Abstract
Patients with bloodstream infections (BSI) are associated with high mortality rates. Due to tigecycline has shown excellent in vitro activity against most pathogens, tigecycline is selected as one of the candidate drugs for the treatment of multidrug-resistant organisms infections. The purpose of this study was to evaluate the effectiveness and safety of the use of tigecycline for the treatment of patients with BSI. The PubMed and Embase databases were systematically searched, to identify published studies, and we searched clinical trial registries to identify completed unpublished studies, the results of which were obtained through the manufacturer. The primary outcome was mortality, and the secondary outcomes were the rate of clinical cure and microbiological success. 24 controlled studies were included in this systematic review. All-cause mortality was lower with tigecycline than with control antibiotic agents, but the difference was not significant (OR 0.85, [95% confidence interval (CI) 0.31–2.33; P = 0.745]). Clinical cure was significantly higher with tigecycline groups (OR 1.76, [95% CI 1.26–2.45; P = 0.001]). Eradication efficiency did not differ between tigecycline and control regimens, but the sample size for these comparisons was small. Subgroup analyses showed good clinical cure result in bacteremia patients with CAP. Tigecycline monotherapy was associated with a OR of 2.73 (95% CI 1.53–4.87) for mortality compared with tigecycline combination therapy (6 studies; 250 patients), without heterogeneity. Five studies reporting on 398 patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae BSI showed significantly lower mortality in the tigecycline arm than in the control arm. The combined treatment with tigecycline may be considered the optimal option for severely ill patients with BSI.
Similar content being viewed by others
Background
Bloodstream infections (BSI) are potentially life-threatening diseases. BSI was defined as at least 1 positive blood culture for a recognized pathogen and clinical symptoms consistent with bacteraemia. They can cause serious secondary infections, such as infective endocarditis and osteomyelitis, and may result in severe sepsis. Meanwhile, BSI due to multidrug-resistant (MDR) organisms has been associated with multiple poor outcomes, including increased length of hospital stay, health care costs and a high rate of morbidity and mortality.
Tigecycline is a glycylcycline with a broad spectrum of antibacterial activity. The emergence of MDR strains infections has been extensively observed worldwide and has become a priority issue over past decade. Tigecycline is a useful alternative to face the challenges of many MDR organisms. Tigecycline has a large volume of distribution of 7–10 l/kg [1], penetrating well into different tissues, it has been approved for the treatment of complicated skin and soft-structure infections (cSSSI), complicated intra-abdominal infections (cIAI), and community-acquired bacterial pneumonia (CAP). Tigecycline is not indicated for treatment of diabetic foot infection or for hospital-acquired or ventilator-associated pneumonia [2]. The use of tigecycline in bacteremia is controversial because of its low serum levels with standard dosing [3].
Attention should be paid by clinicians, because tigecycline was associated with higher mortality than comparator antibiotics [4–6]. However, a recent meta-analysis showed that the drug was not associated with significantly higher mortality than comparator antibiotics and was as effective as comparators when the analysis was restricted to patients who received tigecycline for approved indications [7]. A prospective study demonstrates that tigecycline plus prolonged infusion standard-dose imipenem/cilastatin, showed good clinical efficacy on VAP patients with XDR-Ab VAP bacteremia [8]. The increased mortality associated with tigecycline is not yet well understood in the treatment of BSI. Therefore, we systemically searched and analysed the current available evidence to assess clinical effectiveness of tigecycline for the treatment of BSI.
Methods
Literature search
Relevant studies were identified through PubMed, Embase and hand-searched from inception until October 2016.The search terms were:“(tigecycline OR TGC OR tygacil) and (bacteraemia OR bacteremia OR bloodstream infection OR sepsis OR septicaemia)”. No language restrictions were applied.
Study selection
Any article providing the clinical outcomes of patients treated for bloodstream infections caused by any etiological agent was considered eligible for inclusion in the review. Prospective and retrospective observational cohort studies examining the association between tigecycline use (on hospital admission or previous users) and the outcomes of bacteremic patients were included. The outcome of interest was overall hospital mortality at the longest follow-up at each single study. Case reports and case series including fewer than 10 infected patients treated with tigecycline were excluded from the review.
