Abstract
Background
Protein supplements have been widely used among those who are struggling with sarcopenic obesity among older adults. However, despite their popularity, there is still a lack of concrete evidence on both the potential benefits and side effects of protein supplementation and exercise on sarcopenic obesity (SO).
Objective
Thus, we aimed to determine the impacts of protein supplementation and exercise in older adults with sarcopenic obesity.
Method
A systematic database search was conducted for randomised controlled trials, quasi experimental study and pre-post study design addressing the effects of protein supplementation in improving sarcopenic obesity among older adults. This scoping review was conducted based on PRISMA-Scr guidelines across PubMed, Embase, Web of Science and Cochrane Library databases. To assess record eligibility, two independent reviewers performed a rigorous systematic screening process.
Results
Of the 1,811 citations identified, 7 papers met the inclusion criteria. Six studies were randomised controlled trials and one study was a pre-post test study design. The majority of studies discussed the use of both protein supplements and exercise training. The included studies prescribed protein intake ranging from 1.0 to 1.8 g/kg/BW/day for the intervention group, while the duration of exercise performed ranged from 2 to 3 times per week, with each session lasting for 1 hour. Whey protein supplementation has been shown to be effective in improving sarcopenic conditions and weight status in SO individuals. The combination of exercise training especially resistance training and the used of protein supplement provided additional benefits in terms of lean muscle mass as well as biomarkers. The study also revealed a lack of consistency in exercise design among interventions for sarcopenic obesity.
Conclusion
Overall, it appears to be a promising option for SO individuals to improve their sarcopenic condition and weight status through the combination of resistance exercise and whey protein supplementation. However, it also highlights the need for caution when it comes to high amounts of protein intake prescription. Future research is warranted to investigate the optimal exercise design for this population, given the limited research conducted in this specific area.
Similar content being viewed by others
Introduction
With the rapid growing of older adult population worldwide, age-related health problems have become a global concern. According to the United Nations, the global population aged 60 years or over numbered 962 million in 2017, which is approximately 13% of the global population [1]. The World Health Organization (WHO) reported that the number had increased to one billion in 2020, outnumbering children younger than five years old. [2]. This figure is anticipated to double by 2050, reaching 2.1 billion [2]. The issue of ageing population is not only limited to high-income countries. In low- and middle-income countries, the number of people aged 60 years and over is growing faster than in high-income countries [3]. The pace of population ageing is much faster than in the past, as a result it is estimated that 80% of older people will be living in low- and middle-income countries [2].
Research has shown that older adults are at a considerably higher risk of health problems [4] including sarcopenia [5,6,7] and sarcopenic obesity [8, 9]. Sarcopenia is a condition that affects older people more frequently than younger people, with prevalence rates ranging from 5 to 50% [5] depending on diagnostic criteria and geographic location. For instance, among Asian countries, Thailand, Malaysia and Singapore showed prevalence rates of sarcopenia of 22.2%, 59.8% and 32.2%, respectively [7, 10, 11]. As a high-risk geriatric syndrome, by using different definitions, a study conducted in Canada reported prevalence of sarcopenic obesity ranging from 0.1 to 85.3% in males, and from 0 to 80.4% in females [9]. It is noted that the prevalence of sarcopenic obesity is increasing in adults aged 65 years and older [8] and it appears to be particularly common among older women [12].
Ageing people may experience change in visceral fat distribution into the intra-abdominal region due to adipose inflammation, which can also promote fat infiltration inside the skeletal muscles, ultimately resulting in loss of overall strength and functional ability [13]. This disease is referred to as sarcopenia, which is the loss of muscle mass that occurs with ageing and is significantly linked to an increased risk of injury occurrences [14], poor mental health, cognitive decline, decreased physical activity [15] and overall increased mortality [14, 16]. On the other hand, sarcopenic obesity refers to the combination of sarcopenia and obesity [17]. Sarcopenia can occur in obese individuals at any age as a result of the detrimental effects of adipose tissue-dependent metabolic abnormalities, such as oxidative stress, inflammation, and insulin resistance, all of which have a significant negative impact on muscle mass [18].
Sarcopenia and obesity are considered as double health burden as it can independently pose increased risks for adverse health outcomes. For example, individuals with obesity have a high prevalence of chronic non-communicable diseases that negatively impact muscle metabolism [19, 20]. Compared to individuals who only have sarcopenia or obesity, individuals with sarcopenic obesity have greater risks of metabolic disorders, higher CVD prevalence, higher mortality rates and reduced physical performance [21,22,23]. The health hazards may be increased synergistically when these two disorders are present [8, 23]. Moreover, individuals with sarcopenic obesity have a higher risk of developing chronic conditions such as systemic inflammation, full-blown sarcopenia, cachexia as well as systemic insulin resistance and other related clinical issues [13].
