Abstract
Background
Retinal biomarkers of Alzheimer’s disease (AD) have been extensively investigated in recent decades. Retinal nervous and vascular parameters can reflect brain conditions, and they can facilitate early diagnosis of AD.
Objective
Our study aimed to evaluate the difference in retinal neuro-layer thickness and vascular parameters of patients with AD and healthy controls (HCs).
Methods
Non-invasive optical coherence tomography angiography (OCTA) was used to determine the combined thickness of the retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL), as well as the full retinal thickness (FRT). The vascular branching (VB), vascular curvature (VC), and vascular density (VD) for AD and HC groups were also obtained. The Mini-Mental State Examination (MMSE) was used to evaluate the cognitive performance of all the participants. After obtaining all the parameters, two-way analysis of variance (ANOVA) was used to compare the mean values of all the retinal parameters of the patients with AD and the HCs. Pearson's correlation was used to test the association between retinal parameters, MMSE scores, and vascular parameters.
Results
Seventy-eight eyes from 39 participants (19 AD and 20 HC; male, 52.6% in AD and 45.0% in HC; mean [standard deviation] age of 73.79 [7.22] years in AD and 74.35 [6.07] years in HC) were included for the analysis. The average RNFL + GCL thickness (106.32 ± 7.34 μm), FRTs of the four quadrants (290.35 ± 13.05 μm of inferior quadrant, 294.68 ± 9.37 μm of superior quadrant, 302.97 ± 6.52 μm of nasal quadrant, 286.02 ± 13.74 μm of temporal quadrant), and retinal VD (0.0148 ± 0.003) of patients with AD, compared with the HCs, were significantly reduced (p < 0.05). Retinal thickness was significantly correlated with the MMSE scores (p < 0.05). Meanwhile, retinal VD was significantly correlated with the average RNFL + GCL thickness (r2 = 0.2146, p < 0.01). When the vascular parameters were considered, the sensitivity of the AD diagnosis was increased from 0.874 to 0.892.
Conclusion
Our study suggested that the patients with AD, compared with age-matched HCs, had significantly reduced RNFL + GCL thickness and vascular density. These reductions correlated with the cognitive performance of the participants. By combining nerve and vessel parameters, the diagnosis of AD can be improved using OCTA technology.
Trail registration Name of the registry: Chinese Clinical Trail Registry, Trial registration number: ChiCTR2000035243, Date of registration: Aug. 5, 2020. URL of trial registry record: http://www.chictr.org.cn/index.aspx
Similar content being viewed by others
Background
In 2018, the National Institute of Aging and Alzheimer’s Association (NIA-AA) modified the diagnosis framework, suggesting that Alzheimer’s disease (AD) biomarkers, including β-amyloid (Aβ) deposition, pathologic tau (T), and neurodegenerative/neuronal injury biomarkers (N), can be added to the A/T/N classification system to improve diagnostic accuracy [1]. Furthermore, during the preclinical phase of AD, these biomarkers show abnormalities earlier than behavioral symptoms [2, 3]. However, we found that several biomarkers are assessed using expensive or invasive methods, including Aβ-positron emission tomography (PET), tau-PET, and lumbar puncture for Aβ or tau in cerebrospinal fluid. The retina and brain are connected directly by the axons of the optic nerve, which transport the amyloid precursor protein in retinal ganglion cells [4, 5]. Retinal and brain Aβ depositions are related. Therefore, retinal pathologies directly reflect brain pathologies.
One of the neurodegenerative biomarkers is the retinal structure, which can be evaluated noninvasively using optical coherence tomography (OCT) [6,7,8]. Retinal changes in AD patients are important for diagnosing and monitoring neurogenerative diseases [9]. In an OCT study, participants with AD showed that the ratio of the ellipsoid zone to the retinal pigment epithelium volume correlated with the cognitive assessment scores [10]. A quantitative histopathologic study revealed that the retinal nerve fiber layer (RNFL) was thinner in AD patients than in normal individuals [11]. The ganglion cell layer (GCL) thickness was also significantly reduced in patients with AD [12]. A 27-month pilot study showed a significant positive correlation between RNFL thickness and memory testing clinic questionnaire scores [13].
