Abstract
Background
The literature showed that Grape Products Containing Polyphenols (GPCP) had anti-oxidant activity. However, the effects of GPCP on different biomarkers of oxidative stress are still controversial. In this regard, this systematic review and meta-analysis aimed to evaluate the effect of Grape Products Containing Polyphenols (GPCP) intake on oxidative stress markers.
Methods
PubMed, Scopus, Web of Science, and Google Scholar data bases were searched up to August 20, 2020. A random-effects model, weighted mean difference (WMD), and 95% confidence interval (CI) were applied for data analysis. Meta-analysis was conducted over 17 eligible RCTs with a total of 633 participants. The study registration number is CRD42019116696.
Results
A significant increase was observed in Total Antioxidant Capacity (TAC) (weighted mean difference (WMD) = 1.524 mmol/L, 95% confidence interval (CI): 0.83, 2.21). Intake of GPCP enhanced Superoxide Dismutase (SOD) (WMD = 0.450 mmol/L, 95% CI: 0.23, 0.66), TAC (WMD = 2.829 mmol/L, 95% CI: 0.13, 5.52), and Oxygen Radical Absorbance Capacity (ORAC) (WMD = 0.524 μmol/L, 95% CI: 0.42, 0.62) among healthy participants. Higher GPCP doses increased SOD (WMD = 0.539 U/mgHb, 95% CI: 0.24, 0.82) and ORAC (WMD = 0.377 μmol/L, 95% CI: 0.08, 0.67), whereas longer intervention periods enhanced ORAC (WMD = 0.543 μmol/L, 95% CI: 0.43, 0.64).
Conclusion
GPCP intake may partly improve status of oxidative stress, but further well-designed trials are required to confirm these results.
Similar content being viewed by others
Introduction
Under normal physiological conditions, various enzymatic systems, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) act as antioxidants and protect the cells against free radical damage, including reactive oxygen species (ROS) [1, 2] Free radicals attack the main macromolecules and lead to cell damage and homeostasis. Increased number of free radicals reduce the detoxification capacity of tissues [3] and lead to oxidative stress. Oxidative stress occurs from the imbalance between production of ROS and protective effect of the antioxidant system, which is responsible for their neutralization and/or removal [4, 5]. Oxidative stress can be caused by biological endogenous factors [6, 7] or exogenous environmental factors [8]. High accumulation of free radicals overwhelms the antioxidant capacity of the body and leads to irreversible oxidative damage to nucleic acids, lipids, and proteins [9]. Oxidative damage lead to the cellular damage and cause changes in gene expression, cell proliferation, and apoptosis [9] Consequently, the aging process of the body is influenced and many chronic diseases develop, including cardiovascular disease, neural degeneration, cancer, and diabetes [2, 10]. Endogenous antioxidant defense system involves a network of antioxidant enzymes and non-enzymatic molecules in cytoplasm of organs [2]. Antioxidant enzymes, such as SOD, catalase, and glutathione reductase, transform ROS into more stable molecules and maintain oxidative equilibrium [2]. In addition, reduced glutathione (GSH) is a tripeptide made of glutamine, cysteine, and glycine with protective function against oxidative stress [4]. Malondialdehyde (MDA), as a byproduct of polyunsaturated fatty acids peroxidation [11], can be toxic, potentially mutagenic, and atherogenic due to its reaction with biomolecules such as protein and nucleic acid [12]. It is also a biomarker of oxidative stress [13]. Oxygen radical absorbance capacity (ORAC) has been widely used for measuring the antioxidant activity [14]. Considering the difficulty of measuring plasma antioxidant capacity of each sample and regarding the interactions among different compounds [15], the total antioxidant capacity (TAC) was evaluated [16]. Generally, increased oxidative stress decreases TAC [17].
Human trials showed protective effects of grape products containing polyphenols (GPCP) in disease which oxidative stress involved in them such as cardiovascular disease [18,19,20,21,22], type 2 diabetes [23, 24], metabolic syndrome components [25], dyslipidemia [26], neuro-degenerative [27], and some in-vitro studies investigating several cancers [28,29,30]. For instance, GPCP had anti-inflammatory effects in subjects with stable coronary artery disease [18] and diabetic patients [23], and as well as improving effects on insulin resistance and glycemic control in type 2 diabetic patients [24]. Moreover, GPCP decreased plasma lipids and oxidative stress in women [22] and markers of the metabolic syndrome in obese patients [25].The GPCP contain antioxidants in the form of polyphenols including phenolic acid (e.g. gallic acid), resveratrol, proanthocyanidin, and flavonoids (anthocyanins, flavonols, and quercetin) [31, 32]. These phenols are mostly present in grape crust, stems, leaves, and kernels in comparison with the juicy parts [33,34,35]. However, the results of randomized clinical trials (RCTs) over the effect of GPCP on oxidative stress markers are inconsistent.
Intake of 2 g/d grape polyphenol in overweight and obese participants with type 2 diabetes [24] also 0.5 g/d resveratrol (~ 500 mg polyphenol) supplementation [36] had no significant effect on antioxidant capacity. In addition, consuming 480 g/d grape juice (~ 945 mg polyphenol) [37] and 92 g/d grape powder consumption (~ 62.24 mg polyphenol) had no significant effect on ORAC levels [38]. While 90 g/d raisin (~ 178.75 mg polyphenol) increased ORAC levels [39]. Moreover, some researches indicated that intake of 500 g/d grape juice (~ 1066 mg polyphenol) [40] and GSE 0.15 g/d (~ 150 mg polyphenol) [41] or 0.6 g/d (~ 600 mg polyphenol) [42] significantly reduced the MDA levels. Although, intake of 90 g/d raisin (~ 178.75 mg polyphenol) had no significant effect on MDA levels [43]. SOD levels were significantly lower for subjects receiving 0.35 g/d whole grape extract (~ 350 mg polyphenol) compared to placebo group [1]. In the other study significant increasing and decreasing on SOD and TAC levels was observed respectively by intake of 0.2 g/d GSE (~ 200 mg polyphenol) [44]. Moreover, 0.1 g/d resveratrol supplementation (~ 100 mg polyphenol) could decrease on GPX level in healthy men [45]. Besides, intake of 0.6 g/d GSE (~ 600 mg polyphenol) [23] and 12 g/d grape powder (500 mg total polyphenol) [46] showed a significant increase on GSH.