In 2015, a study was conducted to investigate the effects of a high whey protein, leucine and vitamin D-enriched supplement on muscle mass during intentional weight loss among obese older adults. The outcomes demonstrated that the use of the supplement was successful in maintaining muscle mass while losing weight in this cohort [24]. On the other hand, a six-month experiment among older people with sarcopenia aiming to examine the safety and tolerability of a medical nutrition drink fortified with vitamin D, calcium, and leucine revealed that the drink was safe and well-tolerated by the participants [25]. The results from these studies have suggested that the oral supplement drink containing protein supplement may have prospective advantages for the treatment of sarcopenic obese older people without compromising muscle mass and strength. These studies have highlighted the potential efficacy of protein supplementation in attenuating the negative impact of sarcopenic obesity on muscle health.
However, recent review studies have indicated that protein supplementation alone may not lead to significant changes in parameters associated with sarcopenia [26, 27], which contradicts the findings from previous studies [28]. On the other hand, a meta-analysis review has shown that exercise training alone or in combination with protein supplementation improved muscle mass, grip strength, reduced total fat mass, as well as waist circumference in individuals with sarcopenia [29]. The evidence suggests that exercise and protein supplementation have a synergistic effect on the condition of sarcopenia among individuals with sarcopenia [30, 31]. However, none of these systematic reviews specifically focused on the sarcopenic obesity population, and the potential side effects of protein supplementation were not reported. Among the previous review studies on sarcopenic obesity, greater emphasis was placed on the effects of exercise [32, 33], while the effect of protein supplementation remains relatively underreported. Taken together, the findings regarding the effects of protein supplementation on sarcopenia were inconclusive. Therefore, the aim of the present scoping review is to identify the various types of protein supplements available, assess their effects on sarcopenia and obesity, evaluate the potential side effects, and to determine the impact of exercise among older adults with sarcopenic obesity.
Method
Study design
We conducted a scoping review to summarise the available evidence and provide an overview of protein supplementation intervention and their outcomes related to the sarcopenia and weight status of older adults with sarcopenic obesity. The methodological framework proposed by Arksey and O’Malley [34] was used to conduct this scoping review which involved the following steps: (i) identifying the research question, (ii) identifying relevant studies, (iii) selecting the studies, (iv) charting the information and (v) summarizing the results. The present scoping review was reported in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis extension for scoping review (PRISMA-ScR) guidelines [35].
Identifying research question
This review was led by the following research questions: (i) What are the types of protein supplementation commonly used for sarcopenic obesity? (ii) What are the effects of protein supplementation intervention on sarcopenic obesity in older adults? and (iii) What are the side effects of protein supplementation?
Identifying relevant studies
A comprehensive search was performed using the databases in PubMed, Embase, Web of Science and Cochrane Library. We searched for articles that were published between 25 December 2012 and 1 February 2023 (last 10 years). The search was limited to the last 10 + years because authors perceived those interventions earlier than 10 + years might not be as relevant in the current scenario. Additionally, an internet search was conducted using various combinations of relevant search terms in the Google search engine, reviewing the first 10 pages of search results to identify any potentially relevant articles. This review examined the effectiveness of protein supplementation, compared protein supplement with control, and provided quantitative measurements of muscle strength, body composition, or frailty. Randomised or quasi-randomised controlled trials that included adults with sarcopenic obesity were used in this review. Protein-supplemented or control groups which co-ingested other potentially anabolic agents (e.g. testosterone, creatine) were not considered. The search plan followed the following search string and key search terms used in the search for articles are as listed in Table 1.
Study selection
The screening process consisted of two stages: (1) a title and abstract screening and; (2) full-text screening. Dietary supplementation of protein or amino acids from all sources was considered. Sarcopenic obesity was deemed eligible if the article clearly mentioned its definition. Articles were included based on predefined PICOS (Participants, Intervention, Comparison, Outcomes, Study design) criteria. The description of the PICOS criteria used to define the research question is presented in Table 2.
Studies meeting the following criteria were included: (1) participants aged 55 years or above; (2) healthy participants with sarcopenia, defined with at least one of the following indicators: muscle mass loss, low muscle strength, or poor physical performance; (3) the intervention group was with protein or amino acid supplementation, and the comparison group was exercise alone or with placebo supplementation; (4) study design: RCTs; and (5) outcome: muscle strength, muscle mass, and physical performance.