In addition to the decrease in retinal thickness in AD patients, there is an association between the degree of reduction in retinal thickness and the clinical severity of dementia [14,15,16]. In contrast, primary open angle glaucoma affects cognitive neurological functions besides vision; more visual field loss correlates with lower cognitive assessment scores [17].
The retinal vasculature is also important in AD patients [18, 19]. The participants with AD, compared with the patients with mild cognitive impairment (MCI) and controls, showed a significant reduction in retinal vascular density (VD, which was defined as the ratio of the number of pixels in the perfused retinal vascular area to the number of pixels of the entire retina), and changes in the vascular branches (VB, which was defined as the number of branches in the retinal central vascular system) and curvature (VC, which was defined as the average curvature of four main stem vessels in the retinal central vascular system) [20]. However, using only the retinal vasculature may not discriminate patients with AD from controls [21].
OCT has enabled us to visualize and assess the structures of the longitudinal retina and optic nerve, which is comparable to tissue slices [22]. En face OCT can reveal changes in structures parallel to the surface of the retina [23, 24]. OCT and en face OCT have been breakthroughs for observing portions of or the entire structures of the retina and optic nerve, and they have improved our perspective on the effects of diseases on the retina. Split-spectrum amplitude-decorrelation angiography was subsequently developed to observe retinal blood flow, and it has facilitated studies on the structure and function of the retina [25, 26]. This technology covers the conditions of the retina, optic nerve, and blood vessels to show the characteristics of the retina more comprehensively. The new generation of OCT angiography (OCTA) for clinical research has pioneering significance for the detection of neurodegenerative diseases.
In this study, we considered the coupling effect of blood vessels and nerves, and combined the vessel parameters to improve the diagnostic accuracy. We used OCTA to assess the retinal nerve and vasculature and comprehensively evaluate the diagnostic value of the fundus parameters. The combined thickness of the RNFL and GCL and the full retinal thickness (FRT) were used to evaluate the differences between the patients with AD and healthy controls (HCs). The correlations of retinal thickness with retinal vasculature were also investigated using Pearson’s correlation tests. In addition to neurological characteristics, vascular parameters can indicate neurological dysfunction. More objective assessments, apart from neuropsychological scales and especially the noninvasive modalities, are needed to increase the specificity and sensitivity of AD diagnosis.
Results
Demographic and cognitive characteristics of participants
After obtaining informed consent, 39 participants, including 19 patients with AD and 20 HCs, were recruited for this study. The demographics and clinical characteristics of the participants are detailed in Table 1. The mean age of the AD patients was 73.79 ± 7.22 years, 52.6% were male, body mass index (BMI) was 20.94 ± 2.34, duration of education was 9.00 ± 2.67 years, MMSE score was 12.79 ± 5.44 and mean disease course was 5.05 ± 3.17 years. There were significant differences in the mean MMSE scores between the AD and HC groups (p < 0.01). There were no differences in age, BMI, or level of education between the groups (p > 0.05).