Data extraction
The extracted data consisted of the main characteristics of a study (first-author name, year of publication, country, study period, and design), main characteristics and underlying diseases of the study population, number of patients with infections BSI, the causative pathogen(s), sites of infections, and antibiotic treatment (combination therapy or monotherapy). Clinical outcomes (mortality, treatment failure) of patients in each treatment group were recorded as well.
Statistical analysis
We chose mortality as the primary outcome, because of the high mortality rates among patients with BSI, while the secondary outcomes were: clinical response, microbiological response, adverse effects, and emergence of resistance. Microbiological response was defined as successful when eradication or sterile culture results were obtained during or after the antibiotic therapy. Because there are no standard criteria to assess clinical response and adverse events, we accepted the criteria as reported in each study.
All statistical analyses were performed using the comprehensive meta-analysis V2.2 (BioStat, Englewood, NJ). Among the controlled studies, the between-study heterogeneity was assessed using the I2 test, whereby I2 values >50% were defined as indicating heterogeneity. Either fixed-effects (Mantel–Haenszel method) or random-effects (DerSimonian and Laird method) models were used, depending on the heterogeneity result. If no heterogeneity was found, meta-analysis was done using the Mantel–Haenszel fixed-effects model. Binary outcomes from controlled studies were expressed as odds ratios (OR) with their 95% confidence intervals (CI), and continuous outcomes were expressed as the mean difference between 2 groups. Egger regression, as well as the Begg methods, was used to evaluate publication bias. All P values were two-tailed, and a P value of ≤0.05 was considered statistically significant. Some statistical analysis was performed by using the SPSS statistical software (version 19; SPSS Inc., Chicago, IL). Categorical variables were evaluated by using the χ2 test or 2-tailed Fisher’s exact test, as appropriate. Subgroup analyses for mortality and clinical cure were planned for bacteraemic patients. Comparisons were subcategorized by the type of infection. A funnel plot was used to assess small-study effects.
Results
Literature search results
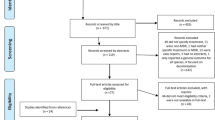
1540 potential articles were identified; 56 case reports and clinical series including less than 10 infected patients were excluded; 41 duplicates and 18 single-arm studies were excluded; 22 studies were ruled out because they did not present clear treatment regimens or detailed clinical outcomes; 24 articles were excluded due to few patients in each group. Ultimately 24 studies met the inclusion criteria, 24 controlled studies (1961 patients) included in this systematic review.
Study characteristics
The features of the 24 trials are described in Table 1. Five of them were prospective cohort studies, 7 were retrospective studies. All of the included controlled studies had an NOS score >3. Most patients in the included studies were critically ill, with most of them in ICU.
Mortality
As shown in Fig. 1, no significant difference was noted when tigecycline was compared with control groups in terms of all-cause mortality (14 studies; 1502 patients) [OR 0.841, 95% confidence interval (CI) 0.517–1.367; P = 0.485]. Because statistical heterogeneity existed among studies (X2 = 32.76, df = 13, (P = 0.002), I2 = 60.3%), a random-effects model of analysis was used. No publication bias was detected by Egger regression (t = −0.39; df = 12.0; P = 0.701) or Begg (z = 0.55; df = 12.0; P = 0.584).
Table 2 shows the subgroup analysis of the controlled studies. A significant difference was observed between the tigecycline monotherapy therapy group and the tigecycline combination therapy group in terms of mortality (6 studies; 250 patients) (OR 2.733, [95% CI 1.533–4.873; P = 0.001]; I2 = 8.7%). A significantly higher mortality was noted in the monotherapy group than in the combination therapy group in cases of blood stream infection. The mortality in the combination of tigecycline plus colistin based group was not significantly lower than that in the other antibiotics combination group (OR 0.68, [95% CI 0.407–1.135; P = 0.14]; I2 = 0.0%).