Main reasons for exclusion of articles from the scoping review were: (1) undefined classification of sarcopenic obesity; (2) description study, observation study, animal study; (3) clinical research with patient populations diagnosed with chronic and acute disorders or receiving treatments that may independently lead to catabolic changes in protein turnover with negative effects on skeletal muscle mass/function.
Two investigators independently read the full texts of the articles that were not excluded at the initial stage, then selected the studies that met the inclusion criteria. Any disagreements in article selection were resolved through discussion and consensus.
Charting the data
Data charting was primarily completed by two independent reviewers (K.J. and L.J.) using a pre-established template. Data were extracted from this review based on the following categories: (a) study characteristics, (b) methodological characteristics, (c) intervention strategies, and (d) targeted outcomes.
Collating, summarizing, and reporting the results
A thematic narrative synthesis of included articles summarising the effectiveness of each intervention strategy on sarcopenic obesity was carefully extracted from each included article.
Ethics
Ethical approval was not required from the Medical Research and Ethics Committee as data were collected from existing publications (i.e., secondary data) and no humans were directly contacted.
Results
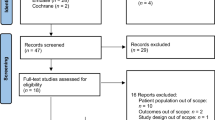
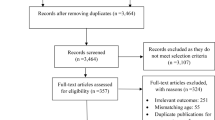
A total of 1,811 records were identified through the electronic searches. Of these, 97 were deemed eligible and were assessed for study abstract. Articles were removed because they were case studies or did not include a sarcopenic obesity sample (n = 58), sarcopenic obesity was not the primary reported results of an intervention (n = 18), the articles were reviews or study design did not fit inclusion criteria (n = 10), and wrong intervention setting (n = 7). Additional one article was added from a hand searched method. Thus, a total of 7 trials were analysed in this scoping review. A flow diagram of the study selection procedure is shown in Fig. 1.
Characteristics of included articles
Among the 7 included studies, none of the studies recruited both sexes, three studies recruited male subjects [36, 39, 40] while four studies recruited female as subjects [37, 38, 41, 42]. The sample size ranged from 16 to 139 subjects. Four out of six studies assessed the effectiveness of protein supplementation with combination of exercise training [36,37,38,39, 41]. One study evaluated the whole-body electromyostimulation and protein supplementation [40], while another study prescribed low calorie diet with protein supplementation to determine its effects on sarcopenic obesity [42]. Of these, six studies were randomised controlled trials [36,37,38,39,40,41] and one was a pre-post study design [42]. The majority (26%) of the studies was reported from Italy [38, 42] and German [39, 40], one from Canada [36], one from Japan [37], and one from Brazil [41]. The characteristics of the included studies have been summarized in Table 3.
Definition of sarcopenic obesity
In view of the definition of sarcopenia, different studies used different sarcopenia diagnoses. Skeletal muscle mass (Appendicular skeletal mass, ASM), lean body mass, ideal fat free mass, and skeletal muscle mass index were commonly used in most studies to determine sarcopenic condition [36,37,38, 41]. Three studies mentioned the used of the EWGSOP (European Working Group on Sarcopenia in Older People) as diagnostic criteria for sarcopenia in older adults [39, 40, 42]. In terms of obesity, the body fat percentage used was ranged between 27 and 38% [37,38,39,40,41,42]. Only one study that used body mass index (BMI) > 30 kg/m2 to determine the weight status of the subjects [36].
Types of protein supplementation & protein intake
These studies included interventions of protein supplementation (Leucine enriched essential amino acid, EAA; whey protein) [36,37,38,39,40,41,42]. In one study, participants underwent a 3-month intervention involving the combination of catechin and a protein supplement [37]. The protein intake prescription by these studies ranged from 1.0 to 1.8 g/kg/BW/day for intervention group, for control group the protein intake was typically between 0.8 and 1.0 g/kg/BW/day [36, 38,39,40,41,42].
Effects of different interventions on sarcopenic obesity
The effectiveness of the intervention in older adults was assessed through various sarcopenia evaluations. The most used sarcopenia measurement was lean muscle mass [36, 38] muscle strength [38, 42] handgrip strength [39, 42]. Measurement of body fat mass was seen in majority of the studies [36, 37, 39, 42]. In addition, body weight [36, 38], body trunk fat [36, 40, 42], and waist circumference [40, 42], were also measured to determine the effects on obesity.