Although several clinical trials investigated the effect of GPCP intake on oxidative stress markers, no conclusive result exists on this issue. Furthermore, no systematic review and meta-analysis has ever been conducted in this area. Therefore, this systematic review and meta-analysis aimed to investigate the effect of GPCP on oxidative stress.
Materials and methods
Search strategy
This systematic review and meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guidelines) [47].
The protocol of this study was also registered on PROSPERO, an International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO) with the registration no of CRD42019116696.
We searched PubMed, Scopus, Web of Science, and Scholar up to August 20, 2020 using Medical Subject Heading terms (MeSH) and non-MeSH terms to evaluate the effect of GPCP supplementation on oxidative stress biomarkers. The following keywords were used in the search:
(grape* OR “grape polyphenol” OR “grape seed extract” OR “grape seed” OR “Grape Seed Proanthocyanidins” OR raisin* OR polyphenol* OR “Vitis vinifera” OR raisin* OR “grape extract” OR wine* OR “grape polyphenols” OR “grape powder” OR “concord grape juice” OR “grape juice”) AND (“ oxidative stress “ OR “ Superoxide Dismutase” OR “ Superoxide Peroxidase “ OR “oxidative stress indices” OR “Glutathione Peroxidase” OR “ oxidative stress markers” OR “oxidative mediators” OR “oxidative biomarker” OR “F2-isoprostanes” OR “ isoprostanes “ OR malondialdehyde* OR MDA OR “Catalase” OR CAT OR “ total antioxidant capacity “ OR “ total antioxidant status” OR “total oxidant status “ OR TAS OR TOS OR Glutathione* OR TBARS OR “ Reduced Glutathione” OR “ Thiobarbituric Acid Reactive Substances “ OR ORAC OR “Oxygen Radical Absorbance Capacity “ OR TAC OR GPX OR SOD OR GSH) AND (trial* OR “randomized controlled trials” OR RCT OR “ Clinical Trials as Topic “ OR “clinical trials” OR “randomized controlled clinical trial” OR “randomized clinical trials” OR “controlled clinical trials” OR intervention OR Intervention OR randomized OR randomised OR random OR randomly OR placebo OR assignment OR cross-over OR parallel) NOT (animal* OR rat OR rats OR rabbit* OR rattus OR monkey* OR mice* OR mouse* OR hen* OR chicken* OR duck* OR pig* OR cow* OR “cell line” OR non-human OR “In-vitro” OR “In-vivo”). Furthermore, Reference lists of the related original and review articles were also carefully checked to obtain other eligible studies.
Selection criteria
The inclusion criteria for the studies were: 1) having an RCT design; 2) evaluating the effect of GPCP on oxidative stress biomarkers versus placebo or other intervention, such as water or usual wine; 3) reporting the dose of GPCP; 4) having participants with 18 years of age or older, and 5) being in English.
Study selection
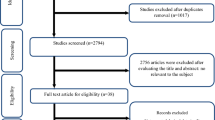
Two researchers separately performed the initial screening on the basis of the titles and abstracts of the articles. In the next step, the full texts of all related articles were investigated by reviewers to find studies over the effect of GPCP on oxidative stress. Eventually, any disagreements were discussed and resolved by consensus with third researcher (Fig. 1).
Data extraction
Two independent researchers summarized the data on the studies’ author’s family name, publication year, sample size and rate of sample loss, dose and type of intervention, study duration, cross-over or parallel study design, gender, age and healthy status of participants, as well as mean and SD of oxidative stress biomarkers levels at the baseline and end of trial. The collected information was double-checked by a third researcher.
Quality assessment
At this stage, two reviewers independently evaluated the methodological quality of the eligible studies through Cochrane Collaboration’s tool including six domains of: 1) random sequence generation (selection bias); 2) allocation concealment (selection bias); 3) blinding of participants and personnel (performance bias); 4) blinding of outcome assessment (detection bias); 5) incomplete outcome data (attrition bias); and 6) selective reporting (reporting bias). Each domain was classified to three categories: low risk of bias, high risk of bias and unclear risk of bias. According to the mentioned domains, the overall quality of each study was considered as good (low risk for more than 2 item), fair (low risk for 2 item), or weak (low risk for less than 2 item) [48].
Data synthesis and analysis
Statistical analyses were conducted using STATA software, version 11.2 (STATA Corp, College Station, TX). The random effects model which takes the between-study heterogeneity into account was used to calculate the weighted mean difference (WMD) and its 95% confidence intervals (CIs) [49]. To incorporate between-study variation, a random effects model was also applied to combine effect sizes. This model takes between-study heterogeneity into account. To evaluate heterogeneity among studies, I2 and Q statistic were used. If I2 > 50% and p-value of Q statistic < 0.05, statistically significant heterogeneity was recognized [50]. Subgroup analysis was conducted to explore the possible source of heterogeneity among the studies for all of oxidative biomarkers were addressed in our meta-analysis. Subgroup analysis included the following: duration, grape polyphenol doses, study design (parallel and crossover), study quality (weak, fair, good), and health status of study population (healthy individuals: people with no clinical condition versus unhealthy individuals: overweight; obese; chronic obstructive pulmonary disease; chronic kidney disease; coronary artery disease or ≥ 1 cardiac risk factor; type 2 diabetes).
Publication bias was assessed by examination of the funnel plot and formal testing for “funnel plot” asymmetry using Begg’s test and Egger’s test [51]. Sensitivity analysis was performed to identify whether a specific study or a particular group of studies affected the outcomes [51]. P values of less than 0.05 were considered significant.
Meta-regression
Meta-regression was conducted to evaluate the association of estimated effect size with dose and duration of the GPCP intake.
