Abstract
Background
Individuals with metabolic syndrome face elevated cardiovascular and mortality risks, and there is ongoing debate regarding the cardiovascular effects of niacin and its impact on the prognosis of metabolic syndrome.
Exposure
Levels of dietary niacin intake based on 24-hour dietary recall.
Methods
Kaplan-Meier survival curves were used to compare survival status among quartiles of dietary niacin intake. Weighted Cox proportional hazards models and restricted cubic splines were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of all-cause and CVD mortality associated with the exposure.
Results
This cohort study included 8,744 participants, and during a median follow-up period of 106 months, 1,552 (17.7%) deaths were recorded, with 511 attributed to cardiovascular disease. Kaplan-Meier curves comparing quartiles of dietary niacin intake showed significant differences in both all-cause and cardiovascular mortality rates (log-rank p < 0.001). In the fully adjusted model, the highest quartile of dietary niacin intake was associated with HRs of 0.68 (95% CI: 0.54, 0.87, P = 0.002) for all-cause mortality and 0.63 (95% CI: 0.39, 0.78, P < 0.001) for cardiovascular mortality.
Conclusion
The results of this cohort study suggest that higher dietary niacin intake is associated with reduced cardiovascular and all-cause mortality risks in the metabolic syndrome population. Furthermore, there appears to be a dose-response relationship between dietary niacin intake and the risks of all-cause and cardiovascular mortality.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a cluster of interrelated and accumulating risk factors for cardiovascular disease (CVD), including abnormal blood glucose, lipid abnormalities, hypertension, and central obesity [1, 2]. The prevalence of metabolic syndrome varies significantly globally [3], with surveys showing that the prevalence in the United States has exceeded 30%, continuously increasing over the past 20 years [4]. Patients with metabolic syndrome face a high risks of all-cause and cardiovascular mortality [5, 6]. Studying how to reduce the mortality risk among individuals with metabolic syndrome is of paramount importance.
Dietary niacin, also known as vitamin B3, is considered beneficial for lowering blood lipids [7,8,9] and plays a crucial role in cellular metabolism [10]. It can improve endothelial dysfunction [11]. Although niacin aids in lipid management [12,13,14,15], its cardiovascular protective effect is not universally recognized. On the contrary, one study found an increased risk of developing hypertension with a daily niacin intake exceeding 15.6 mg/d [16]. Several meta-analyses suggest that niacin lacks significant cardiovascular benefits and may even increase the risk of cardiovascular mortality [17, 18]. A recent study suggests that niacin’s adverse effects on the cardiovascular system are mediated through its metabolites and inflammatory effects [19]. Considering the existence of the complex effects niacin may have on lipids and cardiovascular disease, and the lack of large-sample studies on niacin intake among individuals with metabolic syndrome who face a high risk of cardiovascular mortality, this study utilized the National Health and Nutrition Examination Survey(NHANES) database, representative of the US population, to investigate the long-term effects of niacin intake on all-cause and cardiovascular mortality rates among individuals with metabolic syndrome, which was able to make some informative conclusions about the nutritional intake of this population with metabolic syndrome.
Materials and methods
Study design and participants
The data were derived from ten interview cycles of theNHANES, spanning from 2003 to 2018. The NHANES database employs a stratified, multistage probability sampling design to systematically collect health-related data that is representative of the civilian, non-institutionalized US population. The datasets analyzed in this study are accessible at https://www.cdc.gov/nchs/nhanes/index.html [20]. Among the 80,312 participants from the NHANES dataset spanning from 2003 to 2018, a total of 69,595 individuals completed at least one dietary intake interview and reported niacin intake. Among them, 8,904 individuals met the criteria for metabolic syndrome. From these 8,904 subjects, 150 participants under the age of 18 and 10 participants with missing mortality data were excluded, resulting in a final inclusion of 8,744 participants. The participants selection flowchart is shown in Fig. 1. The study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Exposure variable
The measurement of dietary niacin intake was conducted through the Dietary Interview component referred to as What We Eat in America (WWEIA), which is a collaboration between the United States Department of Agriculture (USDA) and the Department of Health and Human Services (DHHS). All eligible NHANES participants undergo two 24-hour dietary recall interviews to report the types and amounts of foods consumed in the preceding 24 h (from midnight to midnight). Food energy and 64 nutrients/food components from each food/beverage as calculated using USDA’s Food and Nutrient Database for Dietary Studies. The first recall is conducted face-to-face at the Mobile Examination Center (MEC), while the second recall takes place approximately 3 to 10 days later via telephone interview. The Total Nutrient Intake dataset provides summary records of nutrient intake for each individual. In this study, participants with two niacin intake assessments have their values averaged, while those with only one niacin intake assessment are determined by their single reported value.