In the patients infected with Klebsiella pneumoniae (Kp) BSI, tigecycline seemed to have a lower mortality than comparator drugs, but the difference was not significant (OR 0.678, [95% CI 0.457–1.006; P = 0.054]; I2 = 0.0%; [P = 0.556]). Five studies (398 patients) reported data on carbapenemase-producing Kp BSI, and a significant difference with respect to overall mortality was observed between the tigecycline therapy group and the controls (OR 0.636, [95% CI 0.417–0.971; P = 0.036]; I2 = 0.0%; [P = 0.507]). Three controlled studies (221 patients) reported Acinetobacter BSI, no difference was seen between patients who received tigecycline as therapy and others in mortality (OR 0.967, [95% CI 0.096–0.759; P = 0.978]; I2 = 91.6%; [P = 0.001]).
Clinical cure
There was a significant differences were observed between the tigecycline and control groups in this regard (OR 1.76, [95% CI 1.26–2.45; P = 0.001]; I2 = 29.2%; [P = 0.159]; Fig. 2). Clinical cure was significantly higher in the tigecycline population. In the subgroup analysis, for analysis by type of infection, without statistical significance was found in patients with cIAI (OR 0.97, [95% CI 0.52–1.80; P = 0.919]; I2 = 0.0%; [P = 0.953]) and cSSSI (OR 0.71, [95% CI 0.26–1.90; P = 0.494]; I2 = 0.0%; [P = 0.821]), but in trials assessing patients with CAP, for the rate of clinical cure, the efficacy of tigecycline was better than that of comparator regimens (OR 2.44, [95% CI 1.20–4.94; P = 0.013]; I2 = 0.0%; [P = 0.821]). As shown in Fig. 2.
Microbiological response
As shown in Fig. 2, tigecycline group did not differ significantly compared with the comparators in the rate of microbiological success (OR 2.07, [95% CI 0.56–7.70; P = 0.279]; I2 = 0.0%; [P = 0.854]) (Fig. 2).
Adverse effects
There were not sufficiently effective data to be recoded, so that the common adverse effects of tigecycline (nausea, vomiting, and diarrhea) could not be extracted in any of the studies.
Discussion
We conducted this systematic review and meta-analysis to investigate the effectiveness and safety of tigecycline for the treatment of BSI. Numerous studies have established bacteremia as a marker of severe infection and a risk for adverse outcomes in multiple treatment settings [9, 10], but there were some positive elements about the treatment of BSI with tigecycline.
To our knowledge, this was the first systematic review to assess the efficacy of tigecycline in treating BSI. Although all-cause mortality was lower with tigecycline than with the control regimens, the difference was not significant. Tigecycline seemed to be better than levofloxacin for treatment of community-acquired pneumonia, and worse than control regimens for cIAI and cSSSI, but these differences were not significant. However, drug safety guidelines published by the FDA refer to an increased mortality risk associated with intravenous tigecycline compared with other drugs used to treat serious infections (risk difference = 0.6%, 95% CI 0.1–1.2) [2]. This result has been confirmed by a study that associated the increased risk mortality with resistant pathogens, hospital-acquired pneumonia, and increased age of patients [11]. However the type of serious infections didn’t include BSI. We used the same effect metric to assess our results, and noted that the risk difference of all-cause mortality was not significant (−3.5%, 95% CI −13 to −6; I2 = 85.4%, P = 0.001).
Although the overall mortality did not differ between tigecycline and the control groups, subgroup analysis found the mortality was significantly lower in the tigecycline combination group than in the tigecycline monotherapy therapy group. Tigecycline in combination with colistin, carbapenem in combination with colistin, and tigecycline in combination with gentamicin were the commonly administered antibiotic treatment regimens among the included studies and might result in lower mortality than other combinations of antibiotics. The most common combination was tigecycline with colistin in tigecycline combination therapy group, yet this data did not necessarily predict tigecycline plus polymyxins based therapy was significantly better than other antibiotics combination therapy. For the patients with KPC-Kp BSI, antibiotic therapy with tigecycline was associated with lower mortality.