Results
Literature search
Our search in the databases of Google Scholar, PubMed, Web of Science, and Scopus resulted in 6670 articles. After removing the duplicate studies 4375 papers remained. Later after screening the included articles’ tittles and abstracts, 4332 other studies were excluded since they hadn’t RCT design (n = 3306), didn’t evaluate the effect of grape products as interventions (n = 983) and they were animal studies (n = 3), in Persian (n = 3). Review/meta-analysis studies (n = 37) with topics that appeared not to be related to our research question for example they addressed effects of dietary polyphenols [52,53,54,55,56,57,58,59], polyphenol-rich interventions [54, 60], antioxidant supplements [61], flavonoids [62, 63], resveratrol [64,65,66,67], alcoholic beverage [68,69,70,71,72,73,74,75,76,77], grape products [78,79,80,81,82,83,84,85,86,87], or fruit and vegetable juices consumption [88] which did not address our objects linking to oxidative stress biomarkers. However, reference lists of the related review articles were also carefully checked to obtain eligible studies. Later, full texts of the selected studies were reviewed and 26 papers were excluded since: they did not report the amount of oxidative biomarker’s levels [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104], unit [105], or the dose of GPCP [106], assessed the effect of grape product along with other fruits [107,108,109,110,111,112] or drug [113], the age of participants were 9–14 year old [41]. Finally, 17 studies were included in our systematic review and meta-analysis (Fig. 1).
Study characteristics
Characteristics of all studies that entered our systematic review and meta-analysis are indicated in Table 1. All studies were published from 2006 to 2018. The total number of included participants was 633 (intervention group: N = 376, control group: N = 355). The articles were conducted in Europe [43, 114,115,116,117,118], America [1, 37,38,39, 45, 119], and Asia [32, 40, 42, 44]. All studies had a randomized controlled trial with parallel [1, 32, 37, 40, 42,43,44,45] or cross over design [36, 38, 39, 114,115,116,117,118]. The trials lasted from 2 to 16 weeks and the dose of GPCP ranged from 0.1 g to 500 g. GPCPs were also administered in different forms such as [42, 44, 119], grape extract [1, 116], grape powder [38], juice [40], raisin [39, 43], resveratrol [36, 45], red wine [114, 115, 117, 118]. Considering the Cochrane risk of bias, 8, 2, and 7 articles had good, poor and fair quality, respectively (Table 2).
Effect of GPCP on SOD levels
According to the data meta-analysis, some studies [1, 32, 36, 44, 45, 115,116,117] showed that GPCP had no significant effect on SOD levels (WMD = 0.003 U/mgHb; 95% CI: − 0.002, 0.007; P = 0.29) (Fig. 2). This finding did not change after sensitivity analysis Supplementary Fig. 1), but a significant heterogeneity was observed among the studies (P < 0.001, I2 = 96.66). Although funnel plots showed publication bias for the related studies (Supplementary Fig. 2), asymmetry tests showed no publication bias (Begg’s test, P = 0.10 and Egger‘s test, P = 0.09). According to the subgroup analysis, intake of ≥400 mg/d grape polyphenol doses had a significant effect on SOD level (Dose < 400 mg/d: WMD < 0.001 U/mgHb; 95% CI: − 0.001, 0.001; P = 0.75; ≥ 400 mg/d: WMD = 0.539 U/mgHb; 95% CI: 0.24, 0.82; P < 0.001) (Table 3). The impact of supplementation with GPCP was significant only among the healthy participants (Healthy individuals: WMD = 0.450 U/mgHb; 95% CI: 0.23, 0.66; P < 0.001; Unhealthy participants: WMD < 0.001 U/mgHb; 95% CI: − 0.001, 0.001; P = 0.99) (Table 3). Furthermore, GPCP had no significant effect on SOD in supplementation durations of ≥10 weeks versus < 10 weeks (Duration < 10 wk.: WMD = 0.001 U/mgHb; 95% CI: − 0.006, 0.007; P = 0.86; Duration ≥10 wk.: WMD = 0.085 U/mgHb; 95% CI: − 0.01, 0.18; P = 0.09) (Table3). Cross-over studies (Cross-over: WMD = 0.539 U/mgHb; 95% CI: 0.24, 0.82; P < 0.001; Parallel: WMD < 0.001 U/mgHb; 95% CI: − 0.001, 0.001; P = 0.75) (Table 3) and poor quality studies indicated a significant effect on SOD (Good quality: n = 4, WMD = 0.085 U/mgHb; 95% CI: − 0.01, 0.18; P = 0.08; Fair quality: n = 3, WMD < -0.001 U/mgHb; 95% CI: − 0.001, 0.001; P = 0.75; Weak quality: n = 1, WMD = 2.399 U/mgHb; 95% CI: 2.01, 2.78; P < 0.001) (Table 3). Meta-regression analysis also showed a significant association between the administered dose of GPCP and SOD concentrations (slope = 0.00001; 95% CI: < 0.00001, 0.00002; P = 0.034), while the GPCP dosage had no significant relationship with the supplementation duration (slope = 0.00002; 95% CI: − 0.00031, 0.00035; P = 0.924) (Supplementary Figs. 3 A, B).
