Abstract
Background
The diagnosis of invasive pulmonary aspergillosis is challenging. It is unclear whether galactomannan (GM) results from bronchial wash (BW) and bronchoalveolar lavage (BAL) samples differ in a clinically meaningful way.
Results
Ninety-six paired (BAL and BW) samples from 85 patients were included. The average age was 53 years, 61 % of the patients were male, and 74.1 % had an underlying diagnosis of AML/MDS (ALL 7.1 %, other hematologic malignancy 18.8 %). 57 (67.1 %) patients were neutropenic, and 56 (65.9 %) patients were receiving mold-active drugs at least 48 h prior to bronchoscopy. The overall agreement between GM detection from BW and BAL was 63.5 % (K = 0.152; 95 % CI 0.008–0.311) and 73 % (K = 0.149; 95 % CI 0.048–0.348) at cut off ≥0.5 and ≥1.0, respectively. Among 43 positive samples, using a GM cut-off of 0.5, 39 (90.5 %) were positive in BW samples whereas 12 (29.3 %) were positive in BAL samples. The median level of GM in BW (0.28) samples was significantly higher than in BAL (0.20) samples among 53 samples with negative results (P = 0.001). There was no statistically significant difference in the median GM values between the BW and BAL samples with positive results (P = 0.08). There was no significant difference in GM detection between samples with positive and negative results with regard to antifungal, beta lactam antibacterial treatment or neutropenia (60.5 vs 56.6 %; 53.9 vs 46 %; 65.1 vs 54.7 %, respectively).
Conclusion
This retrospective study examining two collection techniques suggests that BW may have higher diagnostic yield compared to bronchoalveolar lavage for GM detection.
Similar content being viewed by others
Findings
The mortality rate of invasive pulmonary aspergillosis (IPA) remains high in part due to barriers to early diagnosis [1–3]. A definite diagnosis of IPA requires histological evidence of invasive disease or positive cultures from sterile sites [4]. However, respiratory cultures have poor sensitivity and tissue biopsy is not always feasible [5, 6]. Therefore, current diagnostic approaches include non-culture/histopathologic modalities including combination of chest CT imaging and non-invasive, non-culture based tools such as galactomannan (GM) antigen detection in bronchoalveolar lavage (BAL) and bronchial wash (BW) [7–11]. BW samples are defined as the first obtained aliquot of BAL fluid after instillation of small amount of saline into a major airway; BAL samples are collected from the distal bronchioles and alveoli following instillation of a large volume of saline often greater than 140 ml in several aliquots [12]. It is unclear whether GM results from BW and BAL samples differ in a clinically meaningful way. It is hypothesized that bronchial airways may contain more aspergillus hyphae with more subsequent GM release than in alveoli. Alternatively, GM in BAL fluid may be diluted by lavage to an undetectable level by ELISA [13, 14].
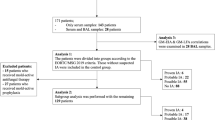
In this study, we aimed to compare the performance characteristics of GM antigen tests obtained from BW and BAL samples. This retrospective study included all adult patients with hematologic malignancies at the University of Maryland Greenebaum Cancer Center who underwent bronchoscopy for evaluation of new pulmonary infiltrates while receiving broad spectrum antibiotics or progression of existing lung infiltrates from October 2010 to September 2013. Only patients who had concurrent paired BAL and BW samples separately tested for GM antigen during the same procedure were included. The Platelia Aspergillus enzyme immunoassay (Bio-Rad Laboratories, Hercules, CA, USA) was used to measure GM antigen levels, using different cutoffs for positivity (≥0.5 and ≥1). A patient who underwent repeated sampling in separate bronchoscopic procedures was included in the analysis if bronchoscopy had been performed on the basis of a new pulmonary infiltrate at least 2 months after the first procedure. Plasmalyte solution was not used to perform BAL. Kappa statistics were used to assess the agreement between the GM results obtained from BW and BAL samples. The Wilcoxon test and McNemar’s test were used, as appropriate. All data analyses were performed using SAS V.8.0. 96 paired samples (BAL and BW) were analyzed from 85 patients (AML/MDS 74.12 %, ALL 7.06 % and the others 18.82 %) comprising 52 males and 33 females with an average age of 53 years (range 20–81). 57 (67.06 %) patients were neutropenic (absolute neutrophil count <500//mm3) and 56 patients (58.3 %) were receiving mold-active drugs at least 48 h prior to bronchoscopy. Piperacillin-tazobactam was used in nine patients, none had a positive GM test from BAL or BW. Concurrent serum GM assays were performed in only 14 patients (16 %) and three of which were positive at a cutoff ≥0.5. Of these three, all three were positive in samples collected by BW but only one was positive in samples collected by BAL (using OD cutoff value of ≥0.5). Of the 11 negative serum GM results, four were positive by BW samples alone, and one was positive by BAL sample alone; the remainder were negative by both BAL and BW. Of the total four culture-positive BAL samples, one yielded aspergillus fumigatus (with a GM of ≥1 from both BAL and BW), and three yielded penicillium species, of which only one had a positive GM >0.5 from BW sample alone.