The included intervention studies have consistently shown that exercise combined with protein supplement interventions, can lead to significant improvements in sarcopenic conditions such as muscle mass, strength, and physical function. Two studies utilised resistance training exercise in the intervention [36, 41], while one study combined both resistance exercise and aerobic exercise in the intervention [37]. The duration of exercise performed ranged from 2 to 3 times per week, with each session lasting 1 h [36, 37, 41]. However, one article did not report the duration of the exercise [36]. The exercise training also demonstrated a significant weight loss, loss of fat mass or trunk mass while preserving the lean muscle mass [36, 37, 41]. The results are the same as the intervention studies that utilised electromyostimulation (EMS) as an alternative to exercise [39]. Protein supplementation alone also showed improvement in sarcopenia measurements [38, 39, 42] as well as decrease in fat mass [36, 37], weight status [28], and waist circumference [42].
Effects of interventions on metabolic and inflammatory biomarkers
Five studies examined the effects of intervention on metabolic and inflammatory biomarkers such as total cholesterol (CHOL), triglycerides (TG), low density lipoprotein, high density lipoprotein (HDL), C-reactive protein (CRP), and Interleukin-6 (IL-6) on the subjects [36, 37, 40,41,42]. Two studies reported no significant changes in cardiometabolic parameters and inflammatory biomarkers throughout the intervention period [36, 37, 42]. However, one study showed that EMS intervention improved the HDL and IL-6 level [40]. Another study showed that resistance training exercise increased the HDL level while the level of fasting glucose, TG, and CRP were decreased [41].
Side effects of protein supplementation
No side effects have been reported in the clinical trials of included articles [36,37,38,39,40,41]. However, one study reported that there was some but not significant adverse effects on the subjects’ BUN, serum creatinine and eGFR with daily protein intake of 1.38 g/kg for 45 days [42].
Discussion
The objective of this scoping review was to summarise the protein supplementation and exercise interventions that have demonstrated positive effectiveness in treating sarcopenic obesity (SO) among older adults. The results showed that protein supplementation alone (1.5 months to 4 months) improved the body weight, waist circumference, muscle strength and muscle mass [36,37,38,39,40,41,42]. Interventions that combined exercise and protein supplementation intervention demonstrated additional benefits, including improved inflammation markers, blood lipid profiles, fasting glucose levels, and greater impact on weight loss while preserving lean muscle mass in the sarcopenic obesity population [41]. By incorporating resistance exercise, which promotes muscle growth and strength [43], with whey protein supplementation, providing essential amino acids for muscle protein synthesis [28, 44], SO individuals have the potential to enhance muscle mass and function while managing their weight.
Numerous studies have examined the effects of whey and leucine protein supplementation as a nutritional intervention for sarcopenic obesity [37, 39,40,41,42]. Whey protein is a high-quality protein source that contains all the essential amino acids needed to support muscle growth and repair. Leucine, a branched-chain amino acid found in whey protein, plays a critical role in stimulating muscle protein synthesis. In combination, systematic review study has reported association between these protein supplementations and sarcopenia [45, 46]. Our study revealed whey and leucine supplementation (L-EAA) can increase muscle mass, improve muscle strength and function, and reduce body fat in individuals with sarcopenic obesity [37, 39,40,41,42]. On the other hand, protein supplement has been found to have a positive impact on metabolic health markers in combination with exercise training [36]. Unfortunately, only a small number of research have examined its impact on biomarkers in sarcopenic obesity-affected older adults.
The results of this scoping review are consistent with recent systematic reviews in older adults (50–70 years old) with sarcopenic obesity which have found that combining exercise with nutritional interventions provide advantages in reducing fat mass [33]. Most of the included studies were complimented with exercise intervention, this implies the importance of exercise training for sarcopenic obesity. Indeed, in line with previous studies, exercise interventions have demonstrated the ability to improve muscle mass, muscle strength, physical performance, and reduce fat mass [29, 32]. Resistance exercise can stimulate muscle hypertrophy due to training stimulus and lead to improvement in muscle strength and physical performance [47]. Resistance training exercise has been recognised as the primary treatment for sarcopenia in older adults, with a recommended frequency of two exercise sessions per week (1–3 sets of 6–12 repetitions) [48]. The recommended exercise prescription is largely consistent with our findings and previous research [33]. However, there is variation in the design of the exercise interventions, particularly in terms of the required sets, and the specific body parts targeted. [36, 37, 41], and no specific guidelines have been provided in this regard. On the other hand, combining resistance training with aerobic exercise may have potential benefits for sarcopenic obesity [37]. This approach has demonstrated improvements in ectopic fat deposition as well as physical and metabolic function among older adults with obesity [49].