Effect of GPCP on TAC levels
Meta-analysis of six RCTs [1, 40, 42, 114, 118, 119] showed that GPCP supplementation increased the TAC levels significantly (WMD = 1.524 mmol/L; 95% CI: 0.83, 2.21; P < 0.001) (Fig. 3). This result remained significant in sensitivity analysis) supplementary Fig. 4). A significant heterogeneity was observed among the studies (P < 0.001, I2 = 99.57). No publication bias was determined based on the Funnel plot and symmetry tests (Begg’s test, p = 0.70; Egger‘s test, P = 0.28) among the related studies (Supplementary Fig. 5). According to the subgroup analysis, duration of study (Duration ≤6 wk.: WMD = 0.084 mmol/L; 95% CI: − 0.36, 0.52; P = 0.71; Duration > 6 wk.: WMD = 3.814 mmol/L; 95% CI: − 4.14, 11.77; P = 0.34) (Table 3) and grape polyphenol dose (Dose ≤650 mg/d: WMD = 2.595 mmol/L; 95% CI: − 2.53, 7.72; P = 0.32; Dose > 650 mg/d: WMD = − 0.058 mmol/L; 95% CI: − 0.22, 0.11; P = 0.49) had no significant impact on TAC (Table 3). The GPCP supplementation significantly increased TAC only among the healthy participants (healthy subjects: WMD = 2.829 mmol/L; 95% CI: 0.13, 5.52; P = 0.04; unhealthy subjects: WMD = − 0.254 mmol/L; 95% CI: − 0.65, 0.14; P = 0.21) (Table 3). The cross-over type of study had a significant effect on TAC (Cross-over: WMD = 2.785 mmol/L; 95% CI: 0.01, 5.55; P = 0.04; Parallel: WMD = − 0.164 mmol/L; 95% CI: − 0.65, 0.33; P = 0.51) (Table 3). Moreover, poor and good quality studies had significant effects on TAC (Good quality: n = 3, WMD = − 0.145 mmol/L; 95% CI: − 1.18, − 0.10; P < 0.001; Fair quality: n = 2, WMD = 0.266 mmol/L; 95% CI: − 0.22, 0.75; P = 0.28; Weak quality: n = 1, WMD = 8.018 mmol/L; 95% CI: 7.54, 8.49; P < 0.001) (Table 3). The duration (slope = − 0.02; 95% CI: − 0.02, − 0.01; P < 0.00001) and dose of GPCP supplementation (slope = − 0.001; 95% CI: − 0.0014, − 0.0010; P < 0.00001) had a significant association with TAC in meta-regression analysis (Supplementary Figs. 6 A, B).
Effect of GPCP on ORAC levels
Meta-analysis of five RCTs [37,38,39, 116, 117] demonstrated no significant effect of GPCP on ORAC levels (WMD = 0.210 μmol/L; 95% CI: − 0.15, 0.57; P = 0.25) (Fig. 4). This result did not change after the sensitivity analysis) Supplementary Fig. 7). A significant heterogeneity was seen among the studies (P < 0.001, I2 = 96.02). No significant publication bias was found with regard to the related studies based on the funnel plots (Supplementary Fig. 8) and asymmetry tests (Begg’s test, P = 0.80 and Egger‘s test, P = 0.94). According to the subgroup analysis, intake of GPCP had a significant effect on healthy individuals (Healthy participants: WMD = 0.524 μmol/L; 95% CI: 0.42, 0.62; P < 0.001; Abnormal wt. subjects: WMD = − 0.03 μmol/L; 95% CI: − 0.46, 0.40; P = 0.89) (Table 3). Higher durations and grape polyphenol doses of GPCP supplementation had a significant impact on ORAC (Duration < 10 wk.: WMD = 0.089 μmol/L; 95% CI: − 0.26, 0.44; P = 0.62; Duration ≥10 wk.: WMD = 0.543 μmol/L; 95% CI: 0.43, 0.64; P < 0.001) (Dose ≤400 mg/d: WMD = 0.161 μmol/L; 95% CI: − 0.26, 0.58; P = 0.46; Dose > 400 mg/d: WMD = 0.377 μmol/L; 95% CI: 0.08, 0.67; P = 0.01) (Table 3). Supplementation with GPCP had no significant effect on ORAC in parallel and cross-over studies (Cross-over: WMD = 0.210 μmol/L; 95% CI: − 0.15, 0.57; P = 0.25; Parallel: WMD = − 140.0 μmol/L; 95% CI: − 651.9, 371.9; P = 0.59) (Table 3). Moreover, poor, fair, and good quality studies had a significant effect on ORAC (Good quality: n = 2, WMD = 0.365 μmol/L; 95% CI: 0.01, 0.71; P = 0.03; Fair quality: n = 2, WMD = − 0.251 μmol/L; 95% CI: − 0.36, − 0.13; P < 0.001; Weak quality: n = 1, WMD = 0.377 μmol/L; 95% CI: 0.08, 0.67; P = 0.01) (Table 3). The dose (slope = 0.0008; 95% CI: 0.0005, 0.0012; P < 0.0001), and duration of GPCP supplementation (slope = 0.069; 95% CI: 0.03, 0.10; P = 0.0002) (Supplementary Figs. 9 A, B) indicated a significant association with ORAC in meta-regression analysis.
Effect of GPCP on MDA levels
According to the meta-analysis of seven RCTs [32, 40, 42,43,44, 115, 119], GPCP had no significant impact on MDA levels (WMD = − 0.178 μmol/L; 95% CI: − 0.46, 0.11; P = 0.22) (Fig. 5). This finding did not change after the sensitivity analysis) Supplementary Fig. 10). A significant heterogeneity was observed among the studies (P = 0.002, I2 = 71.454). Funnel plots and asymmetry tests indicated no publication bias in the related studies (Begg’s test, P = 1.00 and Egger‘s test, P = 0.30) (Supplementary Fig. 11). The findings of subgroup analysis demonstrated no significant effect of GPCP on MDA in healthy and unhealthy participants (Healthy subjects: WMD = − 0.214 μmol/L; 95% CI: − 0.62, 0.19; P = 0.30; Unhealthy participants: WMD = − 0.092 μmol/L; 95% CI: − 0.50, 0.32; P = 0.66) (Table 3). Moreover, GPCP impact was not significant with regard to different supplementation durations (Duration < 8 wk.: WMD = 0.096 μmol/L; 95% CI: − 1.14, 1.33; P = 0.88; Duration ≥8 wk.: WMD = − 0.149 μmol/L; 95% CI: − 0.47, 0.17; P = 0.36) and grape polyphenol doses (Dose < 600 mg/d: WMD = − 0.034 μmol/L; 95% CI: − 0.88, 0.81; P = 0.93; Dose ≥600 mg/d: WMD = − 0.237 μmol/L; 95% CI: − 0.58, 0.11; P = 0.18) (Table 3). Parallel and cross-over types of study (Cross-over: WMD = 0.003 μmol/L; 95% CI: − 0.05, 0.05; P = 0.90; Parallel: WMD = − 0.328 μmol/L; 95% CI: − 0.79, 0.14; P = 0.17) (Table 3) as well as Quality of studies (Good quality: n = 4, WMD = − 0.072 μmol/L; 95% CI: − 0.55, 0.40; P = 0.77; Fair quality: n = 3, WMD = − 0.313 μmol/L; 95% CI: − 0.67, 0.05; P = 0.09) (Table 3) had no significant effect on MDA. Meta-regression analysis showed that MDA levels had a significant association with GPCP supplementation duration (slope = 0.05; 95% CI: 0.022, 0.094; P = 0.001), but this relationship was not significant with the GPCP supplementation dose (slope = − 0.0003; 95% CI: − 0.0008, 0.00001; P = 0.17) (Supplementary Figs. 12 A, B).