With regard to clinical response, the evidence that we could compile from studies was that tigecycline therapy may have no clinical advantage over comparator therapy, but may result in better clinical cure in treatment of CAP presenting with bacteremia.
Tigecycline had good eradication ability for most pathogens recorded at baseline, as a novel glycylcycline antibiotic, it has a broad spectrum of antimicrobial activity, ranging from aerobic to anaerobic bacteria, and gram-positive, gram-negative (exceptions of Pseudomonas aeruginosa and Proteus mirabilis), and atypical organisms [12]. Eradication was better than with control regimens in all cases, although no significant difference was found when tigecycline was compared with the comparators.
Previous studies have shown that the most common adverse effects of tigecycline had increased incidence in the tigecycline group, such as nausea, vomiting, and diarrhea [13, 14]. According to a recently published review, tigecycline induces acute pancreatitis, indicating that surveillance for adverse events from the digestive system is needed during treatment [15]. But lack of data from all trials results can not be obtained about adverse events outcomes in our meta-analysis.
Small non-comparative series have reported relatively poor clinical and microbiological outcomes with tigecycline for tigecycline-susceptible CR-Ab bacteremia [16–18]. The high severity of illness and the notable delays in initiation of effective antimicrobial therapy could also explain these results. In a pooled, retrospective data analysis of phase 3 clinical trials, 91 patients being treated with tigecycline had secondary bacteremia detected, tigecycline appeared safe and well tolerated in the treatment of secondary bacteremia associated with cSSSI, cIAI, and CAP; cure rates were similar to comparative standard therapies [19]. Recently, a high-dose regimen (loading dose 200 mg followed by 100 mg every 12 h) has been successfully and safely used in critically ill patients with severe infections due to multi drug resistant bacteria although the number of primary bacteremia was anecdotal [20].
Several potential limitations should be taken into consideration when interpreting the present results. Firstly, the number of subjects included was not large enough. We would have preferred to contact researchers directly for missing data, but this approach was not attempted because of time constraints. Secondly, in some subgroup analyses, the sample size was small, which may have reduced the power of the statistical analysis. Another important issue is that the administrations of the antibiotics differed among the studies with regard to the duration of infusion or the total daily dose. Thirdly, due to the included studies did not provide relevant data, we were unable to assess the impact of tigecycline on adverse drug reactions. Accordingly, these differences might influence the clinical outcomes. Last, the matter of the emergence of resistance during therapy was not raised by any of the included studies.
In conclusion, based on a review of published cases, tigecycline appears to have produced some favourable clinical and microbiological outcomes in patients with BSI, even when used as monotherapy. This research was needed to clarify whether tigecycline was suitable for treatment such infections when other antibiotics fail, especially because indications for increased risk of all-cause mortality have been reported in patients treated with this drug. The FDA has recently reported an increased risk of death when intravenous tigecycline is used for FDA approved purposes [21], which may be explained by a worsening infection or potential complications [11].
The available evidence suggests that combination antibiotic treatment may offer a comparative advantage over monotherapy with regard to the mortality of critically ill patients with severe infections due to BSI. The number of currently available appropriate antimicrobial agents is limited, combination therapy with tigecycline, it could be a fine option for the treatment of BSI, especially in patients with KPC-Kp BSI.
Abbreviations
- BSI:
-
bloodstream infection
- cIAI:
-
complicated intra-abdominal infections
- cSSSI:
-
complicated skin and skin-structure infections
- CAP:
-
community-acquired pneumonia
- CR-Kp:
-
carbapenem-resistant K. pneumoniae
- CVC-CoNS:
-
central venous catheter-related coagulase-negative staphylococcal
- KPC-Kp:
-
Klebsiella pneumoniae carbapenemase-producing K. pneumoniae
- MRSA:
-
methicillin-resistant Staphylococcus aureus
- VAP:
-
ventilator-associated pneumonia
- VRE:
-
vancomycin-resistant enterococci
- XDR-Ab:
-
extensively drug-resistant Acinetobacter baumannii
References
Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother. 2004;49(1):220–9.
FDA drug safety communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. http://www.fda.gov/drugs/drugsafety/ucm224370.htm.