In this review, we noticed some studies utilised the whole-body electromyostimulation (WB-EMS) as an alternative to exercise, [39, 41] which has shown to possibly improve muscle mass and strength among non-athletic adults [50]. Due to the limited number of studies that have utilised WB-EMS as an intervention, drawing conclusive findings based on the available evidence is challenging. Nonetheless, our results demonstrated that exercise interventions, particularly resistance exercise performed 2–3 times per week for 60 min per session, resulted in weight loss, reduction in body fat, trunk fat, and waist circumference. Considering the condition of obesity, further research is necessary to assess the exercise design, duration and types of exercise (i.e. incorporating aerobic exercise) necessary to improve the sarcopenic obesity as well as the efficacy of WB-EMS among sarcopenic obesity population.
This scoping review identified that three studies used the European Working Group on Sarcopenia in Older People (EWGSOP) for the definition of sarcopenia [39, 40, 42]. The EWGSOP recommends the use of a combination of different methods to diagnose sarcopenia based on the presence or absence of low muscle mass, low muscle strength, and/or low physical performance [51]. The other studies [36,37,38, 41] considered lean mass as diagnostic criteria for sarcopenia. This is in line with diagnostic criteria proposed by the European Society for Clinical Nutrition and Metabolism [52] and Society of Sarcopenia, Cachexia and Wasting Disorders [53] which considered lean muscle mass and gait speed are important predictors of mortality and physical disability in people with sarcopenia.
For obesity, different ranges of body fat percentage (27–38%) were used [37,38,39,40,41,42] except for one study [36] which used BMI as diagnostic criteria. The percentage of body fat index (PBF) has been considered as a more accurate standard than BMI to determine being overweight or obese because it measures body fat directly and BMI does not always reflect the true body fat in our body [54]. This distinction is particularly significant for sarcopenic elderly individuals, who often exhibit low muscle mass and high body fat while maintaining a seemingly normal BMI [13]. Consequently, it is crucial to utilize PBF (fat mass/total mass × 100) whenever possible when assessing the obesity status of sarcopenic older adults. Thus, by incorporating PBF measurements, healthcare professionals can obtain a more precise understanding of body composition and better identify and address obesity in this population.
The consensus guideline has recommended that for healthy older adults the protein intake should be at least 1.0 to 1.2 g of protein per kilogram of body weight (BW) per day [55, 56]. More protein is required (1.2–1.5 g/kg BW/d) for those who have acute or chronic diseases as suggested by PROT-AGE Study Group [55] and The Society of Sarcopenia, Cachexia and Wasting Disorders, which recommends the intake of 1.0 to 1.5 g/kg BW/day for older adults to maintain muscle mass [57]. This study showed the overall protein intake for intervention groups ranged between 1.0 and 1.8 g/kg BW/day which is slightly higher than 1.5 g/kg BW/day as recommended. One of the included articles reported some adverse effects on subjects’ renal profile with the daily protein intake of 1.38 g/kg BW during the intervention period [7]. Although the adverse effects of high protein intake did not appear to be significant, it is important to be cautious about recommending protein intake levels above 1.4 g per kilogram of body weight in older adults with sarcopenic obesity. In addition to protein, the role of micronutrients is also crucial for individuals with sarcopenic obesity. Insufficient intake of specific micronutrients, such as magnesium, selenium, calcium [58], vitamin B complex, vitamin D, and, iron [59] have been associated with the development of sarcopenia.
This scoping review has some limitations. The main shortcoming of this review was including studies that used different definitions for sarcopenic obesity which may reduce the comparability of results. However, the lack of a consensus on diagnostic criteria for sarcopenic obesity is an unavoidable challenge that needs to be addressed. Second, six of the seven included studies were conducted in Western countries, where body composition may differ from that of Asians and Caucasians. Therefore, the generalisability of the results might be limited. Third, the intervention periods of the included studies may be short to be representative of long-term effects. Studies on the role of protein supplements and exercise are of great public health importance and should be a priority.
Conclusion
This scoping review provides an overview of nutritional and exercise approaches for managing sarcopenic obesity. Studies have demonstrated the effectiveness of exercise, specifically resistance exercise, and protein supplementation, such as whey protein, in addressing sarcopenic obesity in older adults, who experience age-related muscle mass and strength loss. However, exercise training may provide additional benefits beyond those provided by protein alone. In particular, exercise has been shown to have a positive impact on inflammatory markers, body weight, body fat trunk, and waist circumference. Therefore, a combination of resistance exercise (2–3 times/week) and protein supplementation may be the most effective approach for improving sarcopenic obesity and promoting healthy ageing. We suggest that a moderately high protein intake (1-1.3 g/kg BW/day) that take protein supplementation into account will be able to preserve muscle mass in individuals with sarcopenic obesity. Intake of more than (1.4 g/kg BW/day) should be prescribed with caution. Further research is needed to determine the optimal exercise and whether aerobic exercise should be incorporated for individuals with sarcopenic obesity.