Retinal neuro-layer thickness in patients with AD and HCs
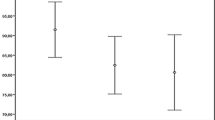
The RNFL + GCL thickness and FRT of the two groups were obtained using OCTA. Figure 1 shows the comparison of the retinal thicknesses of the AD and HC groups using two-way ANOVA. The average RNFL + GCL thickness (106.32 ± 7.34 μm vs. 119.01 ± 6.71 μm, Fig. 1A, F (1, 35) = 30.87, p < 0.001) and RNFL + GCL thicknesses of the inferior (111.33 ± 8.67 μm vs. 128.97 ± 6.44 μm, Fig. 1B, F (1, 35) = 49.44, p < 0.001), superior (109.04 ± 7.35 μm vs. 123.44 ± 7.07 μm, Fig. 1C, F (1, 35) = 41.28, p < 0.001), nasal (102.83 ± 10.16 μm vs. 113.24 ± 9.11 μm, Fig. 1D, F (1, 35) = 11.21, p = 0.002), and temporal (102.26 ± 9.82 μm vs. 110.18 ± 10.39 μm, Fig. 1E, F (1, 35) = 5.61, p = 0.024) quadrants were significantly lower in the AD group than in the HC group. FRTs of the inferior (290.35 ± 13.05 μm vs. 315.11 ± 13.74 μm, Fig. 1G, F (1, 35) = 32.93, p < 0.001), superior (294.68 ± 9.37 μm vs. 311.57 ± 11.3 μm, Fig. 1H, F (1, 35) = 25.33, p < 0.001), nasal (302.97 ± 6.52 μm vs. 307.84.11 ± 6.51 μm, Fig. 1I, F (1, 35) = 5.39, p = 0.026) and temporal (286.02 ± 13.74 μm vs. 308.29 ± 10.72 μm, Fig. 1J, F (1, 35) = 30.03, p < 0.001) quadrants were also significantly lower in the AD patients. There was no significant difference between the FRT center thickness in the AD patients and the HCs (255.52 ± 9.77 μm vs. 255.72 ± 11.42 μm, Fig. 1F, F (1, 35) = 0.005, p = 0.946).
RNFL + GCL thickness (A–E) and FRT (F–J) in different regions in the AD and HC groups. The average RNFL + GCL thickness (A, p < 0.001), the RNFL + GCL thicknesses in the inferior (B, p < 0.001), superior (C, p < 0.001), nasal (D, p < 0.01) and temporal (E, p < 0.05) quadrants, and the FRTs of the inferior (G, p < 0.001), superior (H, p < 0.001), nasal (I, p < 0.05) and temporal (J, p < 0.001) quadrants were lower in the AD group than in the HCs. There was no significant difference between the FRT center thicknesses in the AD patients and the HCs (F, p > 0.05). * p < 0.05, ** p < 0.01, *** p < 0.001. AD, Alzheimer’s disease, HC, healthy control
Retinal vessel parameters of patients with AD and HCs
The vessel parameters, including VB, VC, and VD, of the AD patients and HCs were obtained (Fig. 2). There was a significant difference between the VDs of the AD and HC groups (0.0148 ± 0.003 vs. 0.018 ± 0.005, Fig. 2C, F (1, 35) = 6.054, p = 0.019). However, there were no differences between the VBs and VCs of the AD and HC groups (p > 0.05).
Association of retinal thickness with MMSE
The correlations between the retinal thickness parameters and MMSE scores were determined and are shown in Fig. 3. The MMSE score were significantly correlated with the average of the RNFL + GCL thicknesses of all the five regions of interest (ROIs) (Fig. 3A, p < 0.001), and the RNFL + GCL thicknesses in the inferior (Fig. 3B, p < 0.001) and superior (Fig. 3C, p < 0.01) regions. The Pearson’s coefficients (r2) are presented in the figures. The MMSE scores was also correlated with the FRTs for the inferior (Fig. 3G, p < 0.001), superior (Fig. 3H, p < 0.01), and temporal (Fig. 3J, p < 0.01) regions but not for the central macular region (Fig. 3F, p > 0.05). For all the ROIs, retinal thickness was positively correlated with the MMSE score.
Associations between retinal thickness and vascular parameters
For all the ROIs, Pearson's correlation was used to investigate the associations between retinal thickness and vascular parameters (Fig. 4). VB was negatively correlated with retinal thickness (Fig. 4A). VC and VD were positively correlated with retinal thickness (Fig. 4B–F). The average RNFL + GCL thickness was significantly correlated with VD (r2 = 0.2146, p < 0.01).
