Abstract
Background
There is little evidence to comprehensively summarize the adverse events (AEs) profile of intermittent fasting (IF) despite its widespread use in patients with overweight or obesity.
Methods
We searched the main electronic databases and registry websites to identify eligible randomized controlled trials (RCTs) comparing IF versus control groups. A direct meta-analysis using a fixed-effect model was conducted to pool the risk differences regarding common AEs and dropouts. Study quality was assessed by using the Jadad scale. Pre-specified subgroup and sensitivity analyses were conducted to explore potential heterogeneity.
Results
A total of 15 RCTs involving 1,365 adult individuals were included. Findings did not show a significant difference between IF and Control in risk rate of fatigue [0%, 95% confidence interval (CI), -1% to 2%; P = 0.61], headache [0%, 95%CI: -1% to 2%; P = 0.86] and dropout [1%, 95%CI: -2% to 4%; P = 0.51]. However, a numerically higher risk of dizziness was noted among the IF alone subgroup with non-early time restricted eating [3%, 95%CI: -0% to 6%; P = 0.08].
Conclusions
This meta-analysis suggested that IF was not associated with a greater risk of AEs in adult patients affected by overweight or obesity. Additional large-scale RCTs stratified by key confounders and designed to evaluate the long-term effects of various IF regimens are needed to ascertain these AEs profile.
Similar content being viewed by others
Introduction
Currently, obesity and overweight are considered as widespread chronic metabolic diseases. In China, an estimated 34.3% of the adult population is overweight with another 16.4% being obese [1]; in the USA, 33.3% of adults with obesity [2] and in Europe, 34.5% are overweight and 15.8% are obese [3]. The rate of overweight and obesity is continuing to rise domestically and globally [4], which is undoubtedly associated with a concomitant rise in medical and economic costs [5].
A wide range of treatments are available for weight loss, including intensive lifestyle interventions, public health programs, pharmacotherapies and surgical bariatric therapies [6], among which intermittent fasting (IF), an eating pattern involving periods of voluntary abstinence from calories for a period of time, alternating with periods of caloric consumption, has gained public popularity as a feasible and easy-to-adapt dietary strategy [7, 8].
Previous meta-analyses have shown that IF can effectively decrease body weight [9], regardless of various regimens [10], namely time-restricted eating (TRE), the 5:2 diet and alternate day fasting (ADF). Despite its weight-centric effectiveness, many people are concerned with the adverse effects of IF [11], including long-term uncertain safety implications [12]. In recent years, many randomized controlled trials (RCTs) have been conducted in adults with overweight or obesity to investigate the potential effects of IF. Given that only narrative reviews on the adverse events (AEs) profile of IF [13, 14] were found, we aimed to conduct the first comprehensive meta-analysis to quantitatively assess the AEs profile of IF based on these published RCTs.
Methods
Search strategy and selection criteria
This review protocol was pre-registered on the International Prospective Register of Systematic Reviews database (https://www.crd.york.ac.uk/PROSPERO/; registration ID: CRD42023488573). RCTs to investigate AEs profile of IF were eligible for inclusion in our analysis, without any restrictions in terms of language or publication date. We electronically searched PubMed, Embase, Web of Science Core Collection and Cochrane Library databases on November 15, 2023, using the following search terms (“intermittent fasting” or “time- restricted feeding” or “time-restricted eating” or “alternate day fasting” or “5:2 dieting”) AND Randomized Controlled Trial. We also further conducted searches on the ClinicalTrials.gov register website. The detailed search strategies used for these studies are included in Supplementary Table 2, Additional File 1.
Eligible RCTs had to meet the following inclusion criteria: (i) published as an original article; (ii) study participants are adults with obesity or overweight; (iii) evaluated the effect of any regimen of IF as one of the study interventions compared with the control group; and (iv) reported any data on any AEs and dropouts. Only parallel-arm RCTs were eligible for inclusion. When more than one article reported data from a study with the same registration number, the most updated and relevant study was included.
Data extraction and risk of bias assessment
The following information was extracted from each eligible study in this meta-analysis: (i) first author’s surname and study country; (ii) publication year; (iii) study size; (iv) study population entry criteria; (v) demographics (age, sex and body mass index [BMI]); (vi) IF regimen; (vii) control group regimen; (viii) treatment duration and follow-up duration; (ix) number of subjects with various AEs and dropouts. The reported AEs were further coded using the 23.1-English version of the Medical Dictionary for Regulatory Activities (MedDRA) at preferred terms and system organ class levels.
