Abstract
Background
Previous studies have shown that high-density lipoprotein cholesterol (HDL-C) levels are positively associated with cognitive function across a range of concentrations. However, recent studies have suggested that very high HDL-C levels may lead to poorer outcomes. Therefore, we aimed to investigate the relationship between different concentrations of HDL-C and cognitive impairment risk.
Methods
We collected data from 3632 participants aged over 60 years from the U.S. National Health and Nutrition Examination Survey (NHANES) between 2011 and 2014 to assess the relationship between HDL-C and cognitive function. Cognitive function was evaluated with the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) test, the animal fluency test (AFT), and the digit symbol substitution test (DSST). We used restricted cubic spline models and logistic regression to examine the association between HDL-C and cognitive function.
Results
A U-shaped was observed between HDL-C and cognitive outcomes, individuals with higher risk in those with both low and very high HDL-C levels compared with those with midrange values. Very high HDL-C levels (≥ 2.50 mmol/L) were associated with increased risk of cognitive impairment (OR = 2.19; 95% CI, 1.12–4.28) compared with those with HDL-C levels in the range of 1.50 to 1.99 mmol/L in older adults after adjustment for confounding factors. Interaction test demonstrated that relationship between very high HDL-C and the risk of cognitive impairment was not changed in different sex and race group (P for interaction > 0.05).
Conclusions
Very high HDL-C levels were associated with an increased risk of cognitive impairment. HDL-C may not be a protective factor for maintaining brain health in older adults at very high levels.
Similar content being viewed by others
Introduction
In 2019, 700 million people in the world were over the age of 65, and by 2050, one out of six people will be older adults [1]. An increasing number of old people complain of cognitive decline [2], which is characterized by deficits in working memory, processing speed, and attention [3]. It significantly decreases the quality of life, imposes a disease burden on patients [4], and results in poor clinical outcomes [5,6,7].
At present, the development of cognitive decline involves a diversity of risk factors, such as low education level [8], comorbidities (i.e., metabolic diseases and depression) [9, 10] and health behaviors (i.e., cigarette dietary intake [11]). Among metabolic factors, abnormal lipid metabolism, including high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC) and triglycerides (TG), has been identified as an important factor in cognitive function [12, 13]. In particular, HDL-C, which is well known for its cardioprotective and anti-inflammatory functions [14], is strongly purported to have positive effects on cognitive function across a range of concentrations [12, 15,16,17]. But results form some recent studies indicated that HDL-C is associated with poorer outcomes at very high levels, such as cardiovascular events [18, 19], cerebrovascular disease [20], age-related macular degeneration [21], infections [22, 23], and mortality [18,19,20,21,22,23,24]. However, the relationship between very high concentrations of HDL-C and cognitive function remains unclear. We hypothesized that very high concentrations of HDL-C may lead to a higher risk of cognitive impairment. Accordingly, we conducted an analysis to explore the correlation between HDL-C and cognitive impairment in older individuals with a subsample of the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2014.
Materials and methods
Study population
The data were collected from the NHANES 2011–2014, which is a cross sectional study conducted by the National Centers for Health Statistics (NCHS). According to the inclusion criteria of our study, a total of 3632 participants aged over 60 years form the data of NHANES 2011–2014 were enrolled in the analyses (Fig. 1).
Assessment of cognitive function
The cognitive function of participants was estimated using the following tests [25, 26]: (1) word learning and recall modules from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), consisting of three consecutive learning trials, and a delayed recall. It assesses immediate and delayed learning ability for new verbal information (memory sub-domain); (2) the Animal Fluency test (AFT), which examines categorical verbal fluency, a component of executive function [27]; and (3) the Digit Symbol Substitution test (DSST), a performance module from the Wechsler Adult Intelligence Scale (WAIS III), relies on processing speed, sustained attention, and working memory [28]. These tests are widely used in cohort studies to assess memory, language, speech, and cognitive functions and to screen for risk factors [29,30,31].
To account for the ceiling and floor effect caused by a wide range of cognitive function in the elderly population, commonly known as the scale attenuation effect [32], we devised a method to create a global cognitive score. This was achieved by averaging the standardized scores of three cognitive tests: the CERAD, AFT, and DSST [33, 34]. Since there are no established cutoff points for these tests to determine low or normal cognitive function, we employed the lowest quartile in the study group to indicate cognitive impairment, consistent with methods previously published in literature [35].
