributes to the survival of these strains and the severity of infection [14, 32, 33].
The observable cluster of VNS-S sciuri lacking pvl, demonstrating high level heterogenous antibiotic resistance from peri-urban and rural communities, is a pending threat to public health [4, 14]. This further suggests a stemming spread of VNS-CoNS infection of soft tissue, bloodstream and traumatized wounds.
Increasing resistance to the most commonly used antimicrobial drug associated with these strains is a potential factor for therapeutic failure, particularly among the immuno-compromised patients with implants, catheters, severe or septic wounds, traumatized orthopedic and long-term hospitalized elderly patients [34, 35]. The recorded high resistance to various antimicrobial classes (tetracycline, aminoglycoside, fluoroquinolone, cephalosporin, and penicillin), are indication of the acquisition of a large reservoir of resistance determinants via mobile genetic elements, as a result of human–human (hospital-acquired) or human-animal interactions (zoonotic) [36].
Despite MALDI-TOF MS characterization of the emerging VNS-CoNS with improved detection capability, its discriminatory evaluation revealed low true-positive VNS-CoNS detection (AUC = 0.525). Structural mutation of the VNS-CoNS cell wall could give mass spectra of multiple signals of peaks characterized by low m/z values, poor intensities, and low signal-to-noise ratios. Also, ROC provides a reasonable and accurate differentiation of MALDI-TOF AUC level needed for strain typing that could facilitate CoNS infection diagnosis [37, 38]. Application of the ROC algorithm could be considered a rapid, robust, and easy-to-use method for MALDI-TOF discrimination of CoNS types. This technique provides excellent turn-around time for detection and early diagnosis of CoNS infection, particularly vancomycin non-susceptible strains [39]. Another envisaged limitation for VNS-CoNS detection is the selection of suitable filters, high-dimensional classification, computational programming for a relevant subset characterization and classification problems with a large set of diverse specie-features [40].
As for the high rate of VNS-CoNS and VI-CoNS among the subjects of median age 36.5 years, possible misuse of vancomycin drug or its derivatives for skin abrasion, wound or abscess may be inevitable. This would lead to a steady rise in selective pressure for VNS-CoNS, particularly among the adolescent and adult age groups [41]. Vulnerability of young people to staphylococci infection emanates from poor skin hygiene, unguided application of topical antibiotics and skin cosmetics, disposing them to various dermatologic infections [42].
The increasing proportion of VNS-CoNS could provide predictions for unachievable public health antimicrobial stewardship and ineffective antibiotic therapies associated with increased skin and tissue morbidity, mostly among the vulnerable patient groups [43]. VNS-CoNS distribution among all the ages with up to 75th quartile resistance rating calls for immediate surveillance and more clinical attention. Although, inhibitory or cidal activities of related glycopeptides (such as teicoplanin) which is not commonly prescribed, could be impaired via various resistance mechanism. Resistance mechanisms associated with VanA operons could induce phenotypic resistance, resulting in heterogeneous VNS-CoNS identified in wounds and skin aspirates [44]. Mutational changes to cell wall integrity and regulatory genes associated with RNA synthesis, cell transport, and intracellular protein-binding [45], are possible genetic selections that facilitate sequential resistance to vancomycin. These VNS-CoNS are potential emerging threats to therapeutic formulation needed for the treatment of skin and soft-tissue infections. There is a need to prioritize detection, antibiogram, and prevention of these strains, particularly in hospital settings in low-income countries.
Currently, vancomycin is regarded as the best choice for treating staphylococci infection [46], but the prevalence of pan-drug resistance VNS-CoNS would continue to overwhelm its available treatment options and enhance the development of the extended-resistant spectrum, thereby increasing morbidity and occasional mortality, particularly in bloodstream infections.
Conclusion
Antibiotic-resistant CoNS should be considered significant pathogens rather than mere contaminants and suitable hospital infection prevention and control must be provided. The use of MALDI-TOF MS analytical protocol would enhance diagnosis and aid proper antimicrobial therapy and further improve the definition of VNS-CoNS infections. There is a high tendency for VNS-S. sciuri and VR-S. condimentii to carry antibiotic-resistant and virulent determinants with the possibility of expressing pathological tropism in the skin, soft tissues, blood-stream, and wound infections. An urgent surveillance would be required to curb unexpected outbreaks in peri-urban and rural communities.