Key data were extracted using a standardized data-recording form and the risk for bias was assessed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Supplementary Table 1, Additional File 1) [15]. Three investigators (F.Z, T.Z and X.J) conducted the study search and screening, data extraction, and risk of bias assessment independently by using the revised Cochrane risk of bias tool for randomized trials (ROB2) [16]. Information was checked and adjudicated independently by an additional investigator (W.S.) until agreement was achieved where needed. We also calculated the Jadad score to assess the quality of the included RCTs [17]. The overall quality of evidence for each outcome was also assessed by two independent investigators (F.Z. and W.S.) using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology [18].
Outcomes
The primary outcome in the study was the serious adverse events (SAEs). The secondary outcomes included the most occurred specific AEs and dropouts (named dropout, loss to follow-up, loss of contact or withdrawal for various reasons).
Statistical analysis
We used the statistical software R 4.3.1 (www.r-project.org) along with the ‘meta’ package to conduct the direct meta-analysis [19]. Firstly, a subject-based frequency table by treatment was created to depict each specific adverse event as appropriate. Then distinctions of these binary variables were statistically evaluated using risk difference (RD) with a two-sided 95% confidence interval (CI), displayed by forest plots for each of the common AEs. The various IF or Control regimens were merged separately, and for pooling homogeneous study data, a fixed-effect model was established; otherwise, both fixed-effect and random-effects models (restricted maximum-likelihood [REML] estimator used to explore the between-study variance) following inverse variance method were provided. Sensitivity analysis was conducted as appropriate if BMI data were not clearly available in the RCTs. Continuity correction of 0.5 was utilized in studies with zero cell frequencies as appropriate. Two-sided P values of less than 0.05 were considered statistically significant. Between-study heterogeneity was assessed via I2 (less than 25% for low heterogeneity, within 25% and 50% for moderate heterogeneity, within 50% to 75% for substantial heterogeneity and more than 75% for high heterogeneity) [20] and Q test of Cochran (if P < 0.10 for heterogeneity) [21]. Both statistical measures evaluate the percent variability across studies due to heterogeneity instead of chance. Subgroup analyses according to prespecified diabetes mellitus status (Yes versus No), IF timing (early TRE [eTRE] versus non-eTRE) and study treatment duration (short-term [< 6 months] versus long-term [6 or 12 months]) were conducted to ascertain the effects of potential confounders on AEs, where eTRE refers to time restricted eating whose eating window starts in early morning (not later than 10:00 AM) [22].
Funnel plots for common AEs and dropouts were performed and the Egger’s regression test [23] was also used to statistically assess publication bias. Upon request the R codes are available from the authors.
Results
Search results
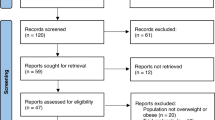
In total, 756 citations were initially identified with the use of our search strategy, and after duplicate record removal, followed by title and abstract screening, 66 full-text reports were included for final eligibility assessment. Eventually 15 reports originating from 15 RCTs (1,365 individuals) [11, 12, 24,25,26,27,28,29,30,31,32,33,34,35,36]met our criteria of inclusion and were involved in this meta-analysis. The whole processes of the relevant study selection are shown in detail in Fig. 1.
Study characteristics
Table 1 displays the study and participants characteristics for the 15 included trials. Among them, four were conducted in patients with diabetes mellitus [11, 24, 25, 29] and the remaining in patients without diabetes mellitus. Study treatments durations in one-third of the trials were twelve or six months while others ran for no shorter than seven [35] or eight [33, 34] weeks. Various regimens of IF were found, with the fasting to eating ratio ranging from 16:8 h (n = 7) or 14:10 h (n = 3) daily to 5:2 days (n = 3) or 4:3 days (n = 2) per week. The mean ages at the study level in included studies were all over thirty years, with the exception of the trial by Liu H et al. (21 ± 1 [mean ± standard deviation] kg/m2, but with hidden obesity [body fat percentage ≥ 30%]) [33] and another study with no mean BMI data of subjects reported [25]. Both sexes were included in all trials other than two studies [33, 35]. Hence, the two RCTs [25, 33] were removed from the sensitivity analysis.