Receiver operating characteristics (ROC) curve for RNFL + GCL, vessel parameters, and the combination of RNFL + GCL and vessel parameters
ROC curves show sensitivities (true-positive rate) and specificities (false-positive rate) of the RNFL + GCL (orange line), vessel parameters (red line), and their combination (blue line) for all participants with AD and HCs. The areas under the curves were 0.874 (95% CI, 0.7378 to 1.0000), 0.696 (95% CI, 0.5238 to 0.8683) and 0.892 (95% CI, 0.7705 to 1.0000) (Fig. 5).
Discussion
Previous studies have used plasma biomarkers to assess the risk of conversion from MCI to AD, such as plasma P-tau181, Aβ42/Aβ40, and neurofilament light [27, 28], and they have often focused on the evaluation of individual biomarkers, rather than systematically determining the best set of “A/T/N” markers for individualized prediction. With technological advances, retina-related AD biomarkers can be incorporated into the diagnostic workup of AD. As confirmed by OCTA quantitative parameters, retinal neurodegenerations, involving the nervous system and vessels, may morphologically reflect and provide insights into brain conditions [29].
In this study, all quantitative retinal nerve parameters (RNFL + GCL thickness and FRT of superior, inferior, nasal, and temporal regions) were reduced in the eyes of AD patients relative to those of the HCs. The MMSE scores, which reflect cognitive performance, were lower in the AD patients than in the HCs. Besides the RNFL + GCL of the inner retina, the volumetric and thickness parameters of the outer retina and the Montreal cognitive assessment scores also decreased synchronously [10]. Although the patients in this study had moderate to severe AD, a significant decrease in the macular RNFL thickness was observed on OCT during the preclinical stage [30]. In clinical practice, the retinal thicknesses of AD patients and normal individuals may be comparable; the retina of normal people may be thin, and the conditions of these patients should be considered. OCTA can detect early alterations of the retina in patients with normal cognition [31]. The observation of retinal changes during the early stages of AD is beneficial for early diagnosis.
The retinal microvasculature was assessed using OCTA. Compared with the HCs, the patients with AD showed lower VDs. This can also be observed in patients with MCI [32, 33]. Furthermore, we investigated the relationship between the retinal microvasculature and thickness. There was a significant correlation between vascular density and RNFL + GCL thickness. These vascular changes may be caused by deposition of Aβ proteins [34]. Local microglial reactivity around Aβ plaques can affect retinal vein morphology and lead to a decrease in vascular density [35]. The retinal vascular and capillary networks can also provide valuable insights into the brain of AD patients [36, 37].
In addition to morphological changes, changes in the concentrations of various proteins and cytokines in the retina of patients with AD retina were present in mice with AD [38]. Moreover, non-invasive technologies are needed for the clinical diagnosis of AD, especially based on anatomical and functional changes [39]. Anatomically, the RNFL and GCL are parts of the third neuron of the inner retina; the RNFL is the axon, while the GCL is the cell body of the third neuron. Therefore, these two layers directly reflect the characteristics of the inner retina. Furthermore, the inner retina and brain share a common embryonic origin, which can directly reflect the characteristics of brain neurons. The OCT parameters of both global and specific retinal regions are needed to evaluate the accuracy of brain trajectory test [40, 41].
Several studies have proposed approaches to medical image procedding, including the elasticity-based state-space approach [42] and the Kalman filter snake method to improve the accuracy of diagnosis [43]. We used OCTA equipment (Cirrus 5000 Angioplex, Zeiss Meditec, Inc, Germany) with the integration of nerve and vessel segmentation methods, including optical microangiograph measurement [44].
This study did not involve patients with early-stage disease; however, the proposed non-invasive approach can be applied to early-stage disease. Patients are unwilling to undergo invasive testing during the early stages of the disease. Therefore, this study provides a non-invasive approach for early detection.