Availability of data and materials
Data sharing is not applicable to this article as no datasets (genomic sequences) were generated or analysed during the current study.
References
Grace JA, Olayinka BO, Onaolapo JA, Obaro SK. Staphylococcus aureus and Coagulase-Negative Staphylococci in Bacteraemia: the epidemiology, predisposing factors Pathogenicity Antimicrobial Resistance. Clin Microbiol. 2019;8:325.
John JF, Davidson RJ, Low DE. Staphylococcus epidermidis and other coagulase-negative staphylococci. Infect Dis Antimicrobial Agents. 2015;45:293–8.
Sheikh AF, Mehdinejad M. Identification and determination of coagulase negative staphylococcal species and antimicrobial susceptibility pattern of isolates from clinical specimens. Afr J Microbiol Res. 2012;6:1669–74.
Akinduti PA, Olasehinde GI, Ejilude O, Taiwo OS, Obafemi YD. Fecal carriage and Phylo-Diversity of Community-Acquired bla TEM Enteric Bacilli in Southwest Nigeria. Infect Drug Resistance. 2018;11:2425–33.
Boamah VE, Agyare C, Odoi H, Adu F, Gbedema SY, Dalsgaard A. Prevalence and antibiotic resistance of coagulase-negative Staphylococci isolated from poultry farms in three regions of Ghana. Infect Drug Resist. 2017;10:175–83.
Zhang Y, Fu Y, Yu J, Ai Q, Li J, Peng N, et al. Synergy of ambroxol with vancomycin in elimination of catheter-related Staphylococcus epidermidis biofilm in vitro and in vivo. J Infect Chemother. 2015;21(11):808–15.
Garrett DO, Jochimsen E, Murfitt K, Hill B, McAllister S, Nelson P, Spera RV, Sall RK, Tenover FC, Johnston J, Zimmer B, Jarvis WR. The emergence of decreased susceptibility to vancomycin in Staphylococcus epidermidis. Infect Control Hosp Epidemiol. 1999;20:167–70.
Ma XX, Wang EH, Liu Y, Luo EJ. Antibiotic susceptibility of coagulase-negative staphylococci (CoNS): Emergence of teicoplaninnon-susceptible CoNS strains with inducible resistance to vancomycin. J Med Microbiol. 2011;60:1661–8.
Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009;134(1–2):45–54.
Asante J, Amoako DG, Abia ALK, Somboro AM, Govinden U, Bester LA, Essack SY. Review of clinically and epidemiologically relevant coagulase-negative Staphylococci in Africa. Microb Drug Resist. 2020;26(8):951–70.
Asante J, Hetsa BA, Amoako DG, Abia ALK, Bester LA, Essack SY. Multidrug-resistant coagulase-negative staphylococci isolated from bloodstream in the uMgungundlovu district of KwaZulu-Natal Province in South Africa: emerging pathogens. Antibiotics 2021;10(2):198.
Szczuka E, Jabłonska L, Kaznowski A. Coagulase-negative staphylococci: pathogenesis, occurrence of antibiotic resistance genes and in vitro effects of antimicrobial agents on biofilm growing bacteria. J Med Microbiol. 2016;65:1405–14.
Gupta V, Singhal L, Chander J. Characterization and antimicrobial susceptibility of coagulase-negative staphylococci isolated from clinical samples. J Lab Physicians. 2018;10:414–9.
Akinduti AP, Osiyemi JA, Banjo TT, Ejilude O, El-Ashker M, Adeyemi AG, et al. Clonal diversity and spatial dissemination of multiantibiotics resistant Staphylococcus aureus pathotypes in Southwest Nigeria. PLoS ONE. 2021;16(2):e0247013.
Santos DC, Costa TM, Rabello RF, Alves FA, Mondino SS, et al. Mannitol-negative methicillin-resistant Staphylococcus aureus from nasal swab specimens in Brazil. Braz J Microbiol. 2015;46(2):531–3.
Laham NAA. Species identification of clinical coagulase-negative Staphylococci isolated in Al-Shifa Hospital Gaza using Matrix-assisted Laser Desorption/ionization-time of flight mass spectrometry. Curr Res Bacteriol. 2017;10:1–8.