Measurement of serum lipid profiles
HDL-C, TC and TG levels in serum were measured by enzymatic assay [36,37,38]. LDL-C was calculated from the measured values of TC, HDL-C, and TG according to the Friedewald formula [38]. And normal range of serum lipids levels are as follows: HDL ≥ 1.04 mmol/L (40 mg/dL); LDL < 3.36 mmol/L (130 mg/dL); TC < 5.17 mmol/L (200 mg/dL); and TG < 3.88 mmol/L (150 mg/dL) [39].
Covariates
We also obtained demographic characteristics, including sex, age, race, body mass index (BMI), educational level, hypertension, diabetes, smoking, alcohol consumption, lipid-lowering therapy and physical activity (PA) information. PA was categorized into low intensity and high intensity [40]. The chronic disease history (hypertension and diabetes) was self-reported based on the diagnosis of the physician. Individuals who had smoked more than 100 cigarettes in their life were classified as smokers. And alcohol consumption was classified as fewer than 12 drinks per year or more than 12 drinks per year.
Statistical analysis
The data were downloaded from the NHANES database (2011–2014). The missing data on demographic information, cognitive function and serum lipid profiles of some people were handled with multiple imputation (MI) to minimize the bias of analysis results. We grouped HDL-C into five categories in accordance with prior study (< 1.0, 1.0 to 1.49, 1.5 to 1.99, 2.0 to 2.49, and ≥ 2.50 mmol/L) [41] and compared baseline characteristics between them. Continuous variables were represented as the mean ± SD and comparisons between participants with and without cognitive impairment were analyzed by t-tests; while categorical variables are represented as N (%) and the chi-square tests were used to compare the differences.
First, we entered HDL-C as a continuous variable and analyzed the possibility of nonlinearity between HDL-C and cognitive function using a restricted cubic spline model. The concentrations of HDL-C associated with the lowest risk of cognitive impairment was the concentrations with the lowest OR ratio in restricted cubic spline regression. Next, we taken HDL-C as a categorical variable and used the multivariable logistic regression to further study the relationship between HDL-C and cognitive function in different concentrations. Individuals with HDL-C levels between 1.50 and 1.99 mmol/L formed the reference. Model 1 was adjusted for sex, age, race, BMI, and educational level, and model 2 was further adjusted for physical activity, diabetes, hypertension, smoking, alcohol consumption, lipid-lowering therapy, LDL cholesterol and triglycerides. Finally, we performed interaction analysis to determine if the association between very high HDL-C and outcomes differed by sex and race after adjusting for aforementioned covariates.
All statistical analyses were performed with SPSS 26.0 and R 4.2.1, and P < 0.05 was considered statistically significant.
Results
Basic characteristics of participants
Table 1 lists the general characteristics of the participants in this study. A total of 3632 adults aged ≥ 60 years old were included in this study, of which 1760 (48.5%) were male and 1872 (51.5%) were female. Characteristics of participants by the HDL-C categories are summarized in the Table 1. Participants with higher levels of HDL-C tended to have lower body mass index, higher educational level, and to be more likely take part in high intensity physical activity and use lipid-lowering agents (Table 1).
Restricted cubic spline regression analysis between HDL-C and cognitive impairment
For the restricted cubic spline model analysis, we found a nonlinear curve relationship between HDL-C and cognitive function (P = 0.045, Fig. 2). The graph shown that the risk of cognitive impairment decreases with increasing concentrations of HDL-C, reaching a minimum risk of approximately 1.70 mmol/L and increasing thereafter. Individuals with lower or higher concentrations of HDL-C had a greater tendency to have poor cognitive outcome. The concentration of HDL-C associated with a lower risk of cognitive impairment was approximately 1.35 to < 2.05 mmol/L.
Multivariable logistic regression analysis between HDL-C and cognitive impairment
As shown in Table 2, after full adjustment for covariates, compared with individuals with HDL-C in the range of 1.55 to 1.99 mmol/L, the OR for risk of cognitive impairment was 2.19 (95%Cl, 1.12–4.28) for individuals with concentrations of HDL-C ≥ 2.50 mmol/L in the model 2 (Table 2).