Limitations
The limitation of this study is that (1) participants were relatively few. The follow-up study will involve a larger sample to improve the diagnostic accuracy. (2) The capillaries were not considered for the vascular density in this study. In further studies, capillaries will be included to allow a more accurate reflection of blood supply [45]. In the future, the severe stages of AD may be studied by considering the different courses of AD.
Conclusion
To improve the accuracy of early diagnosis, biomarkers of AD should be evaluated as comprehensively as possible. In this study, a non-invasive optical method was used to assess retinal nerves and vessels; the conditions of retinal vessels and nerves may indicate conditions in the brain. Our study suggested that the AD patients, compared with age-matched HCs, had significantly reduced RNFL + GCL thickness and vascular density. These reductions correlated with the cognitive performance of the participants. In conclusion, retinal changes should be used as valuable clinical diagnostic information.
Methods
Participants
A total of 19 patients with AD and 20 age-matched HCs were recruited for this study. The AD patients with no retinal diseases aged between 60 and 88 years were diagnosed by two research psychiatrists according to the standards of the National Institution of Neurologic and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDSADRDA) and the Diagnostic and Statistical Manual of Mental Disorders (DSM) [46, 47]. Medical evaluations, including neuroimaging and blood examinations, were performed to exclude alternative causes of dementia. The other inclusion and exclusion criteria, including Parkinson's disease, cerebral small vessel disease, chronic renal failure, cerebral small vessel diseases, white matter hyperintensities, and diabetes mellitus, were presented on the China website of clinical trials (clinical trial registration number: ChiCTR2000035243).
Optical coherence tomography imaging
OCT was used to measure the RNFL + GCL thickness and the FRT of the macular. Macular cube 512 × 128 scan patterns (Cirrus 5000 Angioplex, Zeiss Meditec, Inc., Germany) were used to obtain the parameters of the ROIs, including the peripheral macular region and center of macular region covering the foveal avascular zone (Fig. 6). The microvasculature in en face images were visualized. The VB (which was defined as the number of branches in the retinal central vascular system), VC (which was defined as the average curvature of four main stem vessel in the retinal central vascular system), and VD (defined as the ratio of the pixels covering the perfused retinal vessels to total pixels for the retina) for the area of the ROI were also measured. An image of each eye was obtained for each patient and each healthy participant. Seventy-eight images were obtained in total. The average values for the two eyes were obtained for all the parameters for each participant. The capillaries were not considered in the calculation of the vascular density in this study.
Cognitive assessment
The MMSE scale, with scores range from 0 to 30, was used to evaluate the cognitive performance of all the participants. Lower MMSE scores indicated the poorer cognitive performance. The MMSE scale is currently the most widely used for cognitive examination, and it includes six dimensions: orientation ability, immediate recall, attention and calculation ability, delayed recall, language function, and visual space perception [48]. The scores were categorized as follows: ≥ 27 represented normal cognitive performance; 21–26 represented mild dementia; 10–20 represented moderate dementia; and < 10 represented severe dementia.
Statistical methods
All data were analyzed using SPSS software V.16 (IBM Corp., Armonk, NY, USA) and are presented as the mean ± standard deviation. Differences in the data were assessed using two-way analysis of variance (ANOVA), followed by Bonferroni’s post hoc test (p < 0.05, with Bonferroni correction). An analysis of covariance (ANCOVA) was conducted to control for the effects of gender, age, disease duration, and education. The correlations between retinal parameters, MMSE score, and vascular parameters were tested using Pearson's correlation. A p value of < 0.05 denoted statistical significant.
Availability of data and materials
All data are included in the manuscript. However, the raw data used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Abbreviations
- Aβ:
-
β-Amyloid
- AD:
-
Alzheimer’s disease
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- FRT:
-
Full retinal thickness
- GCL:
-
Ganglion cell layer
- HCs:
-
Healthy controls
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-mental state examination
- OCT:
-
Optical coherence tomography
- OCTA:
-
Optical coherence tomography angiography
- RNFL:
-
Retinal nerve fiber layer
- ROC:
-
Receiver operating characteristics
- ROIs:
-
Regions of interest
- VB:
-
Vascular branching
- VC:
-
Vascular curvature
- VD:
-
Vascular density
References
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804.