Szczuka E, Jabłonska L, Kaznowski A. Coagulase-negative staphylococci: pathogenesis, occurrence of antibiotic resistance genes and in vitro effects of antimicrobial agents on biofilmgrowing bacteria. J Med Microbiol. 2016;65:1405–14.
Qi C, Stratton CW, Zheng X. Phenotypic Testing of Bacterial Antimicrobial Susceptibility. In: Advanced Techniques in Diagnostic Microbiology. Boston: Springer. 2006.
Leclercq R, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect. 2013;19(2):141–60.
Kavanagh A, Ramu S, Gong Y, Cooper MA, Blaskovich MA. Effects of microplate type and broth additives on microdilution MIC susceptibility assays. Antimicrobial Agents Chemotherapy. 2018;63(1):e01760-e1818.
Akinduti PA, Aboderin BW, Oloyede R, Ogiogwa JI, Motayo BO, Ejilude O. High-level multi-resistant and virulent Escherichia coli in abeokuta. Nigeria J Immunoassay Immunochem. 2016;37(2):104–14.
Kirby-bauer Bauer AW, Kirby WM, Sherries JC, Turk M. Antibiotic susceptibility testing by a standard single disc methods. Am J Clin Pathol. 1996;45:493–6.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. 2015.
Thiran E, Di Ciccio PA, Graber HU, Zanardi E, Ianieri A, Hummerjohann J. Biofilm formation of Staphylococcus aureus dairy isolates representing different genotypes. J Dairy Sci. 2017;101:1000–12.
Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, Ruzicka F. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115(8):891–9.
Ibrahim YM, Abouwarda AM, Omar FA. Effect of kitasamycin and nitrofurantoin at subinhibitory concentrations on quorum sensing regulated traits of Chromobacterium violaceum. Antonie Van Leeuwenhoek. 2020;113(11):1601–15.
Robert Weinstein A, Scott FK. Vancomycin-Intermediate and –Resistant Staphylococcus aureus what the infectious disease specialist needs to know. Clin Infect Dis. 2001;32(1):108–15. https://doi.org/10.1086/317542.
Zhou XH, Obuchowski NA, Obushcowski DM. Statistical methods in diagnostic medicine. New York: Wiley & Sons; 2002.
Ezekiel Olugbenga A, Adebayo L. Species distribution and antibiotic resistance in coagulase-negative staphylococci colonizing the gastrointestinal tract of children in Ile-Ife. Nigeria Tropic J Pharmaceut Res. 2010;9(1):35–43.
Szymanek-Majchrzak K, Mlynarczyk A, Mlynarczyk G. Characteristics of glycopeptide-resistant Staphylococcus aureus strains isolated from inpatients of three teaching hospitals in Warsaw, Poland. Antimicrob Resist Infect Control. 2018;7:105.
Manukumar HM, Umesha S. MALDI-TOF-MS based identification and molecular characterization of food associated methicillin-resistant Staphylococcus aureus. Sci Rep. 2017;7:11414.
Ndhlovu GO, Dube FS, Moonsamy RT, Mankahla A, Hlela C, Levin ME, Abdulgader SM. Skin and nasal colonization of coagulase-negative staphylococci are associated with atopic dermatitis among South African toddlers. PLoS ONE. 2022;17(3): e0265326.
Schiffer CJ, Schaudinn C, Ehrmann MA, Vogel RF. SxsA, a novel surface protein mediating cell aggregation and adhesive biofilm formation of Staphylococcus xylosus. Mol Microbiol. 2022;00:1–6.
Becker K, Both A, Weißelberg S, Heilmann C, Rohde H. Emergence of coagulase-negative staphylococci. Expert Rev Anti Infect Ther. 2020;18(4):349–66.
Keim Luiz S, et al. Prevalence, aetiology and antibiotic resistance profiles of coagulase negative staphylococci isolated in a teaching hospital. Braz J Microbiol. 2011;42(1):248–55.
Bora P, Datta P, Gupta V, Singhal L, Chander J. Characterization and antimicrobial susceptibility of coagulase-negative staphylococci isolated from clinical samples. J Lab Physicians. 2018;10:414–9.
Liu X, Su T, Hsu YS, Yu H, Yang HS, Jiang L, Zhao Z. Rapid identification and discrimination of methicillin-resistant Staphylococcus aureus strains via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2021;35(2):30.