Assessment of bias risk
Figure 2 presents the assessment for risk of bias in 15 trials according to Cochrane RoB2 tool. 14 out of them had an overall “some concerns” risk of bias, except the “high” risk in Lin, S. et al.’s study [36], and no study stopped early. There were some concerns about the risk of bias in the trial by Kotarsky et al. [34] for lack of mentioning analysis of intention-to-treat principle and the reporting of only TRE-related AEs. Another included RCT [24] did not describe any specific method to generate the randomization sequence. In addition, by using the Jadad score assessment, we noticed that two studies [24, 34] obtained 2 points, one study [11] obtained 4 points and the rest obtained 3 points. In summary, the overall quality of the included RCTs could be largely defined as good.
Serious adverse events (SAEs)
Six (0.4%) patients in two studies [25, 28] out of the 15 total (1,365 individuals) reported data on any SAEs (2 [0.3%] in the IF group versus 4 [0.5%] in the control group) within the treatment period. All of the reported SAEs were categorized as leading to hospitalization. None of them were considered related to any of the study interventions, and then no specific reported terms were described in the full-text articles. None of the 15 included RCTs reported any major or severe AEs.
Specific adverse events
Twelve included RCTs (N = 1,174) [11, 12, 24, 27,28,29,30,31,32,33,34, 36] reported at least one subject who experienced one of specific AEs, among which the most frequently reported included fatigue, headache, dizziness, constipation and diarrhea (see Supplementary Table 3, Additional File 1 for the complete list of all reported terms).
Fatigue was reported in 87 (14.5%) subjects in the IF group and 124 (16.2%) subjects in the control group (Supplementary Table 3, Additional File 1). No statistically significant difference in risk between IF and Control was found in the overall pooled analysis (0%, 95%CI: -1% to 2%; P = 0.61, Fig. 3a). Similar risk difference in fatigue profile was observed across prespecified subgroups (Supplementary Table 4, Additional File 1).
Headache was reported in 81 (13.5%) subjects in the IF group and 122 (15.9%) subjects in the control group (Supplementary Table 3, Additional File 1). No significant risk difference was equally found in the overall pooled analysis (0%, 95%CI: -1% to 2%; P = 0.86, Fig. 3b). The risk of headache profile appeared similar for the two groups when assessed according to subgroups (Supplementary Table 4, Additional File 1).
In addition, 59 (9.8%) subjects in the IF group and 72 (9.4%) subjects in the control group reported dizziness (Supplementary Table 3, Additional File 1). There was no statistically significant difference in the risk between IF and control groups in the overall pooled analysis (1%, 95%CI: -1% to 3%; P = 0.17, Fig. 3c). In the IF group versus Control, a numerically greater incidence of dizziness was observed among patients with non-eTREs (2%, 95%CI: -0% to 5%; P = 0.07, Supplementary Table 4, Additional File 1) and among patients without diabetes (2%, 95%CI: -0% to 4%; P = 0.08, Supplementary Table 4, Additional File 1). The sensitivity analysis after removing the studies by Obermayer et al. and Liu H et al. [25, 33] revealed similar between-group trends in the occurrence rate of dizziness (data not shown).
Forest plots in the meta-analysis of IF versus Control in terms of (a) fatigue; (b) headache; (c) dizziness. The sizes of the data markers indicate the relative weight of each study in this analysis. The diamond represents the overall estimated effects in each model. Note CI, confidence interval; IF, intermittent fasting; RD, risk difference
Dropouts
All 15 studies reported data on dropout, loss to follow-up, loss due to inability to contact or withdrawal for various reasons. There was no significant difference in the dropout risk between IF and Control (1%, 95%CI: -2% to 4%; P = 0.51, Fig. 4). The overall dropout rate was 11.5% in the IF group, which indicated acceptable adherence given the current study treatment duration.