Victor L, Villemagne SB, Bourgeat P, Brown B, Ellis KA, Salvado O, Cassandra Szoeke S, Macaulay L, Martins R, Maruff P, Ames D, Rowe CC, Masters CL. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:11.
Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, Kile SJ, Blanco A, Fuchs DT, Ashfaq A, Frautschy S, Cole GM, Miller CA, Hinton DR, Verdooner SR, Black KL, Koronyo-Hamaoui M. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2:1.
López-de-Eguileta A, Lage C, López-García S, Pozueta A, García-Martínez M, Kazimierczak M, Bravo M, de Arcocha-Torres M, Banzo I, Jimenez-Bonilla J, Cerveró A, Rodríguez-Rodríguez E, Sánchez-Juan P, Casado A. Ganglion cell layer thinning in prodromal Alzheimer’s disease defined by amyloid PET. Alzheimers Dement (N Y). 2019;5:570–8.
Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, Ma D. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett. 2010;480:69–72.
Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48:2285–9.
Mejia-Vergara AJ, Restrepo-Jimenez P, Pelak VS. Optical coherence tomography in mild cognitive impairment: a systematic review and meta-analysis. Front Neurol. 2020;11:578698.
Gupta VB, Chitranshi N, den Haan J, Mirzaei M, You Y, Lim JK, Basavarajappa D, Godinez A, Di Angelantonio S, Sachdev P, Salekdeh GH, Bouwman F, Graham S, Gupta V. Retinal changes in Alzheimer’s disease- integrated prospects of imaging, functional and molecular advances. Prog Retin Eye Res. 2020;1:100899.
Uchida A, Pillai JA, Bermel R, Bonner-Jackson A, Rae-Grant A, Fernandez H, Bena J, Jones SE, Leverenz JB, Srivastava SK, Ehlers JP. Outer retinal assessment using spectral-domain optical coherence tomography in patients with alzheimer’s and parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:2768–77.
Asanad S, Ross-Cisneros FN, Nassisi M, Barron E, Karanjia R, Sadun AA. The retina in Alzheimer’s disease: histomorphometric analysis of an ophthalmologic biomarker. Invest Ophthalmol Vis Sci. 2019;60:1491–500.
Alber J, Goldfarb D, Thompson LI, Arthur E, Hernandez K, Cheng D, DeBuc DC, Cordeiro F, Provetti-Cunha L, den Haan J, Van Stavern GP, Salloway SP, Sinoff S, Snyder PJ. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dement. 2020;16:229–43.
Cheng DL, Thompson L, Snyder PJ. A potential association between retinal changes, subjective memory impairment, and anxiety in older adults at risk for Alzheimer’s disease: a 27-month pilot study. Front Aging Neurosci. 2019;11:288.
Ito Y, Sasaki M, Takahashi H, Nozaki S, Matsuguma S, Motomura K, Ui R, Shikimoto R, Kawasaki R, Yuki K, Sawada N, Mimura M, Tsubota K, Tsugane S. Quantitative assessment of the retina using OCT and associations with cognitive function. Ophthalmology. 2020;127:107–18.
Kim JI, Kang BH. Decreased retinal thickness in patients with Alzheimer’s disease is correlated with disease severity. PLoS ONE. 2019;14:e0224180.
Choi SH, Park SJ, Kim NR. Macular ganglion cell -inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS ONE. 2016;11:e0162202.
McCoskey M, Addis V, Goodyear K, Sankar PS, Ying GS, Yu Y, Salowe R, Cui QN, Miller-Ellis E, Maguire M, JM OAB, . Association between primary open-angle glaucoma and cognitive impairment as measured by the montreal cognitive assessment. Neurodegener Dis. 2018;18:315–22.