Effect of GPCP on GPX levels
Meta-analysis of five RCTs [36, 44, 45, 115, 116] showed no significant effect of GPCP on GPX levels (WMD = 0.026 U/mgHb; 95% CI: − 0.002, 0.05; P = 0.07) (Fig. 6). This finding remained unchanged after the sensitivity analysis (Supplementary Fig. 13). A significant heterogeneity was observed among the studies (P < 0.0001, I2 = 88.29). Although the funnel plots indicated a publication bias in the studied surveys (Supplementary Fig. 14), asymmetry tests did not confirm this result (Begg’s test, P = 0.08 and Egger‘s test, P = 0.33). According to the subgroup analysis, GPCP had no significant effect among healthy and unhealthy participants (Healthy participants: WMD = 0.044 U/mgHb; 95% CI: − 0.03, 0.12; P = 0.30; Unhealthy participants: WMD = 0.009 U/mgHb; 95% CI: − 0.02, 0.04; P = 0.60) (Table 3). Similarly, GPCP had no significant impact on GPX in various supplementation durations (Duration ≤10 wk.: WMD = 0.068 U/mgHb; 95% CI: − 0.07, 0.20; P = 0.33; Duration > 10 wk.: WMD = − 0.002 U/mgHb; 95% CI: − 0.01, 0.01; P = 0.83) and grape polyphenol doses (Dose < 400 mg/d: WMD < 0.001 U/mgHb; 95% CI: − 0.001, 0.001; P = 0.80; Dose ≥400 mg/d: WMD = 0.063 U/mgHb; 95% CI: − 0.02, 0.15; P = 0.16) (Table 3). Parallel and cross-over types of study (Cross-over: WMD = 0.063 U/mgHb; 95% CI: − 0.02, 0.15; P = 0.16; Parallel: WMD < 0.001 U/mgHb; 95% CI: − 0.001, 0.001; P = 0.80) (Table 3) as well as quality of studies (Good quality: n = 3, WMD = 0.063 U/mgHb; 95% CI: − 0.02, 0.15; P = 0.16; Fair quality: n = 2, WMD < 0.001 U/mgHb; 95% CI: − 0.001, 0.001; P = 0.80) had no significant effect on the GPX level (Table 3). The trial duration (slope = 0.0009; 95% CI: − 0.002, 0.004; P = 0.58) and GPCP dose (slope = 0.00007; 95% CI: 0.00001, 0.00013; P = 0.027) (Supplementary Figs. 15 A, B) showed no significant relationship with the effect of GPCP supplementation on GPX in meta-regression analysis.
Discussion
Findings showed that GPCP intake had a significant effect on increasing the TAC levels; however, it had no significant impact on other oxidative stress biomarkers. In the sub-group analysis, GPCP significantly increased SOD, TAC, and ORAC levels in healthy participants. Furthermore, higher GPCP doses increased ORAC and SOD levels significantly. Longer intervention periods also increased the ORAC levels. Finally, cross-over study design enhanced the TAC and SOD levels.
To the best of our knowledge, this systematic review and meta-analysis is the first study over the effect of supplementation with GPCP on oxidative stress. In the same line with our findings, other systematic reviews and meta-analyses confirmed the beneficial effects of GPCP on the obesity-induced chronic inflammation [120], lipid profile [121], blood glucose [87], systolic blood pressure, heart rate [57, 122], endothelial function [56], liver and heart functions [87], metabolic syndrome, and type 2 diabetes [120]. In addition, the significant impact of GPCP was reported on oxidative stress in patients with Type 2 diabetes [23, 44], pre hypertension [1, 123], overweight [39], acute lymphoblastic leukemia [41], CVD [100, 124], systemic sclerosis [96], Chronic Obstructive Pulmonary Disease [32], hemodialysis patient [46, 125, 126], hyperlipidemia [127], smoker [128], and healthy subjects [21, 26, 40, 42, 90, 92, 94, 101, 102, 105, 114, 115, 118]. In contrast with our results, some RCTs [36, 38, 43, 95, 104, 106, 116, 119, 129] reported no significant effect of GPCP on oxidative stress. The doses and duration of supplementation with GPCP [36], co-administration of minerals and vitamins [36], polyphenols consumption from foods [117], physical activity [130, 131], and age of participants [132] adipose tissue [91], baseline oxidative stress levels [36], and other individual could affect the levels of oxidative stress.
The most notable bioactivity of GPCP is their antioxidant properties [133]. Grape products containing polyphenols use various mechanisms such as sweeping reactive oxygen and nitrogen species [134, 135], chelating metals and minerals, modulating transcription factors, inhibiting and inducing pro-oxidant and antioxidant enzymes, and exerting synergistic effects on other antioxidants to exert their antioxidant activities [136, 137].
In fact, nuclear factor-erythroid-2-related factor-2 (Nrf2) signaling pathway, as an essential pathway for protection against oxidative stress, regulates the expression of many intracellular antioxidant genes [138]. Thus, this pathway is one of the most important therapeutic targets for the prevention and treatment of oxidative stress and its related diseases [139]. The GPCP including grape seed extract proanthocyanidin induce and activate Nrf2 [46, 138]. Grape products containing polyphenols also inhibit lipid peroxidase by increasing paraoxonase activity plasma [140], which consequently inhibits LDL oxidation [141].