Data availability
Not applicable.
References
United Nations. Population ageing and sustainable development. 2017. https://www.un.org/en/development/desa/population/publications/pdf/popfacts/PopFacts_2017-1.pdf. Accessed 15 March 2023.
World Health Organization (WHO). Ageing and health. 2022. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed 15 March 2023.
Sudharsanan N, Bloom DE. The demography of aging in low- and middle-income countries: chronological versus functional perspectives. https://www.ncbi.nlm.nih.gov/books/NBK513069/. Accessed 15 March 2023.
Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5:355. https://doi.org/10.3389/fpubh.2017.00335.
Papadopoulou SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. 2020;12(5):1293. https://doi.org/10.3390/nu12051293.
Chew STH, Tey SL, Yalawar M, Liu Z, Baggs G, How CH, et al. Prevalence and associated factors of sarcopenia in community-dwelling older adults at risk of malnutrition. BMC Geriatr. 2022;22(1):997. https://doi.org/10.1186/s12877-022-03704-1.
Sri-on J, Fusakul Y, Kredarunsooksree T, Paksopis T, Ruangsiri R. The prevalence and risk factors of sarcopenia among thai community-dwelling older adults as defined by the asian working group for sarcopenia (awgs-2019) criteria: a cross-sectional study. BMC Geriatr. 2022;22(1):786. https://doi.org/10.1186/s12877-022-03471-z.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37. https://doi.org/10.1038/s41574-018-0062-9.
Purcell SA, Mackenzie M, Barbosa-Silva TG, Dionne IJ, Ghosh S, Siervo M, et al. Prevalence of sarcopenic obesity using different definitions and the relationship with strength and physical performance in the canadian longitudinal study of aging. Front Physiol. 2021;11:583825. https://doi.org/10.3389/fphys.2020.583825.
Pang BWJ, Wee S-L, Lau LK, Jabbar KA, Seah WT, Ng DHM, et al. Prevalence and associated factors of sarcopenia in singaporean adults—the yishun study. J Am Med Dir Assoc. 2021;22(4):885.e1-.e10 https://doi.org/10.1016/j.jamda.2020.05.029.
Ranee R, Suzana S, You YY, Devinder KAS, Sakian NI. Prevalence and risk factors of sarcopenia among community dwelling older adults in klang valley. Malaysian J Med Health Sci. 2022;18(1):177–86.
Wagenaar CA, Dekker LH, Navis GJ. Prevalence of sarcopenic obesity and sarcopenic overweight in the general population: the lifelines cohort study. Clin Nutr. 2021;40(6):4422–9. https://doi.org/10.1016/j.clnu.2021.01.005.
Li CW, Yu KA-O, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13(2):781–94. https://doi.org/10.1002/jcsm.12901.
Su Y-C, Chang S-F, Tsai H-C. The relationship between sarcopenia and injury events: a systematic review and meta-analysis of 98,754 older adults. J Clin Med. 2022;11:6474. https://doi.org/10.3390/jcm11216474.
Yuenyongchaiwat K, Boonsinsukh R. Sarcopenia and its relationships with depression, cognition, and physical activity in thai community-dwelling older adults. Curr Gerontol Geriatr Res. 2020;2020:8041489. https://doi.org/10.1155/2020/8041489.
Kelley GA, Kelley KS. Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp. Gerontol. 2017;96:100–3. https://doi.org/10.1016/j.exger.2017.06.008.
Stenholm S, Harris Tb Fau - Rantanen T, Rantanen T, Fau - Visser M, Visser M, Fau - Kritchevsky SB, Kritchevsky Sb Fau -, Ferrucci L, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. https://doi.org/10.1097/MCO.0b013e328312c37d.
Hong S-h, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. 2020;21(2):494. https://doi.org/10.3390/ijms21020494.
Fruh SM, Obesity. Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(S1):3–14. https://doi.org/10.1002/2327-6924.12510.
Morgan PT, Smeuninx B, Breen L. Exploring the impact of obesity on skeletal muscle function in older age. Front nutr. 2020;7:569904. https://doi.org/10.3389/fnut.2020.569904.