Sharafi SM, Sylvestre JP, Chevrefils C, Soucy JP, Beaulieu S, Pascoal TA, Arbour JD, Rhéaume MA, Robillard A, Chayer C, Rosa-Neto P, Mathotaarachchi SS, Nasreddine ZS, Gauthier S, Lesage F. Vascular retinal biomarkers improves the detection of the likely cerebral amyloid status from hyperspectral retinal images. Alzheimers Dement (N Y). 2019;5:610–7.
Yan Y, Wu X, Wang X, Geng Z, Wang L, Xiao G, Wu Y, Zhou S, Wei L, Tian Y, Wang K. The retinal vessel density can reflect cognitive function in patients with Alzheimer’s disease: evidence from optical coherence tomography angiography. J Alzheimers Dis. 2021;79(3):1307–16.
Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, Fekrat S. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmol Retina. 2019;3:489–99.
den Haan J, van de Kreeke JA, van Berckel BN, Barkhof F, Teunissen CE, Scheltens P, Verbraak FD, Bouwman FH. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimers Dement (Amst). 2019;11:383–91.
Drexler W, Liu M, Kumar A, Kamali T, Unterhuber A, Leitgeb RA. Optical coherence tomography today: speed, contrast, and multimodality. J Biomed Opt. 2014;19:071412.
Thulliez M, Motulsky EH, Feuer W, Gregori G, Rosenfeld PJ. En face imaging of geographic atrophy using different swept-source OCT scan patterns. Ophthalmol Retina. 2019;3:122–32.
Thouvenin O, Grieve K, Xiao P, Apelian C, Boccara AC. En face coherence microscopy [Invited]. Biomed Opt Express. 2017;8:622–39.
Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical coherence tomography angiography using the optovue device. Dev Ophthalmol. 2016;56:6–12.
Chalam KV, Sambhav K. Optical coherence tomography angiography in retinal diseases. J Ophthalmic Vis Res. 2016;11:84–92.
Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, Chai X, Proctor NK, Eichenlaub U, Zetterberg H, Blennow K, Reiman EM. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–86.
Cullen NC, Leuzy A, Palmqvist S, Janelidze S, Stomrud E, Pesini P, Sarasa L, Allué JA, Proctor NK, Zetterberg H, Dage JL, Blennow K, Mattsson-Carlgren N, Hansson O. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nat Aging. 2021;1:114–23.
Frizziero L, Parrozzani R, Londei D, Pilotto E, Midena E. Quantification of vascular and neuronal changes in the peripapillary retinal area secondary to diabetic retinopathy. Br J Ophthalmol. 2020. https://doi.org/10.1136/bjophthalmol-2020-316468.
Santos CY, Johnson LN, Sinoff SE, Festa EK, Heindel WC, Snyder PJ. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement (Amst). 2018;10:196–209.
O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol. 2018;136:1242–8.
Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, Zheng F, Vanner EA, Lam BL, Rundek T, Wang J. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol. 2018;38:292–8.
Salobrar-Garcia E, Méndez-Hernández C, Hoz R. Ocular Vascular Changes in Mild Alzheimer’s Disease Patients: Foveal Avascular Zone, Choroidal Thickness, and ONH Hemoglobin Analysis. J Pers Med. 2020;10:1.
Masuzzo A, Dinet V, Cavanagh C, Mascarelli F, Krantic S. Amyloidosis in retinal neurodegenerative diseases. Front Neurol. 2016;7:127.
Vandenabeele M, Veys L, Lemmens S, Hadoux X, Gelders G, Masin L, Serneels L, Theunis J, Saito T, Saido TC, Jayapala M, De Boever P, De Strooper B, Stalmans I, van Wijngaarden P, Moons L, De Groef L. The App(NL-G-F) mouse retina is a site for preclinical Alzheimer’s disease diagnosis and research. Acta Neuropathol Commun. 2021;9:6.