Forest plot in the meta-analysis of IF versus Control on dropout. The sizes of the data markers indicate the relative weight of each study in this analysis. The diamond represents the overall estimated effects in each model. Note CI: Confidence Interval; IF: Intermittent Fasting; RD: Risk Difference
IF alone versus usual lifestyle
To further understand whether IF alone could increase the occurrence risk of common AEs and dropouts rate compared with the usual diet or standard care (namely neither any diet intervention provided nor food energy intake changed), we extracted a subset of 11 RCTs involving 820 patients meeting the criteria for subgroup analysis. No significant between-group differences were detected in terms of fatigue (1%, 95%CI: -1% to 3%; P = 0.55), headache (0%, 95%CI: -2% to 2%; P = 0.93) or dizziness (1%, 95%CI: -1% to 4%; P = 0.18). It was also noted that in the IF alone group as compared to usual lifestyle, a numerically higher occurrence rate of dizziness was observed among patients with non-eTREs (3%, 95%CI: -0% to 6%; P = 0.08, Supplementary Table 5, Additional File 1) and among patients without diabetes (3%, 95%CI: -0% to 6%; P = 0.05, Supplementary Table 5, Additional File 1).
Publication bias
Funnel plots and Egger’s tests revealed no evidence of significant publication bias in the current meta-analysis for fatigue, headache, dizziness or dropout (Egger’s test: P = 0.46, 0.11, 0.14 and 0.46, respectively).
Certainty of the evidence
We assessed the certainty of the evidence of our outcome and found that it was moderate in terms of SAEs or low in the other outcomes, which increased confidence in our effect estimate (Table 2).
Discussion
To our knowledge, this is the first meta-analysis to specifically investigate the adverse effects and dropouts of IF compared with the control group in patients with overweight or obesity regardless of diabetes status. Our meta-analysis results suggested that IF was not associated with an increase in the risk of AEs, despite a numerically greater risk of dizziness in the non-eTRE subgroups after treatment with IF. Consistent with our findings on dropouts, another recent meta-analysis [37] revealed no evidence that IF interventions affected dropout in RCTs differently from continuous energy restriction. On the other hand, none of the three recent meta-analyses [9, 10, 37] reported and compared any AEs profile data.
Currently, IF is becoming more popular because it seems to be a simple option to follow in treating several diseases such as overweight and obesity [12]. Like in IF regimens, voluntary abstinence from food has been present throughout human history, such as habits and rituals associated with racial and religious contexts [8]. Hence IF is considered to be safe to some degree, similar to processes with greatly reduced food intake such as hibernation [8]. Very few SAEs (only 2 [0.3%] subjects with IF) were reported in the included RCTs and none of them were judged to be related to the study treatment. None of the 15 involved RCTs reported any major or severe AEs, and 3 of them [25, 26, 35] reported no severe adverse effects. Overall, these data showed that IF regimens are not associated with higher risk of any major AEs when compared to a usual diet or other active comparators.
However, we still found several common AEs (approximately 10% or greater) in the IF group, including fatigue (14.5%), headache (13.5%), constipation (10.2%), dizziness (9.8%) and diarrhea (7.8%). Fatigue is a state of prolonged tiredness, exhaustion, and lack of energy that is not improved by sleep or rest [38]. In general, headache, which is mainly due to hypoglycemia, is a common side effect of fasting [39]. Our data above are apparently in line with the stronger feelings of hunger [30] and desire to eat noted in participants with intermittent energy restriction. In addition, constipation and diarrhea are external gastrointestinal disturbances, which may be caused by irregular TRE regimens compared with habitual eating timing.
Notably, there was a higher greater incidence of dizziness in both subgroups of patients with non-eTRE regimens and without diabetes. Dizziness is primarily caused by a lack of energy and blood volume following fasting and water deprivation [40]. The higher risk of dizziness in the non-eTREs subgroup might originate from a lack of energy intake due to the later eating time, which has been implied in a prior study [41], in which a single case of dizziness was resolved by having a small snack. According to the proposal by Charlot A. et al. [8]. , the food intake should begin at 8 a.m., after the cortisol peak when the activity phase started, and should end no later than 6 p.m., for this purpose of reducing risk of dizziness by obeying the circadian clock. The hypothesized feeding time period started earlier than that in the non-eTRE subgroup, indicating that the non-eTRE may induce a greater risk of dizziness. Therefore, we speculate that early TREs following the circadian clock are beneficial for the prevention of dizziness co-occurring with IF treatment.