Interaction analysis
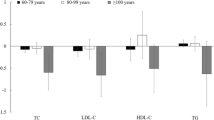
Finally, we further conducted stratified analysis and interaction test of the relationship between HDL-C and cognitive impairment (Fig. 3). As results, there was no significant interaction with sex and race was observed in the group with HDL-C level greater than 2.50 mol/L for cognitive impairment (All P for interaction > 0.05), which indicated that the association between HDL-C and cognitive function was still stable in different sex and race subgroups.
Discussion
Results of our study suggested that in older adults, compared with those with normal HDL-C levels (1.50–1.99 mmol/L), those with very high HDL-C levels (> 2.50 mmol/L) have a higher risk of poor cognitive outcome, independent of traditional risk factors. Our study indicated that high HDL-C is not always a protective factor for brain health. The protective effect of HDL-C may not appear at very high levels, instead of bringing increased risk.
Prior studies have imputed that higher HDL-C levels are associated with better cognitive function status compared with those with normal HDL-C levels [12, 15,16,17], while lower HDL-C levels are related to adverse outcomes [42]. The potential mechanism is that HDL-C is able to bind Aβ and prevent Aβ aggregation into amyloid [43, 44] and then improve the clearance of Aβ from the brain. In this way, the neurotoxicity of Aβ peptides could be decreased [45]. Furthermore, another reason may be the anti-inflammatory and antioxidant properties of apoA-I/HDL [46, 47]. These findings highlight the beneficial role of high HDL-C in maintaining good cognitive function during aging.
However, our data showed that, just as low concentrations of HDL-C can raise the risk of poor cognitive function in the population, too high concentrations of HDL-C (≥ 2.50 mmol/L) are also unfavorable to maintain better cognitive status. Recently, some studies have begun to investigate the correlation between very high concentrations of HDL-C and adverse outcomes. Results from two large cohort study concluded that people with too high concentrations of HDL-C (> 2.07 mmol/L) have a higher frequency of cardiovascular-related risk and mortality [18, 19]. Also, studies on cerebrovascular disease [20], AMD [21], infections [22, 23], and mortality [18,19,20,21,22,23,24] found similar results. European guidelines highlight that HDL-C should not be used as a risk measure when HDL-C levels exceed 90 mg/L (2.3 mmol/L) [48]. In addition, evidence from multiple randomized clinical trials shown that elevating HDL therapies had failed to significantly reduce adverse health outcome [49,50,51].
A study focus on people with Parkinson’s disease found that higher serum HDL-C were associated with poorer cognitive function in females [52]. Another, a large cohort study supported our findings, as they observed that HDL-C above 1.70 mmol/L (65 mg/dL) was significantly related to an increased rapid decline in overall cognition [53]. However, their study was based on a Chinese population with an age range of 50–70 years, whereas analysis of our study contained a much wider range of ages (including an older population over 70 years old) and races (it was multiethnic).
The potential underlying mechanisms by which very high HDL-C levels may be associated with higher cognitive impairment are still not clear. One possible explanation is that very high concentrations are usually caused by genetic variation of HDL-C, such as CETP, LIPC, and SCARB1 [54,55,56,57]. For example, it was shown that HDL-C with certain mutations may be correlate with a higher frequency of adverse cardiovascular [55,56,57] and cerebrovascular outcomes [58]. Another reason may be that the functional properties of HDL-C are impaired in individuals with extreme high HDL-C levels [59]. Generally, mature HDL-C is capable of promoting cholesterol efflux mediated by ABCG1, SR-BI and probably other mechanisms [60]. However, dysfunctional HDL-C reduced the efflux of cholesterol from macrophages by the ABCG1 and activated pro-inflammatory signaling [61]. And very high HDL-C levels (> 2.2 mmol/L) could promote oxidative stress reaction and inflammatory response [62]. These suggest that extremely high concentrations of HDL-C may be related to the adverse cognitive outcomes. However, the exact mechanism needs further investigation.
Our study has some limitations. First, concerning that concentrations of HDL-C varies among different sex (females on average have higher concentrations of HDL-C than males) and the effect of HDL-C may differ by race [63], we performed stratified analysis to study the relationship between HDL-C and cognitive impairment by sex and race. However, numbers in extreme high HDL-C groups (≥ 2.50 mmol/L) were small, limiting statistical power, which may account for the P value not achieve statistical significance in groups ≥ 2.50 mmol/L in subgroups. But interaction test shown that results of our study would not affect by sex and race (P for interaction > 0.05). So we still believe that our results are reliable. Second, as this is an observational cross-sectional study, it is unable to determine whether the association between HDL-C and cognitive impairment is causal. Additionally, the population of our study focuses on people over 60 years old, whether the relationship between HDL-C and cognitive function found in our study can be extended to other age groups is unknown and requires further exploration in the future.