Definition of MetS
The definition of Metabolic Syndrome (MetS) was in accordance with previous guidelines, specifically the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) [21]. Individuals meeting three or more of the following criteria were considered to have MetS: [1] fasting blood glucose (FBG) > 100 mg/dL or undergoing treatment for diabetes mellitus; [2] low levels of high-density lipoprotein cholesterol (HDL-C) (< 50 mg/dL in females, < 40 mg/dL in males) or receiving treatment for reduced HDL-C; [3] plasma triglycerides (TG) > 150 mg/dL or receiving treatment for elevated TG; [4] waist circumference > 88 cm in women or > 102 cm in men; [5] blood pressure > 130/85 mmHg or receiving treatment for hypertension.
Covariates
Population demographics of the participants were obtained from the NHANES database. Socioeconomic characteristics comprised gender (male or female), age, race/ethnicity (Mexican American, Hispanic, non-Hispanic white, non-Hispanic black, or other race), education level (less than high school, high school, and some college or above), and household income-to-poverty ratio (< 1.0, 1–3, > 3). Additionally, lifestyle habits and comorbidity history were assessed, including smoking status (yes or no), alcohol consumption (yes or no), presence of hypertension (yes or no), diabetes (yes or no), history of cancer, and cardiovascular disease (CVD) (Included self-reported coronary heart disease, stroke, congestive heart failure, myocardial infarction, and heart attack)(Yes or no) and chronic kidney disease(CKD). Furthermore, physical and laboratory examinations such as body mass index (BMI), waist circumference(WC), creatinine, triglyceride(TG), high-density lipoprotein cholesterol(HDL-C), low-density lipoprotein cholesterol(LDL-C), total Cholesterol(TC), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were considered as potential confounding factors.
Outcome events
The primary outcomes of this study are all-cause mortality and cardiovascular mortality among individuals with MetS. Mortality data for the follow-up population were obtained from the NHANES public-use linked mortality files as of December 31, 2019. These files are linked to the National Death Index (NDI) through a probabilistic matching algorithm conducted by the National Center for Health Statistics (NCHS). The causes of death were examined using the International Classification of Diseases, 10th Revision (ICD-10), with cardiovascular mortality defined as deaths due to heart disease and cerebrovascular disease, which included the following disease codes: I00-I09 (acute rheumatic fever and chronic rheumatic heart diseases), I11 (hypertensive heart disease), I13 (hypertensive heart and renal disease), I20-I25 (ischemic heart diseases), I26-I28 (pulmonary embolism and other acute pulmonary heart diseases), I29 (various cardiovascular diseases caused by different reasons), I30-I51 (other forms of heart disease), and I60-I69 (cerebrovascular diseases). The follow-up duration was calculated from the initial interview date to the date of patient death or December 31, 2019.
Statistical analysis
Variables with a normal distribution are presented as mean (standard deviation), whereas variables with a non-normal distribution are presented as median (interquartile range). Categorical variables are expressed as numbers (percentages, %). The Kruskal-Wallis test is used to compare non-normally distributed continuous variables, while the chi-square test is used to compare categorical variables with continuous variables.In this study, all analyses accounted for the complex sampling design of NHANES. According to NHANES guidelines, individuals with two dietary intake data points were assigned the average dietary weight, while those with only one dietary intake data point were assigned the weight from the first intake. Stratification and primary sampling units were also included to ensure accurate representation of the population. For categorical variables, data were presented as unweighted frequencies (weighted percentages), while for continuous variables, data were presented as median (interquartile range).
Kaplan-Meier analysis was utilized to compare mortality rate differences among quartiles of niacin intake, and a weighted Cox regression model was employed to determine hazard ratios (HR) and 95% confidence intervals (CI) to examine the association between dietary niacin intake and all-cause mortality as well as cardiovascular mortality. Three models were constructed for analysis. Model 1 was unadjusted. In Model 2, adjustments were made for age, race, and gender. Model 3 included adjustments for race, age, gender, education level, household income-to-poverty ratio, smoking status, alcohol consumption, body mass index (BMI), hypertension, diabetes, CKD, and HDL-C. Restricted cubic spline analysis was conducted to examine the nonlinear association between dietary niacin intake and mortality. Restricted spline analysis was performed using knot = 4 and the best model was selected based on the Akaike information criterion value. Further stratification was performed based on age, gender, education level, household income-to-poverty ratio, smoking status, alcohol consumption, and BMI. The significance of interaction was estimated using the p-value of the product term between dietary niacin intake and stratifying factors. Multiple imputation using chained equations method was employed for covariate imputation for variables with 15% missing data. All statistical analyses were conducted using R software (version 4.2.3), with a two-tailed p < 0.05 considered statistically significant.