Bellanti F, Romano AD, Lo Buglio A, Castriotta V, Guglielmi G, Greco A, et al. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas. 2018;109:6–12. https://doi.org/10.1016/j.maturitas.2017.12.002.
Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–7e2. https://doi.org/10.1016/j.jamda.2019.12.012.
Roh E, Choi KM. Health consequences of sarcopenic obesity: a narrative review. Front Endocrinol. 2020;11:332. https://doi.org/10.3389/fendo.2020.00332.
Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101(2):279–86. https://doi.org/10.3945/ajcn.114.090290.
Bauer JM, Mikušová L, Verlaan S, Bautmans I, Brandt K, Donini LM, et al. Safety and tolerability of 6-month supplementation with a vitamin D, calcium and leucine-enriched whey protein medical nutrition drink in sarcopenic older adults. Aging Clin Exp Res. 2020;32(8):1501–14. https://doi.org/10.1016/j.jamda.2013.05.021.
Jang YJ. The effects of protein and supplements on sarcopenia in human clinical studies: how older adults should consume protein and supplements. J Microbiol Biotechnol. 2023;33:143–50. https://doi.org/10.4014/jmb.2210.10014.
Kamińska MS, Rachubińska K, Grochans S, et al. The impact of whey protein supplementation on sarcopenia progression among the elderly: a systematic review and meta-analysis. Nutrients. 2023;15:2039. https://doi.org/10.3390/nu15092039.
Lee SY, Lee HJ, Lim J-Y. Effects of leucine-rich protein supplements in older adults with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Arch Gerontol Geriatr. 2022;102:104758. https://doi.org/10.1016/j.archger.2022.104758.
Hita-Contreras F, Bueno-Notivol J, Martínez-Amat A, Cruz-Díaz D, Hernandez AV, Pérez-López FR. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: a meta-analysis of randomized controlled trials. Maturitas. 2018;116:24–35. https://doi.org/10.1016/j.maturitas.2018.07.007.
Martone AM, Marzetti EA-O, Calvani R et al. Exercise and protein intake: a synergistic approach against sarcopenia. BioMed Res. Int. 2017; 2017: 2672435. https://doi.org/10.1155/2017/2672435.
Li L, He Y, Jin N, et al. Effects of protein supplementation and exercise on delaying sarcopenia in healthy older individuals in asian and non-asian countries: a systematic review and meta-analysis. Food Chem: X. 2022;13:100210. https://doi.org/10.1016/j.fochx.2022.100210.
Hsu K-J, Liao C-D, Tsai M-W, Chen C-N. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: a meta-analysis. Nutrients. 2019;11(9). https://doi.org/10.3390/nu11092163.
Eglseer D, Traxler M, Schoufour JD, Weijs PJM, Voortman T, Boirie Y, et al. Nutritional and exercise interventions in individuals with sarcopenic obesity around retirement age: a systematic review and meta-analysis. Nutr Rev. 2023;nuad007. https://doi.org/10.1093/nutrit/nuad007.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. https://doi.org/10.1080/1364557032000119616.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (prisma-scr): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850.
Maltais ML, Perreault KF, Courchesne-Loyer A, Lagacé JC, Barsalani R, et al. Effect of resistance training and various sources of protein supplementation on body fat mass and metabolic profile in sarcopenic overweight older adult men: a pilot study. Int J Sport Nutr Exerc Metab. 2016;26(1):71–7. https://doi.org/10.1123/ijsnem.2015-0160.
Kim H, Kim M, Kojima N, Fujino K, Hosoi E, Kobayashi H, et al. Exercise and nutritional supplementation on community-dwelling elderly japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc. 2016;17(11):1011–9. https://doi.org/10.1016/j.jamda.2016.06.016.
Sammarco R, Marra M, Di Guglielmo ML, Naccarato M, Contaldo F, et al. Evaluation of hypocaloric diet with protein supplementation in middle-aged sarcopenic obese women: a pilot study. Obes Facts. 2017;10(3):160–7. https://doi.org/10.1159/000468153.
Kemmler W, Weissenfels A, Teschler M, Willert S, Bebenek M, Shojaa M, et al. Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community-dwelling older men at risk: the randomized controlled FranSO study. Clin Interv Aging. 2017;12:1503–13. https://doi.org/10.2147/CIA.S137987.
Kemmler W, Kohl M, Freiberger E, Sieber C, von Stengel S. Effect of whole-body electromyostimulation and / or protein supplementation on obesity and cardiometabolic risk in older men with sarcopenic obesity: the randomized controlled FranSO trial. BMC Geriatr. 2018;18(1):70. https://doi.org/10.1186/s12877-018-0759-6.