The overall agreement between GM detection from BW and BAL was 63.5 % (K = 0.152; 95 % CI 0.008–0.311) and 73 % (K = 0.149; 95 % CI 0.048–0.348) at a diagnostic cut off ≥0.5 and ≥1.0, respectively. Among 43 positive samples, using a GM cut-off of 0.5, 39 (90.5 %) were positive in BW samples whereas 12 (29.3 %) were positive in BAL samples. The GM assay performance for BW and BAL fluid samples for different cut-off OD indexes is shown in Table 1.
The median levels of GM in BW (0.28) samples were significantly higher than in BAL (0.20) samples among 53 samples with negative results (P = 0.001). Though there was no statistically significant difference in median GM values between the BW and BAL samples with positive results (median OD index of 1.3 for the BW samples vs 3.5 for the BAL samples, P = 0.08), there was a higher number of positive GM results (OD index cut-off of 0.5) only detectable by BW (54.1 %) compared to those detectable only by BAL (9.3 %, P < 0.0001). There was no significant difference in GM detection between samples with positive and negative results with regard to antifungal, antibacterial treatment or neutropenia (60.5 vs 56.6 %; 53.9 vs 46 %; 65.1 vs 54.7 %, respectively).
Conclusions
To our knowledge only two studies have compared GM antigen detection yield in BAL and BW with conflicting results [15, 16]. Similar to the results observed by Seyfarth et al., our study demonstrated that BW and BAL results are not comparable in terms of detection of GM [15].
The poor concordance between the results of GM detection from the two sample methods in our retrospective single center study suggests that the addition of BW to BAL GM detection enhances positive results or it might be a better single diagnostic sample for GM antigen detection. However, we were unable to establish test specificity/sensitivity due to lack of a gold standard, insensitivity of aspergillus species culture and lack of concurrent serum GM assay (proven and probable cases). Further studies are needed to confirm the complementary role of the BW and to evaluate sensitivity, specificity of GM detection in BW and BAL in IPA diagnosis.
Availability of supporting data
The data supporting the results of this study are included within this article.
Abbreviations
- GM:
-
galactomannan antigen
- BW:
-
bronchial wash
- BAL:
-
bronchoalveolar lavage
- OD:
-
optical density
References
Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Amé S, Fohrer C, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–84.
Gallien S, Fournier S, Porcher R, Bottero J, Ribaud P, Sulahian A, et al. Therapeutic outcome and prognostic factors of invasive aspergillosis in an infectious disease department: a review of 34 cases. Infection. 2008;36:533–8.
Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–15.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the european organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46(12):1813–21.
Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22.
Reichenberger F, Habicht JM, Gratwohl A, Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19:743–55.
Musher B, Fredricks D, Leisenring W, Balajee SA, Smith C, Marr KA. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J Clin Microbiol. 2004;42:5517–22.
Kono Y, Tsushima K, Yamaguchi K, Kurita K, Soeda S, Fujiwara A, et al. The utility of galactomannan antigen in the bronchial washing and serum for diagnosing pulmonary aspergillosis. Respir Med. 2013;107:1094–100.
Guo YL, Chen YQ, Wang K, Qin SM, Wu C, Kong JL. Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: a bivariate metaanalysis and systematic review. Chest. 2010;138:817–24.
Park SY, Lee SO, Choi SH, Sung H, Kim MN, Choi CM, et al. Aspergillus galactomannan antigen assay in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Infect. 2010;61:492–8.
Norkin M, Wingard JR. Diagnostic strategies for invasive fungal infections in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J Natl Compr Canc Netw. 2013;11:941–9.
Baselski VS, Wunderink RG. Bronchoscopic diagnosis of pneumonia. Clin Microbiol Rev. 1994;7:533–58.
Klont RR, Mennink-Kersten MA, Verweij PE. Utility of Aspergillus antigen detection in specimens other than serum specimens. Clin Infect Dis. 2004;39:1467–74.
Kauffman HF, Beaumont F, Meurs H, van der Heide S, de Vries K. Comparison of antibody measurements against Aspergillus fumigatus by means of double-diffusion and enzyme-linked immunosorbent assay (ELISA). J Allergy Clin Immunol. 1983;72:255–61.
Seyfarth HJ, Nenoff P, Winkler J, Krahl R, Haustein UF, Schauer J. Aspergillus detection in bronchoscopically acquired material. Significance and interpretation. Mycoses. 2001;44:356–60.
Racil Z, Kocmanova I, Toskova M, Buresova L, Weinbergerova B, Lengerova M, et al. Galactomannan detection in bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in patients with hematological diseases—the role of factors affecting assay performance. Int J Infect Dis. 2011;15:e874–81.
Authors’ contributions
MT and PR designed the study. MT, EW and PR collected the data; MT analyzed and interpreted the data. MT and PR wrote the manuscript. BG and MK critically reviewed the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Taremi, M., Kleinberg, M.E., Wang, E.W. et al. Galactomannan antigen detection using bronchial wash and bronchoalveolar lavage in patients with hematologic malignancies. Ann Clin Microbiol Antimicrob 14, 50 (2015). https://doi.org/10.1186/s12941-015-0111-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-015-0111-3