Results
Baseline characteristics
This study included a total of 8744 diagnosed cases of metabolic syndrome, divided into four groups based on quartiles of dietary niacin intake, with baseline characteristics summarized in Table 1. Compared to participants with the lowest niacin intake, those with higher dietary niacin intake were more common among Non-Hispanic Whites, males, aged between 30 and 60, with higher BMI and higher education levels. There were no significant differences in cholesterol and triglycerides between the groups based on niacin intake.
Niacin Intake, all-cause and CVD mortality
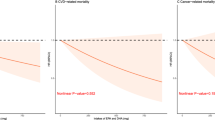
During a median follow-up of 107 months, 1552 (17.7%) deaths were recorded, of which 511 (5.8%) were attributed to cardiovascular deaths. Kaplan-Meier analysis shown in Fig. 2 demonstrated significant differences in both all-cause and cardiovascular mortality risks among the different dietary niacin intake groups (log-rank P < 0.001), with a gradual reduction in all-cause and cardiovascular mortality risks as dietary niacin intake increased. As depicted in Table 2, in Model 1, compared to the reference group, the hazard ratio (HR) for all-cause mortality was 0.49 (95% CI, 0.38–0.62) (P < 0.001) for the fourth quartile, and the HR for cardiovascular mortality was 0.41 (95% CI, 0.28–0.61) (P < 0.001). After adjusting for age, sex, and race, in Model 2, the HR for all-cause mortality was 0.55 (95% CI, 0.39–0.78) (P < 0.001), and the HR for cardiovascular mortality was 0.62 (95% CI, 0.45–0.85) (P < 0.001). In the fully adjusted model, the HR for all-cause mortality for the fourth quartile was 0.68 (95% CI, 0.54–0.87) (P = 0.002), and the HR for cardiovascular mortality was 0.63 (95% CI, 0.39–0.78) (P < 0.001). The dose-response association between dietary niacin intake and both all-cause and CVD mortality rates is depicted in Fig. 3, where within the restricted cubic spline curves, there was a dose-response relationship between dietary niacin intake and all-cause mortality (P = 0.01) and CVD mortality (P = 0.02), with no significant nonlinear relationship observed (P for non-linearity > 0.05).
Subgroup analyses
The subgroup analysis results were presented in Table S1 and Table S2, where no interaction was found with age, gender, BMI, education level, smoking, and alcohol consumption status (P for interaction > 0.05).
Discussion
As the first study, the impact of niacin intake on long-term survival in the metabolic syndrome population, in this large-scale national cohort study involving American adults, it was found that higher niacin intake is associated with lower mortality risk among individuals with metabolic syndrome.
Despite being one of the earliest lipid-lowering drugs, the existence of the “niacin paradox” — the contradiction between niacin’s impact on lipid levels and its challenging achievement of the expected cardiovascular protective effects — has led to ongoing controversy regarding its cardiovascular effects [19]. Recent studies suggest the need to consider niacin’s adverse effects on the cardiovascular system. Based on current research, this study focused on individuals with metabolic syndrome and found a dose-response relationship between higher levels of niacin intake and decreased all-cause and cardiovascular mortality rates. This finding aligns with conclusions drawn from previous studies: increased niacin and B-complex vitamin intake can lower the incidence of metabolic syndrome [22]; individuals in the metabolic syndrome group have lower niacin levels [23, 24]; niacin treatment in patients with metabolic syndrome can improve HDL endothelial protective effects [25]; a clinical trial found that the 15-year total mortality rates for patients with metabolic syndrome receiving niacin and placebo treatments were 60% and 64%, respectively (risk ratio: 0.86) [26].
MetS incorporates risk factors for cardiovascular disease, and insulin resistance, oxidative stress, and chronic low-grade inflammation may play an important role in its pathophysiology [27]. According to the diagnostic criteria for metabolic syndrome, disturbances in carbohydrate and lipid metabolism play a more critical role. The trend of niacin-mediated normalization of mixed dyslipidemia associated with atherosclerosis, along with the alleviation of inflammation [28], improvement in insulin resistance status [29], and enhancement of endothelial function in a lipotoxic environment [30], may explain why niacin exerts a protective effect in individuals with metabolic syndrome.