Conclusions
In conclusion, our study indicated that very high levels of HDL-C may be associated with a greater risk of cognitive impairment. At present, HDL-C may not a good target for drug-based treatment. In the future, more analyses is needed to confirm the association between HDL-C and cognitive impairment in old people and to uncover the exact mechanism linking them.
Data availability
The publicly available data can be obtained here: https://www.cdc.gov/nchs/nhanes/index.htm.
References
United Nations. Department of Economic and Social Affairs. Ageing populations: we are living longer lives, but are we healthier? 2021. New York: United Nations.
Llibre Rodriguez JJ, Ferri CP, Acosta D, Guerra M, Huang Y, Jacob KS, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372:464–74.
Majer R, Adeyi O, Bagoly Z, Simon V, Csiba L, Kardos L, et al. Neuropsychiatric symptoms, quality of life and caregivers’ burden in dementia. Open Med (Wars). 2020;15:905–14.
Klimova B. Computer-based cognitive training in aging. Front Aging Neurosci. 2016;8:313.
2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327–406.
Heun R, Schoepf D, Potluri R, Natalwala A. Alzheimer’s disease and co-morbidity: increased prevalence and possible risk factors of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry. 2013;28:40–8.
Yao S, Liu Y, Zheng X, Zhang Y, Cui S, Tang C, et al. Do nonpharmacological interventions prevent cognitive decline? A systematic review and meta-analysis. Transl Psychiatry. 2020;10:19.
Zhang Q, Wu Y, Han T, Liu E. Changes in cognitive function and risk factors for cognitive impairment of the Elderly in China: 2005–2014. Int J Environ Res Public Health. 2019; 16.
Tahmi M, Palta P, Luchsinger JA. Metabolic syndrome and cognitive function. Curr Cardiol Rep. 2021;23:180.
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–61.
Eshkoor SA, Hamid TA, Mun CY, Ng CK. Mild cognitive impairment and its management in older people. Clin Interv Aging. 2015;10:687–93.
He Q, Li Q, Zhao J, Wu T, Ji L, Huang G, et al. Relationship between plasma lipids and mild cognitive impairment in the elderly Chinese: a case-control study. Lipids Health Dis. 2016;15:146.
Crichton GE, Elias MF, Davey A, Sullivan KJ, Robbins MA. Higher HDL cholesterol is associated with better cognitive function: the Maine-Syracuse study. J Int Neuropsychol Soc. 2014;20:961–70.
Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res. 2014;55:168–79.
Formiga F, Ferrer A, Chivite D, Pinto X, Badia T, Padrós G, et al. Serum high-density lipoprotein cholesterol levels correlate well with functional but not with cognitive status in 85-year-old subjects. J Nutr Health Aging. 2012;16:449–53.
Formiga F, Ferrer A, Chivite D, Pinto X, Cuerpo S, Pujol R. Serum high-density lipoprotein cholesterol levels, their relationship with baseline functional and cognitive status, and their utility in predicting mortality in nonagenarians. Geriatr Gerontol Int. 2011;11:358–64.
Bates KA, Sohrabi HR, Rainey-Smith SR, Weinborn M, Bucks RS, Rodrigues M, et al. Serum high-density lipoprotein is associated with better cognitive function in a cross-sectional study of aging women. Int J Neurosci. 2017;127:243–52.
Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38:2478–86.
Liu C, Dhindsa D, Almuwaqqat Z, Sun YV, Quyyumi AA. Very high high-density lipoprotein cholesterol levels and Cardiovascular Mortality. Am J Cardiol. 2022;167:43–53.
Li H, Qian F, Zuo Y, Yuan J, Chen S, Wu S, et al. U-Shaped relationship of High-Density Lipoprotein Cholesterol and Incidence of Total, ischemic and hemorrhagic stroke: a prospective cohort study. Stroke. 2022;53:1624–32.
Colijn JM, den Hollander AI, Demirkan A, Cougnard-Grégoire A, Verzijden T, Kersten E, et al. Increased high-density lipoprotein levels Associated with Age-Related Macular Degeneration: evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology. 2019;126:393–406.