Our subgroup analyses showed a significant increase in the ORAC levels in longer-term studies; this finding is confirmed by other studies [32, 44, 119]. Meta-regression analysis also indicated a significant relationship between duration of GPCP consumption and TAC, ORAC, and MDA levels. The literature indicated that supplementation duration affected the efficiency of the dietary polyphenols. Furthermore, the supplementation duration should be sufficiently long to influence the oxidative stress markers. In order to demonstrate the dose-response relationship, long-term studies are needed to exert significant effects on the antioxidant biomarkers [31, 32, 142]. The impact of grape seed extract on blood pressure was also more pronounced in longer study [83].
Our results indicated a significant elevation in SOD and ORAC levels in higher doses of grape polyphenols. The meta-regression showed that the changes in SOD, TAC, ORAC, and GPX levels were dependent on grape polyphenols supplementation dose. Similar to our results, a study indicated that different GPCP supplementation doses had different effects. For example, the flow-mediated dilation (FMD) improved better at a higher dose of red grape powder, while the diastolic blood pressure decreased better at a lower dosage of this product [123]. Contrary to our results, a meta-analysis showed that systolic and diastolic blood pressure decreased significantly at lower doses of GSE [83]. Moreover, 25 and 50% (v / v) concentrations of GPCP showed similar antioxidant activity. These concentrations were better than the 10% (v / v) concentration. These results show that the antioxidant capacity of the phenols is probably in the saturated concentration range and does not increase with higher concentrations [143].
The significant increase in SOD levels in studies with cross over designs might be due to the fact that these trials (range: 0.4–300 g, mean: 150.22 g) had significantly higher GPCP doses than the parallel trials (range: 0.1–0.35 g, mean: 0.8 g).
Subgroup analysis also indicated a significant effect of GPCP on TAC, SOD, and ORAC in healthy individuals. Similar to our results, other studies showed that the antioxidant capacity of the studied population may vary with their health status [1]. A meta-analysis found that the effect of grape seed extract on blood pressure depended on the individuals’ initial blood pressure level, although the confounding effect of the patient’s medications should be considered in unhealthy individuals [83]. Another meta-analysis reported that the elevated levels of FMD were different between the individuals with cardiovascular risk factors and the healthy participants [56]. In addition, the dose-response mechanism of the grape products may vary based on the individuals’ health status [144]. According to a previous study, supplementation with GPCP had different effects on smokers since they need higher levels of antioxidants; consequently higher doses of GPCP are required for clearer results [43]. In health status, a balance exists between production of free radicals and the antioxidant defense system that prevents the disease. In disease conditions, the balance shifts towards producing free radicals and increasing oxidative stress [145]; consequently, unhealthy people are expected to have higher levels of oxidative stress [1] and require higher doses of GPCP to improve antioxidant macros.
The present research has some strength. This is the first study over the effect of GPCP on oxidative stress. Subgroup analyses were also conducted on the study type, duration, and quality, the products’ dosage, and the participants’ health status. However, this meta-analysis had several limitations. Few oxidative biomarkers evaluated in most RCTs also, oxidative markers had rapid mechanism of oxidation, future studies should evaluate all of related biomarkers especially TAC, therefore, the results will be more accurate. The RCTs included in the present study had limited follow-up periods. Moreover, the investigated articles were heterogeneous considering their populations’ characteristics as well as the administered type and doses of GPCP. So, further clinical trials are needed over the effect of grape polyphenol on the oxidative biomarkers as primary outcome using different doses and type of GPCP. Polyphenol contents in grape products are varied widely because many factors influence their contents, such as grape cultivars, season, processing, storage condition, and duration. Future researchers are suggested to report the amount of grape polyphenol in their test products and serum levels of polyphenols in participants. Since most studies did not consider the effects of confounders, including lifestyle, diet, physical activity, smoking, health/disease, age and medications, we were unable to evaluate these effects.
Conclusions
In conclusion, the results of this study demonstrated that supplementation with GPCP had a significant effect on increasing the TAC levels, but it had no effect on other oxidative stress biomarkers. The effect of GPCP on SOD, GPX, ORAC, and TAC levels depended on the administered dosage. In the same regard, the supplementation duration affected MDA, TAC, and ORAC levels. However, further well-designed RCTs with larger sample sizes and longer-durations are required in this area.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- FMD:
-
Flow-mediated dilation
- GPCP:
-
Grape Products Containing Polyphenols
- GPX:
-
Glutathione Peroxidase
- GSE:
-
Grape Seed Extract
- GSH:
-
Reduced Glutathione
- MDA:
-
Malondialdehyde
- ORAC:
-
Oxygen Radical Absorbance Capacity
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines
- RCT:
-
Randomized Clinical Trial
- SOD:
-
Superoxide Dismutase
- TAC:
-
Total Antioxidant Capacity
- USA:
-
United States of America
- WMD:
-
Weighted Mean Difference
References
Evans M, Wilson D, Guthrie N. A randomized, double-blind, placebo-controlled, pilot study to evaluate the effect of whole grape extract on antioxidant status and lipid profile. J Funct Foods. 2014;7:680–91.
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:1–19.
Trachootham D, Lu W, Ogasawara MA, Valle NR-D, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–74.
Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–90.
Sies H. Role of reactive oxygen species in biological processes. Klin Wochenschr. 1991;69:965–8.
Knutson MD. Non-transferrin-bound iron transporters. Free Radic Biol Med. 2019;133:101–11.
Stoian I, Oros A, Moldoveanu E. Apoptosis and free radicals. Biochem Mol Med. 1996;59:93–7.
Fu S, DAVIES MJ, STOCKER R, DEAN RT. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J. 1998;333:519–25.
Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–93.
Belli R, Amerio P, Brunetti L, Orlando G, Toto P, Proietto G, Vacca M, Tulli A. Elevated 8-isoprostane levels in basal cell carcinoma and in UVA irradiated skin. Int J Immunopathol Pharmacol. 2005;18:497–502.