Admittedly, our study has several potential limitations. First, it was based on reported aggregate data rather than individual patient data, which may not provide a robust estimation of the comparative risk. The quality of our study relied on the quality of each RCT included. As a result, we only included RCTs in our analysis. RCTs might provide a possibility to estimate the net adverse effect of IF in contrast with the control group when only the usual diet or background treatment shared with the IF group is included in the control group. Second, these 15 RCTs were conducted with different diabetes statuses, various IF regimens, possible concomitant background treatment or physical activity, shorter or longer study treatment durations, multiple countries with diverse dietary cultures, impacts of the recent Coronavirus Disease 2019(COVID-19) pandemic [31, 35] and so on; all of which may represent major potential sources of heterogeneity for our analysis. Therefore, we considered several prespecified subgroup analyses and sensitivity analyses. Nevertheless, we agreed that the certainty of evidence should be low and that the study findings should be applied with caution. Third, none of these 15 RCTs obtained a high score in the Jadad assessment, which was mainly attributed to the unavailability of a double-blinded design due to the nature of the intervention [24,25,26, 34, 36]. Fourth, grading criteria for AEs was rarely mentioned in included RCTs, let alone severity to be collected and analyzed for AEs. For the purpose of standardized pooling, we performed a medical coding for all AEs terms extracted from included RCTs.
In summary, our meta-analyses showed that IF was not associated with significantly increased risk of AEs in patients with overweight or obesity, regardless of diabetes status, timing and duration of IF regimens. Additional large-scale RCTs stratified by key confounders, matched with circadian clocks and designed to evaluate the long-term effects of various IF regimen were needed to confirm these findings, including AEs profile [8, 42].
Additional file 1: Supplementary Table (1) PRISMA 2020 Checklist. Supplemental Table (2) Key words for literature search on intermittent fasting using PubMed, Embase, Web of Science Core Collection, Cochrane and Clinicaltrials.gov. Supplemental Table (3) Number of studies or subjects with PTs reported in all of 15 included randomized controlled trials. Supplemental Table (4) Subgroup analyses between IF and Control of fatigue, headache and dizziness by pre-defined study characteristics. Supplemental Table (5) Subgroup analyses between IF alone and usual diet of fatigue, headache and dizziness by pre-defined study characteristics. Supplemental Fig. 1. Funnel plots in the meta-analysis of IF versus Control in terms of (a) Fatigue; (b) Headache; (c) Dizziness; (d) Dropout.
Data availability
No datasets were generated or analysed during the current study.
References
Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–92. https://doi.org/10.1016/S2213-8587(21)00045-0.
National Center for Chronic Disease Prevention and Health Promotion, Division of Nutrition, Physical Activity, and, Obesity. https://www.cdc.gov/nccdphp/dnpao/data-trends-maps/index.html.
Diamantis DV, Karatzi K, Kantaras P, Liatis S, Iotova V, Bazdraska Y, Tankova T, Cardon G, Wikstrom K, Rurik I, et al. Prevalence and Socioeconomic Correlates of Adult Obesity in Europe: the Feel4Diabetes study. Int J Environ Res Public Health. 2022;19(19). https://doi.org/10.3390/ijerph191912572.
Wang JS, Xia PF, Ma MN, Li Y, Geng TT, Zhang YB, Tu ZZ, Jiang L, Zhou LR, Zhang BF, et al. Trends in the prevalence of metabolically healthy obesity among US adults, 1999–2018. JAMA Netw Open. 2023;6(3):e232145. https://doi.org/10.1001/jamanetworkopen.2023.2145.
Anekwe CV, Jarrell AR, Townsend MJ, Gaudier GI, Hiserodt JM, Stanford FC. Socioeconomics of obesity. Curr Obes Rep. 2020;9(3):272–9. https://doi.org/10.1007/s13679-020-00398-7.
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–138. https://doi.org/10.1161/01.cir.0000437739.71477.ee.
Small S, Iglesies-Grau J, Gariepy C, Wilkinson M, Taub P, Kirkham A. Time-restricted eating: a Novel Dietary Strategy for Cardiac Rehabilitation. Can J Cardiol. 2023;39(11S):S384–94. https://doi.org/10.1016/j.cjca.2023.09.017.
Charlot A, Hutt F, Sabatier E, Zoll J. Beneficial effects of early time-restricted feeding on metabolic diseases: importance of aligning Food habits with the circadian clock. Nutrients. 2021;13(5). https://doi.org/10.3390/nu13051405.
Sun JC, Tan ZT, He CJ, Hu HL, Zhai CL, Qian G. Time-restricted eating with calorie restriction on weight loss and cardiometabolic risk: a systematic review and meta-analysis. Eur J Clin Nutr. 2023;77(11):1014–25. https://doi.org/10.1038/s41430-023-01311-w.