The study provided some basis for the recommended amount of dietary niacin in the metabolic syndrome population, and indeed for the niacin paradox itself, there may be some dose-derived factors. According to the National Institutes of Health (NIH), the recommended dietary allowance (RDA) for niacin is 16 milligrams per day for adult males and 14 milligrams per day for adult females. In fact, nearly one-third of Americans do not meet this standard. In this study, the group with niacin intake exceeding 20.1 mg/day (the third and fourth quartiles) showed a significant reduction in all-cause and cardiovascular mortality risk compared to the reference group (the first quartile). Consideration of dose-related controversies in this study’s conclusions and previous research is warranted. It is noteworthy that in this high-quality meta-analysis, no cardiovascular protective effect of niacin was found. The niacin intake in the literature used as the control group ranged from 500 mg to 4000 mg per day [18], far exceeding the dietary intake in this study, and recent findings suggest that the pro-inflammatory effect of excess niacin metabolism can partially explain the niacin paradox [19]. The maximum daily niacin intake in this study was 143.3 mg, much lower than the daily dose of 2000 mg typically used in clinical randomized controlled trials [31]. Therefore, there may be a “U-shaped” relationship between niacin and cardiovascular effects - where risk increases after exceeding a certain threshold dose. This issue warrants further exploration in future research and requires a more comprehensive sample to elucidate the broader cardiovascular effects of niacin. This study was a nationwide and large-sample study. In conclusion, considering the current controversies surrounding niacin and its status as an easily supplemented essential nutrient, this study has made a certain contribution to the future health of the metabolic syndrome population.
Limitations
Physical activity has been shown to affect the outcomes of metabolic syndrome [32]; however, due to incomplete physical activity data and the assumption that the missing values occurred randomly, this variable was not included in the study, potentially introducing some bias. Additionally, the niacin intake used in this study was based on 24-hour recall, which may be subject to recall bias and selective reporting. Furthermore, niacin intake is also associated with supplemental niacin use; however, since niacin intake as a nutritional supplement was not reported in NHANES before 2005–2006, this important variable was not included, posing another source of bias in this study. Nevertheless, the population consuming niacin dietary supplements is relatively small, hence the impact on the results is considered to be minimal.
Conclusion
This cohort study, conducted using nationally representative data, suggests that increasing niacin intake may reduce the risk of mortality, providing guidance for dietary niacin intake in this population.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MetS:
-
Metabolic syndrome
- CVD:
-
Cardiovascular disease
- NHANES:
-
National Health and Nutrition Examination Survey
- WWEIA:
-
What We Eat in America
- USDA:
-
United States Department of Agriculture
- DHHS:
-
Department of Health and Human Services
- MEC:
-
Mobile Examination Center
- NCEP ATP III:
-
National Cholesterol Education Program Adult Treatment Panel III
- FBG:
-
Fasting blood glucose
- HDL-C:
-
High-density lipoprotein cholesterol
- TG:
-
Triglycerides
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- LDL-C:
-
Low-density lipoprotein cholesterol
- TC:
-
Total Cholesterol
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- NDI:
-
National Death Index
- NCHS:
-
National Center for Health Statistics
- ICD-10:
-
International Classification of Diseases 10th Revision
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- NIH:
-
National Institutes of Health
- RDA:
-
Recommended dietary allowance
References
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–80.
Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822.
Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, Tounouga DN, Tianyi FL, Foka AJ, Ndoadoumgue AL, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924.
Yang C, Jia X, Wang Y, Fan J, Zhao C, Yang Y, Shi X. Trends and influence factors in the prevalence, intervention, and control of metabolic syndrome among US adults, 1999–2018. BMC Geriatr. 2022;22(1):979.
Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32.
Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–67.
Zeman M, Vecka M, Perlík F, Staňková B, Hromádka R, Tvrzická E, Širc J, Hrib J, Žák A. Pleiotropic effects of niacin: current possibilities for its clinical use. Acta Pharm. 2016;66(4):449–69.
Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258(2):94–114.
Kirkland JB. Niacin status, NAD distribution and ADP-ribose metabolism. Curr Pharm Des. 2009;15(1):3–11.
Sahebkar A. Effect of niacin on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Vasc Med. 2014;19(1):54–66.