Speer T, Zewinger S. High-density lipoprotein (HDL) and infections: a versatile culprit. Eur Heart J. 2018;39:1191–3.
Madsen CM, Varbo A, Tybjærg-Hansen A, Frikke-Schmidt R, Nordestgaard BG. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J. 2018;39:1181–90.
Bowe B, Xie Y, Xian H, Balasubramanian S, Zayed MA, Al-Aly Z. High density lipoprotein cholesterol and the risk of all-cause mortality among U.S. Veterans. Clin J Am Soc Nephrol. 2016;11:1784–93.
Centers for Disease Control and Prevention. 2011–2012 Data Documentation, Codebook, and Frequencies: Cognitive Functioning (CFQ-G) https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CFQ_G.htm Accessed on 26 March 2023.
Centers for Disease Control and Prevention. 2013–2014 Data Documentation, Codebook, and Frequencies: Cognitive Functioning (CFQ H) https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CFQ_H.htm Accessed on 26 March 2023.
Strauss E, Sherman EMS, Spreen OA. Compendium of Neuropsychological Tests: Administration, Norms and Commentary (3rd edition). 2006.
Wechsler D. WAIS Manual-Third Edition. New York: Psychological Corporation; 1997.
Lee S, Min JY, Kim B, Ha SW, Han JH, Min KB. Serum sodium in relation to various domains of cognitive function in the elderly US population. BMC Geriatr. 2021;21:328.
Botelho J, Leira Y, Viana J, Machado V, Lyra P, Aldrey JM et al. The role of inflammatory Diet and vitamin D on the link between Periodontitis and cognitive function: a mediation analysis in older adults. Nutrients. 2021;13.
Katzman EW, Nielsen SJ. The Association between Peanut and Peanut butter consumption and cognitive function among Community-Dwelling older adults. J Prev Alzheimers Dis. 2021;8:436–41.
Lim CR, Harris K, Dawson J, Beard DJ, Fitzpatrick R, Price AJ. Floor and ceiling effects in the OHS: an analysis of the NHS PROMs data set. BMJ Open. 2015;5:e007765.
Li H, Wang Z, Fu Z, Yan M, Wu N, Wu H, et al. Associations between blood cadmium levels and cognitive function in a cross-sectional study of US adults aged 60 years or older. BMJ Open. 2018;8:e020533.
Scherr M, Kunz A, Doll A, Mutzenbach JS, Broussalis E, Bergmann HJ, et al. Ignoring floor and ceiling effects may underestimate the effect of carotid artery stenting on cognitive performance. J Neurointerv Surg. 2016;8:747–51.
Chen SP, Bhattacharya J, Pershing S. Association of Vision Loss with Cognition in older adults. JAMA Ophthalmol. 2017;135:963–70.
National Health and Nutrition Examination Survey Home Page. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/TCHOL_G.htm [Accessed on January 6, 2023].
National Health and Nutrition Examination Survey Home Page. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/HDL_G.htm Accessed on January 6, 2023.
National Health and Nutrition Examination Survey Home Page. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/TRIGLY_G.htm Accessed on January 6, 2023.
Handelsman Y, Jellinger PS, Guerin CK, Bloomgarden ZT, Brinton EA, Budoff MJ, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of Dyslipidemia and Prevention of Cardiovascular Disease algorithm – 2020 executive Summary. Endocr Pract. 2020;26(10):1196–224.
U.S. Depatment of Health and Human Services. 2008 Physical activity guidelines for Americans. Hyattsville, MD: US Department of Health and Human Services (2008) http://www.health.gov/paguidelines/guidelines/default.aspx Accessed January 6, 2023.
Hamer M, O’Donovan G, Stamatakis E. High-density lipoprotein cholesterol and mortality: too much of a good thing? Arterioscler Thromb Vasc Biol. 2018;38:669–72.
Lv YB, Yin ZX, Chei CL, Brasher MS, Zhang J, Kraus VB, et al. Serum cholesterol levels within the high normal range are Associated with Better Cognitive performance among Chinese Elderly. J Nutr Health Aging. 2016;20:280–7.