El-Ashker M, Gwida M, Monecke S, Ehricht R, Elsayed M, El-Gohary F, Reißig A, Müller E, Paul A, Igbinosa EO, Beshiru A, Maurischat S. Microarray-based detection of resistance genes in coagulase-negative staphylococci isolated from cattle and buffalo with mastitis in Egypt. Trop Anim Health Prod. 2020;52(6):3855–62.
Flores-Treviño S, Garza-González E, Mendoza-Olazarán S, et al. Screening of biomarkers of drug resistance or virulence in ESCAPE pathogens by MALDI-TOF mass spectrometry. Sci Rep. 2019;9:18945.
Guyon I, Elisseeff A. An introduction to variable and feature selection. J Mach Learn Res. 2003;3:1157–82.
Nunes AP, Teixeira LM, Iorio NL, Bastos CC, Sousa Fonseca L, Souto-Padron T, Santos KR. Heterogeneous resistance to vancomycin in Staphylococcus epidermidis, Staphylococcus haemolyticus and Staphylococcus warneri clinical strains characterisation of glycopeptide susceptibility profiles and cell wall thickening. Int J Antimicrob Agents. 2006;27:307–15.
Henshaw EB, Olasode OA, Ogedegbe EE, Etuk I. Dermatologic conditions in teenage adolescents in Nigeria. Adolesc Health Med Ther. 2014;4(5):79–87.
Tina H. Dao, Ramzi Alsallaq, Joshua B. Parsons, Jose Ferrolino, Randall T. Hayden, Jeffrey E. Rubnitz, Iftekhar M. Rafiqullah, D. Ashley Robinson, Elisa B. Margolis, Jason W. Rosch, Joshua Wolf. Vancomycin Heteroresistance and Clinical Outcomes in Bloodstream Infections Caused by Coagulase-Negative Staphylococci. Antimicrobial Agents Chemotherapy; 2020;64(11):e00944-20
Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003;302(5650):1569–71.
Wang HY, Lee TY, Tseng YJ, Liu TP, Huang KY, Chang YT, Chen CH, Lu JJ. A new scheme for strain typing of methicillin-resistant Staphylococcus aureus on the basis of matrix-assisted laser desorption ionization time-of-flight mass spectrometry by using machine learning approach. PLoS ONE. 2018;13(3): e0194289.
Ibrahim SF, El-Banna TE, El-Aziz AAA, El-Ekhnawy E. Biological characters of vancomycin resistant staphylococcus aureus isolates from a university hospital in Egypt. Arch Clin Microbiol. 2018;9(1):72.
Acknowledgements
The authors thank the management of Federal Medical Centre, Abeokuta for assisting data collection, Dr. H. Wichmann-Schauer and Dr. S Maurischat of German Federal Institute for Risk Assessment, Unit Microbial Toxins, NRL for Coagulase Positive Staphylococci, Department Biological Safety, Berlin, Germany for their technical assistance.
Funding
Deutsche Forschungsgemeinschaft (DFG), Germany in cooperation with the World Academy of Science (TWAS), Italy for providing sponsorship for the genomic analysis at the Staphylococci Unit, BfR, Germany. The publication was partly supported by the CUCRID, Covenant University, Ota, Nigeria. The aforementioned support agents had no role in study design, data collection and analysis, interpretation of data, the decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Each author has made a substantial contribution as follows; PAA, OA, and YDO provide conception of the study; PAA, OA, ME, HU, CJA, AO, BSJP and SUO analysed and interpreted the data; PA and YDO performed genomic analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written ethical permission for the study was obtained from Federal Medical Centre, Abeokuta Nigeria which serves as a referral clinic for internal medicine in Southwest Nigeria with approval number: FMCA/470/HREC/09/2017 and NHREC/08/10–2015.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Biotyper data processing of spectra signature characterizing the strains.
Additional file 2: Tabel S1
. Primers and probes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Akinduti, P.A., Obafemi, Y.D., Ugboko, H. et al. Emerging vancomycin-non susceptible coagulase negative Staphylococci associated with skin and soft tissue infections. Ann Clin Microbiol Antimicrob 21, 31 (2022). https://doi.org/10.1186/s12941-022-00516-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-022-00516-4