Chen J, Zeng L, Xia T, Li S, Yan T, Wu S, Qiu G, Liu Z. Toward a biomarker of oxidative stress: a fluorescent probe for exogenous and endogenous malondialdehyde in living cells. Anal Chem. 2015;87:8052–6.
Riggins JN, Pratt DA, Voehler M, Daniels JS, Marnett LJ. Kinetics and mechanism of the general-acid-catalyzed ring-closure of the malondialdehyde− DNA adduct, N 2-(3-Oxo-1-propenyl) deoxyguanosine (N 2 OPdG-), to 3-(2 ‘-Deoxy-β-d-erythro-pentofuranosyl) pyrimido [1, 2-α] purin-10 (3 H)-one (M1dG). J Am Chem Soc. 2004;126:10571–81.
Khoubnasabjafari M, Ansarin K, Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts: BI. 2015;5:123.
Huang D, Ou B, Hampsch-Woodill M, Flanagan JA. Prior RL: high-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 2002;50:4437–44.
Cao G. Prior RL: comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309–15.
Nagy G, Ward J, Mosser DD, Koncz A, Gergely P, Stancato C, Qian Y, Fernandez D, Niland B, Grossman CE. Regulation of CD4 expression via recycling by HRES-1/RAB4 controls susceptibility to HIV infection. J Biol Chem. 2006;281:34574–91.
Woodford F, Whitehead T. Is measuring serum antioxidant capacity clinically useful? Ann Clin Biochem. 1998;35:48–56.
Albers AR, Varghese S, Vitseva O, Vita JA, Freedman JE. The antiinflammatory effects of purple grape juice consumption in subjects with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2004;24:e179–80.
Coimbra S, Lage S, Brandizzi L, Yoshida V, Da Luz P. The action of red wine and purple grape juice on vascular reactivity is independent of plasma lipids in hypercholesterolemic patients. Braz J Med Biol Res. 2005;38:1339–47.
Freedman JE, Parker Iii C, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–8.
O'Byrne DJ, Devaraj S, Grundy SM, Jialal I. Comparison of the antioxidant effects of Concord grape juice flavonoids α-tocopherol on markers of oxidative stress in healthy adults. Am J Clin Nutr. 2002;76:1367–74.
Zern TL, Wood RJ, Greene C, West KL, Liu Y, Aggarwal D, Shachter NS, Fernandez ML. Grape polyphenols exert a cardioprotective effect in pre-and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911–7.
Kar P, Laight D, Rooprai H, Shaw K, Cummings M. Effects of grape seed extract in type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabetic Med. 2009;26:526–31.
Hokayem M, Blond E, Vidal H, Lambert K, Meugnier E, Feillet-Coudray C, Coudray C, Pesenti S, Luyton C, Lambert-Porcheron S. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2013;36:1454–61.
Moreno-Indias I, Sánchez-Alcoholado L, Pérez-Martínez P, Andrés-Lacueva C, Cardona F, Tinahones F, Queipo-Ortuño MI. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016;7:1775–87.
Sano A, Uchida R, Saito M, Shioya N, Komori Y, Tho Y, Hashizume N. Beneficial effects of grape seed extract on malondialdehyde-modified LDL. J Nutr Sci Vitaminol. 2007;53:174–82.
Krikorian R, Boespflug EL, Fleck DE, Stein AL, Wightman JD, Shidler MD, Sadat-Hossieny S. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem. 2012;60:5736–42.
Akhtar S, Meeran SM, Katiyar N, Katiyar SK. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clin Cancer Res. 2009;15:821–31.
Hudson TS, Hartle DK, Hursting SD, Nunez NP, Wang TT, Young HA, Arany P, Green JE. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67:8396–405.
Singletary KW, Jung K-J, Giusti M. Anthocyanin-rich grape extract blocks breast cell DNA damage. J Med Food. 2007;10:244–51.
Kay CD. The future of flavonoid research. Br J Nutr. 2010;104:S91–5.
Lu M-C, Yang M-D, Li P-C, Fang H-Y, Huang H-Y, Chan Y-C, Bau D-T. Effect of oligomeric proanthocyanidin on the antioxidant status and lung function of patients with chronic obstructive pulmonary disease. in vivo. 2018;32:753–8.
Farhadi K, Esmaeilzadeh F, Hatami M, Forough M, Molaie R. Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chem. 2016;199:847–55.
Xia E-Q, Deng G-F, Guo Y-J, Li H-B. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11:622–46.
Yilmaz Y, Göksel Z, Erdoğan SS, Öztürk A, Atak A, Özer C. Antioxidant activity and phenolic content of seed, skin and pulp parts of 22 grape (V itis vinifera L.) cultivars (4 common and 18 registered or candidate for registration). J Food Processing Preservation. 2015;39:1682–91.
Saldanha JF, Leal VO, Rizzetto F, Grimmer GH, Ribeiro-Alves M, Daleprane JB, Carraro-Eduardo JC, Mafra D. Effects of resveratrol supplementation in Nrf2 and NF-κB expressions in nondialyzed chronic kidney disease patients: a randomized, double-blind, placebo-controlled, crossover clinical trial. J Ren Nutr. 2016;26:401–6.
Hollis JH, Houchins JA, Blumberg JB, Mattes RD. Effects of concord grape juice on appetite, diet, body weight, lipid profile, and antioxidant status of adults. J Am Coll Nutr. 2009;28:574–82.
Zunino SJ, Peerson JM, Freytag TL, Breksa AP, Bonnel EL, Woodhouse LR, Storms DH. Dietary grape powder increases IL-1β and IL-6 production by lipopolysaccharide-activated monocytes and reduces plasma concentrations of large LDL and large LDL-cholesterol particles in obese humans. Br J Nutr. 2014;112:369–80.
Rankin J, Andreae M, Oliver Chen CY, O’keefe S. Effect of raisin consumption on oxidative stress and inflammation in obesity. Diabetes Obes Metab. 2008;10:1086–96.