Elortegui Pascual P, Rolands MR, Eldridge AL, Kassis A, Mainardi F, Le KA, Karagounis LG, Gut P, Varady KA. A meta-analysis comparing the effectiveness of alternate day fasting, the 5:2 diet, and time-restricted eating for weight loss. Obes (Silver Spring). 2023;31(Suppl 1):9–21. https://doi.org/10.1002/oby.23568.
Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab (Lond). 2021;18(1):88. https://doi.org/10.1186/s12986-021-00613-9.
Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386(16):1495–504. https://doi.org/10.1056/NEJMoa2114833.
Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, Contaldo F, Pasanisi F, Ghigo E, Bo S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16(1):371. https://doi.org/10.1186/s12967-018-1748-4.
Harris L, Hamilton S, Azevedo LB, Olajide J, De Brun C, Waller G, Whittaker V, Sharp T, Lean M, Hankey C, Ells L. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database Syst Rev Implement Rep. 2018;16(2):507–47. https://doi.org/10.11124/JBISRIR-2016-003248.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. https://doi.org/10.1002/jrsm.1411.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. https://doi.org/10.1016/0197-2456(95)00134-4.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. https://doi.org/10.1136/ebmental-2019-300117.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. https://doi.org/10.2307/3001666.
Dote-Montero M, Merchan-Ramirez E, Oses M, Echarte J, Clavero-Jimeno A, Alcantara JM, Camacho-Cardenosa A, Cupeiro R, Rodriguez-Miranda MLN, Lopez-Vazquez A, et al. Efficacy of different 8 h time-restricted eating schedules on visceral adipose tissue and cardiometabolic health: a study protocol. Nutr Metab Cardiovasc Dis. 2023. https://doi.org/10.1016/j.numecd.2023.09.014.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Pavlou V, Cienfuegos S, Lin S, Ezpeleta M, Ready K, Corapi S, Wu J, Lopez J, Gabel K, Tussing-Humphreys L, et al. Effect of time-restricted eating on weight loss in adults with type 2 diabetes: a Randomized Clinical Trial. JAMA Netw Open. 2023;6(10):e2339337. https://doi.org/10.1001/jamanetworkopen.2023.39337.
Obermayer A, Tripolt NJ, Pferschy PN, Kojzar H, Aziz F, Muller A, Schauer M, Oulhaj A, Aberer F, Sourij C, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)-A Randomized Controlled Trial. Diabetes Care. 2023;46(2):463–8. https://doi.org/10.2337/dc22-1622.
Suthutvoravut U, Anothaisintawee T, Boonmanunt S, Pramyothin S, Siriyothin S, Attia J, McKay GJ, Reutrakul S, Thakkinstian A. Efficacy of Time-Restricted Eating and Behavioral Economic Intervention in reducing fasting plasma glucose, HbA1c, and cardiometabolic risk factors in patients with impaired fasting glucose: a Randomized Controlled Trial. Nutrients. 2023;15(19). https://doi.org/10.3390/nu15194233.
Chair SY, Cai H, Cao X, Qin Y, Cheng HY, Ng MT. Intermittent fasting in weight loss and cardiometabolic risk reduction: a Randomized Controlled Trial. J Nurs Res. 2022;30(1):e185. https://doi.org/10.1097/jnr.0000000000000469.
Teong XT, Liu K, Vincent AD, Bensalem J, Liu B, Hattersley KJ, Zhao L, Feinle-Bisset C, Sargeant TJ, Wittert GA, et al. Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: a randomized controlled trial. Nat Med. 2023;29(4):963–72. https://doi.org/10.1038/s41591-023-02287-7.
Overland J, Toth K, Gibson AA, Sainsbury A, Franklin J, Gauld A, Wong J. The safety and efficacy of weight loss via intermittent fasting or standard daily energy restriction in adults with type 1 diabetes and overweight or obesity: a pilot study. Obes Med. 2018;12:13–7. https://doi.org/10.1016/j.obmed.2018.11.001.
Sundfor TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr Metab Cardiovasc Dis. 2018;28(7):698–706. https://doi.org/10.1016/j.numecd.2018.03.009.