Robert J, Button EB, Stukas S, Boyce GK, Gibbs E, Cowan CM, et al. High-density lipoproteins suppress Aβ-induced PBMC adhesion to human endothelial cells in bioengineered vessels and in monoculture. Mol Neurodegener. 2017;12:60.
Robert J, Button EB, Yuen B, Gilmour M, Kang K, Bahrabadi A et al. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels. Elife. 2017;6.
Van Valkenburgh J, Meuret C, Martinez AE, Kodancha V, Solomon V, Chen K, et al. Understanding the exchange of systemic HDL particles into the brain and vascular cells has diagnostic and therapeutic implications for neurodegenerative diseases. Front Physiol. 2021;12:700847.
Keeney JT, Swomley AM, Förster S, Harris JL, Sultana R, Butterfield DA. Apolipoprotein A-I: insights from redox proteomics for its role in neurodegeneration. Proteom Clin Appl. 2013;7:109–22.
Schrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O, et al. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Neurobiol Dis. 2013;59:100–10.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Nicholls SJ, Lincoff AM, Barter PJ, Brewer HB, Fox KA, Gibson CM, et al. Assessment of the clinical effects of cholesteryl ester transfer protein inhibition with evacetrapib in patients at high-risk for vascular outcomes: Rationale and design of the ACCELERATE trial. Am Heart J. 2015;170:1061–9.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Bakeberg MC, Gorecki AM, Kenna JE, Jefferson A, Byrnes M, Ghosh S, et al. Elevated HDL levels linked to poorer cognitive ability in females with Parkinson’s Disease. Front Aging Neurosci. 2021;13:656623.
An Y, Zhang X, Wang Y, Wang Y, Liu W, Wang T, et al. Longitudinal and nonlinear relations of dietary and serum cholesterol in midlife with cognitive decline: results from EMCOA study. Mol Neurodegener. 2019;14:51.
Motazacker MM, Peter J, Treskes M, Shoulders CC, Kuivenhoven JA, Hovingh GK. Evidence of a polygenic origin of extreme high-density lipoprotein cholesterol levels. Arterioscler Thromb Vasc Biol. 2013;33:1521–8.
Agerholm-Larsen B, Nordestgaard BG, Steffensen R, Jensen G, Tybjaerg-Hansen A. Elevated HDL cholesterol is a risk factor for ischemic heart disease in white women when caused by a common mutation in the cholesteryl ester transfer protein gene. Circulation. 2000;101:1907–12.
Andersen RV, Wittrup HH, Tybjaerg-Hansen A, Steffensen R, Schnohr P, Nordestgaard BG. Hepatic lipase mutations,elevated high-density lipoprotein cholesterol, and increased risk of ischemic heart disease: the Copenhagen City Heart Study. J Am Coll Cardiol. 2003;41:1972–82.
Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–71.
Anderson CD, Falcone GJ, Phuah CL, Radmanesh F, Brouwers HB, Battey TW, et al. Genetic variants in CETP increase risk of intracerebral hemorrhage. Ann Neurol. 2016;80:730–40.
Gillard BK, Rosales C, Xu B, Gotto AM Jr., Pownall HJ. Rethinking reverse cholesterol transport and dysfunctional high-density lipoproteins. J Clin Lipidol. 2018;12:849–56.
Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–70.
Rosenson RS, Brewer HB Jr., Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13:48–60.
Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–6.
Zakai NA, Minnier J, Safford MM, Koh I, Irvin MR, Fazio S, Cushman M, Howard VJ, Pamir N. Race-Dependent Association of High-Density Lipoprotein Cholesterol levels with Incident Coronary Artery Disease. J Am Coll Cardiol. 2022;80(22):2104–15.
Acknowledgements
The authors have no acknowledgments to report.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82271235) and the Natural Science Foundation of Hunan Province (No. 2021JJ31008).
Author information
Authors and Affiliations
Contributions
The authors’ contributions were as follows: Y.L. designed the research; H.H. and B.Y. analyzed the data; H.H. wrote the paper; W.O. and J.T. contributed to the manuscript revision. Y.L. had primary responsibility for the final content.
Corresponding author
Ethics declarations
Ethical approval (Consent to participate and consent to publish)
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, H., Yang, B., Yu, R. et al. Very high high-density lipoprotein cholesterol may be associated with higher risk of cognitive impairment in older adults. Nutr J 23, 79 (2024). https://doi.org/10.1186/s12937-024-00983-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-024-00983-9