Nabuco HCG, Tomeleri CM, Fernandes RR, Sugihara Junior P, Cavalcante EF, Cunha PM, et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: a randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN. 2019;32:88–95. https://doi.org/10.1016/j.clnesp.2019.04.007.
Camajani EA-O, Persichetti A, Watanabe MA-O, Contini S, Vari M, Di Bernardo S, et al. Whey protein, l-leucine and vitamin d supplementation for preserving lean mass during a low-calorie diet in sarcopenic obese women. Nutrients. 2022;14(9):1884. https://doi.org/10.3390/nu14091884.
Chen N, He X, Feng Y, et al. Effects of resistance training in healthy older people with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Eur Rev Aging Phys Act. 2021;18:23. https://doi.org/10.1186/s11556-021-00277-7.
Gilmartin S, O’Brien N, Giblin LA-O. Whey for sarcopenia: can whey peptides, hydrolysates or proteins play a beneficial role? Foods. 2020;9:750. https://doi.org/10.3390/foods9060750.
Guo Y, Fu X, Hu Q, Chen L, Zuo H. The effect of leucine supplementation on sarcopenia-related measures in older adults: a systematic review and meta-analysis of 17 randomized controlled trials. Front Nutr. 2022;9:929891. https://doi.org/10.3389/fnut.2022.929891.
Chang MA-O, Choo YA-O. Effects of whey protein, leucine, and vitamin d supplementation in patients with sarcopenia: a systematic review and meta-analysis. Nutrients. 2023;15(3):521. https://doi.org/10.3390/nu15030521.
Otsuka Y, Yamada Y, Maeda A, et al. Effects of resistance training intensity on muscle quantity/quality in middle-aged and older people: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2022;13:894–908. https://doi.org/10.1002/jcsm.12941.
Hurst C, Robinson SM, Witham MD, Dodds RM, Granic A, Buckland C, et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. 2022;51:afac003. https://doi.org/10.1093/ageing/afac003.
Waters DL, Aguirre L, Gurney B, Sinacore DR, Fowler K, Gregori G, et al. Effect of aerobic or resistance exercise, or both, on intermuscular and visceral fat and physical and metabolic function in older adults with obesity while dieting. J Gerontol A Biol Sci Med Sci. 2022;77(1):131–9. https://doi.org/10.1093/gerona/glab111.
Kemmler W, Shojaa M, Steele J, Berger J, Fröhlich M, Schoene D, et al. Efficacy of whole-body electromyostimulation (wb-ems) on body composition and muscle strength in non-athletic adults. A systematic review and meta-analysis. Front Physiol. 2021;26(12):640657. https://doi.org/10.3389/fphys.2021.640657.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, et al. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23. https://doi.org/10.1093/ageing/afq034.
Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) cachexia-anorexia in chronic wasting diseases and nutrition in geriatrics. Clin Nutr. 2010;29(2):154–9. https://doi.org/10.1016/j.clnu.2009.12.004.
Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12(6):403–9. https://doi.org/10.1016/j.jamda.2011.04.014.
Stojković M, Heinrich KM, Čvorović A, Jeknić V, Greco G, Kukić F. Accuracy of body mass index and obesity status in police trainees. Eur J Investig Health Psychol Educ. 2022;12(1):42–9. https://doi.org/10.3390/ejihpe12010004.
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14(8):542–59. https://doi.org/10.1016/j.jamda.2013.05.021.
Deutz NEP, Bauer JM, Barazzoni R, Biolo G, Boirie Y, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. 2014;33:929–36. https://doi.org/10.1016/j.clnu.2014.04.007.
Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391–6. https://doi.org/10.1016/j.jamda.2010.04.014.
van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. 2018;19:6–11e13. https://doi.org/10.1016/j.jamda.2017.05.026.
Bhattacharya S, Bhadra R, Schols A, van Helvoort A, Sambashivaiah S. Nutrition in the prevention and management of sarcopenia - a special focus on asian Indians. Osteoporos Sarcopenia. 2022;8:135–44. https://doi.org/10.1016/j.afos.2022.12.002.
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
KhangJin (K.J.), Cheah and Lin Jia (L.J.), Cheah were involved in the whole conception and design process of the study and contributed to the selection of literatures. K.J.-Wrote the manuscript text and L.J.- Synthesised the results and tables. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheah, K., Cheah, L. Benefits and side effects of protein supplementation and exercise in sarcopenic obesity: A scoping review. Nutr J 22, 52 (2023). https://doi.org/10.1186/s12937-023-00880-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-023-00880-7