Jamshed H, Steger FL, Bryan DR, Richman JS, Warriner AH, Hanick CJ, Martin CK, Salvy SJ, Peterson CM. Effectiveness of early time-restricted eating for weight loss, Fat loss, and Cardiometabolic Health in adults with obesity: a Randomized Clinical Trial. JAMA Intern Med. 2022;182(9):953–62. https://doi.org/10.1001/jamainternmed.2022.3050.
Schubel R, Nattenmuller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, Schlett CL, von Stackelberg O, Johnson T, Nabers D, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):933–45. https://doi.org/10.1093/ajcn/nqy196.
Liu H, Chen S, Ji H, Dai Z. Effects of time-restricted feeding and walking exercise on the physical health of female college students with hidden obesity: a randomized trial. Front Public Health. 2023;11:1020887. https://doi.org/10.3389/fpubh.2023.1020887.
Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, Hackney KJ. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep. 2021;9(10):e14868. https://doi.org/10.14814/phy2.14868.
Haganes KL, Silva CP, Eyjolfsdottir SK, Steen S, Grindberg M, Lydersen S, Hawley JA, Moholdt T. Time-restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: a randomized controlled trial. Cell Metab. 2022;34(10):1457–e14711454. https://doi.org/10.1016/j.cmet.2022.09.003.
Lin S, Cienfuegos S, Ezpeleta M, Gabel K, Pavlou V, Mulas A, Chakos K, McStay M, Wu J, Tussing-Humphreys L, et al. Time-restricted eating without calorie counting for weight loss in a racially diverse Population: a Randomized Controlled Trial. Ann Intern Med. 2023;176(7):885–95. https://doi.org/10.7326/M23-0052.
Elsworth RL, Monge A, Perry R, Hinton EC, Flynn AN, Whitmarsh A, Hamilton-Shield JP, Lawrence NS, Brunstrom JM. The Effect of Intermittent Fasting on Appetite: a systematic review and Meta-analysis. Nutrients. 2023;15(11). https://doi.org/10.3390/nu15112604.
Alesci RS, Hecking C, Weissmann MV. Identification of an Unmet Medical need: height of Depression, Hypersomnia, and Sleep Apnea positively correlate with the level of fatigue in patients with Immune Thrombocytopenia. Cureus. 2023;15(10):e47003. https://doi.org/10.7759/cureus.47003.
Shalabi H, Hassan ASt, Al-Zahrani FA, Alarbeidi AH, Mesawa M, Rizk H, Aljubayri AA. Intermittent fasting: benefits, Side effects, Quality of Life, and knowledge of the Saudi Population. Cureus. 2023;15(2):e34722. https://doi.org/10.7759/cureus.34722.
Zhang L, Bu XS, Qiao QQ, Ren YQ, Yu B, Xiao XP, Jia YF, Xia ZY, Zhan LY, Yu SH. Intravenous Administration of Hypertonic Glucose Solution to prevent dizziness in patients undergoing gastrointestinal Endoscopy under General Anesthesia: a Randomized Clinical Trial. Comb Chem High Throughput Screen. 2023;26(8):1571–7. https://doi.org/10.2174/1386207326666230120111036.
Lee SA, Sypniewski C, Bensadon BA, McLaren C, Donahoo WT, Sibille KT, Anton S. Determinants of adherence in Time-restricted feeding in older adults: lessons from a pilot study. Nutrients. 2020;12(3). https://doi.org/10.3390/nu12030874.
Schuppelius B, Peters B, Ottawa A, Pivovarova-Ramich O. Time restricted eating: a Dietary Strategy to prevent and treat metabolic disturbances. Front Endocrinol (Lausanne). 2021;12:683140. https://doi.org/10.3389/fendo.2021.683140.
Acknowledgements
We all thank Dr. Sam Zhong for his generous assistance with the statistical analysis of this study data.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
F.Z., T.Z. and S.W. were involved in the conception and design process of the study, and contributed to the literature selection. F.Z., X.J., and X.C. participated in the data acquisition process and medical coding. T.Z., R.W. and L.S. synthesized the results and tables. F.Z. wrote the manuscript text. S.W. provided suggestions accordingly. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhong, F., Zhu, T., Jin, X. et al. Adverse events profile associated with intermittent fasting in adults with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Nutr J 23, 72 (2024). https://doi.org/10.1186/s12937-024-00975-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-024-00975-9