Ahmed MH. What does the future hold for niacin as a treatment for hyperlipidaemia and cardiovascular disease? J Cardiovasc Med (Hagerstown). 2010;11(11):858–60.
Brooks EL, Kuvin JT, Karas RH. Niacin’s role in the statin era. Expert Opin Pharmacother. 2010;11(14):2291–300.
Boden WE, Sidhu MS, Toth PP. The therapeutic role of niacin in dyslipidemia management. J Cardiovasc Pharmacol Ther. 2014;19(2):141–58.
Stamler J. The coronary drug project–findings with regard to estrogen, dextrothyroxine, clofibrate and niacin. Adv Exp Med Biol. 1977;82:52–75.
Zhang Z, Liu M, Zhou C, He P, Zhang Y, Li H, Li Q, Liu C, Qin X. Evaluation of Dietary Niacin and New-Onset Hypertension among Chinese adults. JAMA Netw Open. 2021;4(1):e2031669.
Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S, et al. Supplemental vitamins and minerals for CVD Prevention and Treatment. J Am Coll Cardiol. 2018;71(22):2570–84.
Schandelmaier S, Briel M, Saccilotto R, Olu KK, Arpagaus A, Hemkens LG, Nordmann AJ. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev. 2017;6(6):Cd009744.
Ferrell M, Wang Z, Anderson JT, Li XS, Witkowski M, DiDonato JA, Hilser JR, Hartiala JA, Haghikia A, Cajka T, et al. A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat Med. 2024;30(2):424–34.
Centers for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 1 July 2023).
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97.
Wu Y, Li S, Wang W, Zhang D. Associations of dietary vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12 and folate equivalent intakes with metabolic syndrome. Int J Food Sci Nutr. 2020;71(6):738–49.
Al-Daghri NM, Al-Attas OS, Alkharfy KM, Alokail MS, Abd-Alrahman SH, Sabico S. Thiamine and its phosphate esters in relation to cardiometabolic risk factors in Saudi arabs. Eur J Med Res. 2013;18(1):32.
Odum EP, Wakwe VC. Plasma concentrations of water-soluble vitamins in metabolic syndrome subjects. Niger J Clin Pract. 2012;15(4):442–7.
Gomaraschi M, Ossoli A, Adorni MP, Damonte E, Niesor E, Veglia F, Franceschini G, Benghozi R, Calabresi L. Fenofibrate and extended-release niacin improve the endothelial protective effects of HDL in patients with metabolic syndrome. Vascul Pharmacol. 2015;74:80–6.
Hughes-Large JM, Pang DK, Robson DL, Chan P, Toma J, Borradaile NM. Niacin receptor activation improves human microvascular endothelial cell angiogenic function during lipotoxicity. Atherosclerosis. 2014;237(2):696–704.
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28.
Adiels M, Chapman MJ, Robillard P, Krempf M, Laville M, Borén J. Niacin action in the atherogenic mixed dyslipidemia of metabolic syndrome: insights from metabolic biomarker profiling and network analysis. J Clin Lipidol. 2018;12(3):810–e821811.
Tenenbaum A, Fisman EZ, Motro M, Adler Y. Atherogenic dyslipidemia in metabolic syndrome and type 2 diabetes: therapeutic options beyond statins. Cardiovasc Diabetol. 2006;5:20.
Canner PL, Furberg CD, McGovern ME. Benefits of niacin in patients with versus without the metabolic syndrome and healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol. 2006;97(4):477–9.
Haynes R, Valdes-Marquez E, Hopewell JC, Chen F, Li J, Parish S, Landray MJ, Armitage J. Serious adverse effects of extended-release Niacin/Laropiprant: results from the Heart Protection Study 2-Treatment of HDL to reduce the incidence of vascular events (HPS2-THRIVE) trial. Clin Ther. 2019;41(9):1767–77.
Chomiuk T, Niezgoda N, Mamcarz A, Śliż D. Physical activity in metabolic syndrome. Front Physiol. 2024;15:1365761.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, F.Y.Q.; software, formal analysis and data curation, X.C.; writing—original draft preparation, F.Y.Q.; writing—review and editing, W.G.F.; visualization, F.Y.Q.; supervision, W.G.F.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional Review Board Statement
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, Y., Xu, C. & Wu, G. Dietary niacin Intake and its association with all-cause and cardiovascular mortality rates in individuals with metabolic syndrome. Nutr J 23, 90 (2024). https://doi.org/10.1186/s12937-024-00993-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-024-00993-7