Chua J, Hu Q, Ke M, Tan B, Hong J, Yao X, Hilal S, Venketasubramanian N, Garhöfer G, Cheung CY, Wong TY, Chen CL, Schmetterer L. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimer’s Res Ther. 2020;12:161.
Bulut M, Kurtuluş F, Gözkaya O, Erol MK, Cengiz A, Akıdan M, Yaman A. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102:233–7.
Grimaldi A, Brighi C, Peruzzi G. Inflammation, neurodegeneration and protein aggregation in the retina as ocular biomarkers for Alzheimer’s disease in the 3xTg-AD mouse model. Cell Death Dis. 2018;9:685.
Szegedi S, Dal-Bianco P, Stögmann E, Traub-Weidinger T, Rainer M, Masching A, Schmidl D. Anatomical and functional changes in the retina in patients with Alzheimer’s disease and mild cognitive impairment. Acta Ophthalmol. 2020;98:e914–21.
Mejia-Vergara AJ, Karanjia R, Sadun AA. OCT parameters of the optic nerve head and the retina as surrogate markers of brain volume in a normal population, a pilot study. J Neurol Sci. 2020;420:117213.
van de Kreeke JA, Nguyen HT, Konijnenberg E, Tomassen J, den Braber A, Ten Kate M, Yaqub M, van Berckel B, Lammertsma AA, Boomsma DI, Tan HS, Visser PJ, Verbraak FD (2020) Longitudinal retinal layer changes in preclinical Alzheimer's disease. Acta Ophthalmol.
Gao Z, Xiong H, Liu X, Zhang H, Ghista D, Wu W, Li S. Robust estimation of carotid artery wall motion using the elasticity-based state-space approach. Med Image Anal. 2017;37:1–21.
Zhao SHEN, GL, Wei ZHANG, JIANJUN GU, . Automatical intima-media border segmentation on ultrasound image sequences using a Kalman filter snake. IEEE Access. 2018;6:40804–10.
Li Y, Choi WJ, Qin W, Baran U, Habenicht LM, Wang RK. Optical coherence tomography based microangiography provides an ability to longitudinally image arteriogenesis in vivo. J Neurosci Methods. 2016;274:164–71.
Song W, Shao W, Yi W, Liu R, Desai M, Ness S, Yi J. Visible light optical coherence tomography angiography (vis-OCTA) facilitates local microvascular oximetry in the human retina. Biomed Opt Expr. 2020;11:4037–51.
Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas. 2013;25:191–2.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia. 2011;7(3):263–9.
Arevalo-Rodriguez I, Smailagic N, Roqué IFM, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;2015:cd010783.
Acknowledgements
An earlier version of manuscript has been presented in Research Square. This version made some improvements, including expanded the sample size.
Funding
This study was supported by the Zhejiang Provincial Natural and Science Fund (LQ19F010003), Natural Science Foundation of Ningbo (2019A610355, 2019A610293) and Zhejiang Medical and Health Science and Technology Project (2021KY1066).
Author information
Authors and Affiliations
Contributions
XM, CZ and CQ performed OCTA experiments, data collection and wrote the manuscript. XM, ZX, and YC performed data analysis. ZC, QZ and CZ proofread the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local Ethics Committee of Ningbo Kangning Hospital (NBKNYY-2018-LC-21) and registered as a clinical trial in the China Clinical Trial Registry (CHICTR: ChiCTR2000035243). All procedures of the study were in accordance with the Helsinki Declaration (2014) ethical standards and regulations for human research. All subjects enrolled in the study signed the written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mei, X., Qiu, C., Zhou, Q. et al. Changes in retinal multilayer thickness and vascular network of patients with Alzheimer’s disease. BioMed Eng OnLine 20, 97 (2021). https://doi.org/10.1186/s12938-021-00931-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12938-021-00931-2