Stein GE, Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis. 2013;75(4):331–6.
Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54(12):1699–709.
Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(9):1963–71.
Food and Drug Administration: Tygacil (tigecycline): drug safety communication-increased risk of death. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm370170.htm.
Vardakas KZRP, Falagas ME. Effectiveness and safety of tigecycline: focus on use for approved indications. Clin Infect Dis. 2012;54(11):1672–4.
Jean SS, Hsieh TC, Hsu CW, Lee WS, Bai KJ, Lam C. Comparison of the clinical efficacy between tigecycline plus extended-infusion imipenem and sulbactam plus imipenem against ventilator-associated pneumonia with pneumonic extensively drug-resistant Acinetobacter baumannii bacteremia, and correlation of clinical efficacy with in vitro synergy tests. J Microbiol Immunol Infect. 2016;49(6):924–33.
Roberts FJ, Geere IW, Coldman A. A three-year study of positive blood cultures, with emphasis on prognosis. Rev Infect Dis. 1991;13(1):34–46.
Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602.
McGovern PC, Wible M, El-Tahtawy A, Biswas P, Meyer RD. All-cause mortality imbalance in the tigecycline phase 3 and 4 clinical trials. Int J Antimicrob Agents. 2013;41(5):463–7.
Nicolau DP. Management of complicated infections in the era of antimicrobial resistance: the role of tigecycline. Expert Opin Pharmacother. 2009;10(7):1213–22.
Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11(11):834–44.
McGovern P, Leister N, Zito E, Tucker N, Mansfield D, Babinchak T. Efficacy of tigecycline versus ceftriaxone and metronidazole for complicated intra-abdominal infections-analysis of pooled clinical trial data. Clin Microbiol Infect. 2010;16(suppl 2):S448.
Hung WY, Kogelman L, Volpe G, Iafrati M, Davidson L. Tigecycline-induced acute pancreatitis: case report and literature review. Int J Antimicrob Agents. 2009;34(5):486–9.
Kim NH, Hwang JH, Song KH, Choe PG, Kim ES, Park SW, Kim HB, Kim NJ, Park WB, Oh MD. Tigecycline in carbapenem-resistant Acinetobacter baumannii bacteraemia: susceptibility and clinical outcome. Scand J Infect Dis. 2013;45(4):315–9.
Guner R, Hasanoglu I, Keske S, Kalem AK, Tasyaran MA. Outcomes in patients infected with carbapenem-resistant Acinetobacter baumannii and treated with tigecycline alone or in combination therapy. Infection. 2011;39(6):515–8.
Gordon NC, Wareham DW. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J Antimicrob Chemother. 2009;63(4):775–80.
Gardiner D, Dukart G, Cooper A, Babinchak T. Safety and efficacy of intravenous tigecycline in subjects with secondary bacteremia: pooled results from 8 phase III clinical trials. Clin Infect Dis. 2010;50(2):229–38.
De Pascale G, Montini L, Pennisi M, Bernini V, Maviglia R, Bello G, Spanu T, Tumbarello M, Antonelli M. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18(3):R90.
FDA drug safety communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning. http://www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
Oliva ME, Rekha A, Yellin A, Pasternak J, Campos M, Rose GM, Babinchak T, Ellis-Grosse EJ, Loh E. A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744]. BMC Infect Dis. 2005;5:88.
Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322–8.
Florescu I, Beuran M, Dimov R, Razbadauskas A, Bochan M, Fichev G, Dukart G, Babinchak T, Cooper CA, Ellis-Grosse EJ, et al. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a phase 3, multicentre, double-blind, randomized study. J Antimicrob Chemother. 2008;62(Suppl 1):i17–28.
Sacchidanand S, Penn RL, Embil JM, Campos ME, Curcio D, Ellis-Grosse E, Loh E, Rose G. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int J Infect Dis. 2005;9(5):251–61.
Liou BH, Lee YT, Kuo SC, Liu PY, Fung CP. Efficacy of tigecycline for secondary Acinetobacter bacteremia and factors associated with treatment failure. Antimicrob Agents Chemother. 2015;59(6):3637–40.
Cheng A, Chuang YC, Sun HY, Sheng WH, Yang CJ, Liao CH, Hsueh PR, Yang JL, Shen NJ, Wang JT, et al. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med. 2015;43(6):1194–204.
Tanaseanu C, Bergallo C, Teglia O, Jasovich A, Oliva ME, Dukart G, Dartois N, Cooper CA, Gandjini H, Mallick R, et al. Integrated results of 2 phase 3 studies comparing tigecycline and levofloxacin in community-acquired pneumonia. Diagn Microbiol Infect Dis. 2008;61(3):329–38.
Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–50.
Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–803.
Bucaneve G, Micozzi A, Picardi M, Ballanti S, Cascavilla N, Salutari P, Specchia G, Fanci R, Luppi M, Cudillo L, et al. Results of a multicenter, controlled, randomized clinical trial evaluating the combination of piperacillin/tazobactam and tigecycline in high-risk hematologic patients with cancer with febrile neutropenia. J Clin Oncol. 2014;32(14):1463–71.
Papadimitriou-Olivgeris M, Marangos M, Christofidou M, Fligou F, Bartzavali C, Panteli ES, Vamvakopoulou S, Filos KS, Anastassiou ED. Risk factors for infection and predictors of mortality among patients with KPC-producing Klebsiella pneumoniae bloodstream infections in the intensive care unit. Scand J Infect Dis. 2014;46(9):642–8.
Breedt J, Teras J, Gardovskis J, Maritz FJ, Vaasna T, Ross DP, Gioud-Paquet M, Dartois N, Ellis-Grosse EJ, Loh E, et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother. 2005;49(11):4658–66.
Babinchak T, Ellis-Grosse E, Dartois N, Rose GM, Loh E. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis. 2005;41(Suppl 5):S354–67.
Ellis-Grosse EJ, Babinchak T, Dartois N, Rose G, Loh E. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis. 2005;41(Suppl 5):S341–53.
Fomin P, Beuran M, Gradauskas A, Barauskas G, Datsenko A, Dartois N, Ellis-Grosse E, Loh E, Three Hundred Six Study G. Tigecycline is efficacious in the treatment of complicated intra-abdominal infections. Int J Surg. 2005;3(1):35–47.
Dartois N, Cooper CA, Castaing N, Gandjini H, Sarkozy D. Tigecycline versus levofloxacin in hospitalized patients with community-acquired pneumonia: an analysis of risk factors. Open Respir Med J. 2013;7:13–20.
Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56(4):2108–13.
Lauf L, Ozsvar Z, Mitha I, Regoly-Merei J, Embil JM, Cooper A, Sabol MB, Castaing N, Dartois N, Yan J, et al. Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. Diagn Microbiol Infect Dis. 2014;78(4):469–80.
Gomez-Simmonds A, Nelson B, Eiras DP, Loo A, Jenkins SG, Whittier S, Calfee DP, Satlin MJ, Kubin CJ, Furuya EY. Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2016;60(6):3601–7.
Maki D, Lentnek A, Sheftel T, Paladino J, Feuerstein S, Jagodzinski L, Schentag J. P284 Prospective multicenter trial of tigecycline for treatment of central venous catheter (CVC)-related coagulase-negative staphylococcal (CoNS) bacteremia. Int J Antimicrob Agents. 2009;34(09):S115.
de Oliveira MS, de Assis DB, Freire MP, do Prado GB, Machado AS, Abdala E, Pierrotti LC, Mangini C, Campos L, Caiaffa Filho HH, et al. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect. 2015;21(2):179.
Authors’ contributions
WJ and PYP designed the study, performed the articles search and screen. WJ wrote the paper. WJ, PYP and XYH performed the Statistical analysis. SJL reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data and material in this review is authentic and available.
Consent for publication
Informed consent was obtained from all individual participants included in the review.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, J., Pan, Y., Shen, J. et al. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 16, 24 (2017). https://doi.org/10.1186/s12941-017-0199-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-017-0199-8