Amoutzopoulos B, Löker GB, Samur G, Çevikkalp SA, Yaman M, Köse T, Pelvan E. Effects of a traditional fermented grape-based drink ‘hardaliye’on antioxidant status of healthy adults: a randomized controlled clinical trial. J Sci Food Agric. 2013;93:3604–10.
El-Dakroory SA, El-Ashry R, Mesbah A, El-Farahaty RM. Doxorubicin Cardiotoxicity in acute lymphoblastic leukemia: possible protective role of grape seed extract Proanthocyanidins. J Advanc Medicine Med Res. 2014:2555–67.
Taghizadeh M, Malekian E, Memarzadeh MR, Mohammadi AA, Asemi Z. Grape seed extract supplementation and the effects on the biomarkers of oxidative stress and metabolic profiles in female volleyball players: a randomized, double-blind, placebo-controlled clinical trial. Iran Red Crescent Med J. 2016;18:1–9.
Kanellos PT, Kaliora AC, Protogerou AD, Tentolouris N, Perrea DN, Karathanos VT. The effect of raisins on biomarkers of endothelial function and oxidant damage; an open-label and randomized controlled intervention. Food Res Int. 2017;102:674–80.
Pourghassem-Gargari B, Abedini S, Babaei H, Aliasgarzadeh A, Pourabdollahi P. Effect of supplementation with grape seed (Vitis vinifera) extract on antioxidant status and lipid peroxidation in patient with type diabetes. J Medicinal Plants Res. 2011;5:2029–34.
Macedo R, Vieira A, Marin D, Otton R. Effects of chronic resveratrol supplementation in military firefighters undergo a physical fitness test–a placebo-controlled, double blind study. Chemico-biological interactions. 2015;227:89–95.
Janiques AGPR, Leal VO, Stockler-Pinto MB, Moreira NX, Mafra D. Effects of grape powder supplementation on inflammatory and antioxidant markers in hemodialysis patients: a randomized double-blind study. Brazilian J Nephrol. 2014;36:496–501.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60.
Hassimotto NMA, Moreira V, Nascimento NG, Souto PCMC, Teixeira C, Lajolo FM. Inhibition of carrageenan-induced acute inflammation in mice by oral administration of anthocyanin mixture from wild mulberry and cyanidin-3-glucoside. Biomed Res Int. 2013;2013:1–10.
Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17:573–86.
Castelli T, Russo GI, Reale G, Privitera S, Chisari M, Fragala E, Favilla V, Cimino S, Morgia G. Metabolic syndrome and prostatic disease: potentially role of polyphenols in preventive strategies. A review. International Braz J Urol. 2016;42:422–30.
Guasch-Ferré M, Merino J, Sun Q, Fitó M, Salas-Salvadó J. Dietary polyphenols, Mediterranean diet, Prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxidative Med Cell Longev. 2017;2017:1–16.
Chiva-Blanch G, Badimon L. Effects of polyphenol intake on metabolic syndrome: current evidences from human trials. Oxidative Med Cell Longev. 2017;2017:1–19.
Li SH, Tian HB, Zhao HJ, Chen LH, Cui LQ. The acute effects of grape polyphenols supplementation on endothelial function in adults: meta-analyses of controlled trials. PLoS One. 2013;8:1–10.
Li SH, Zhao P, Tian HB, Chen LH, Cui LQ. Effect of grape polyphenols on blood pressure: a meta-analysis of randomized controlled trials. PLoS One. 2015;10:1–12.
Li Y, Li S, Meng X, Gan R-Y, Zhang J-J, Li H-B. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9:728.
Santhakumar AB, Battino M, Alvarez-Suarez JM. Dietary polyphenols: structures, bioavailability and protective effects against atherosclerosis. Food Chem Toxicol. 2018;113:49–65.
Marx W, Kelly J, Marshall S, Nakos S, Campbell K, Itsiopoulos C. The effect of polyphenol-rich interventions on cardiovascular risk factors in Haemodialysis: a systematic review and meta-analysis. Nutrients. 2017;9:1–24.
Yasueda A, Urushima H, Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in Cancer treatment: a systematic review. Integrative Cancer Therapies. 2016;15:17–39.
Suen J, Thomas J, Kranz A, Vun S, Miller M. Effect of flavonoids on oxidative stress and inflammation in adults at risk of cardiovascular disease: a systematic review. Healthcare. 2016;4:1–23.
Sequeira IR, Poppitt SD. Unfolding novel mechanisms of polyphenol flavonoids for better glycaemic control: targeting pancreatic islet amyloid polypeptide (IAPP). Nutrients. 2017;9:788.
Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients. 2016;8:1–24.
Sahebkar A. Effects of resveratrol supplementation on plasma lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2013;71:822–35.
Malhotra A, Bath S, Elbarbry F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxidative Med Cell Longev. 2015;2015:1–15.
Pannu N, Bhatnagar A. Resveratrol: from enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed Pharmacother. 2019;109:2237–51.
Huang J, Wang X, Zhang Y. Specific types of alcoholic beverage consumption and risk of type 2 diabetes: a systematic review and meta-analysis. J Diab Investigation. 2017;8:56–68.
Barbhaiya M, Lu B, Sparks JA, Malspeis S, Chang SC, Karlson EW, Costenbader KH. Influence of alcohol consumption on the risk of systemic lupus erythematosus among women in the nurses’ health study cohorts. Arthritis care & research. 2017;69:384–92.
Di Castelnuovo A, Costanzo S, Di Giuseppe R, De Gaetano G, Iacoviello L. Alcohol consumption and cardiovascular risk: mechanisms of action and epidemiologic perspectives. Futur Cardiol. 2009;5:467–77.
Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, van den Brandt PA, Brinton L, Buring JE, Heather Eliassen A. Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol. 2016;45:916–28.
Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–12.
Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. Bmj. 1999;319:1523–8.
Shen Z, Li Y-M, Yu C-H, Shen Y, Xu L, Xu C-F, Chen J-J, Ye H, Xu G-Y. Risk factors for alcohol-related liver injury in the island population of China: a population-based case-control study. World J Gastroenterol: WJG. 2008;14:2255.
Van de Wiel A, De Lange D. Cardiovascular risk is more related to drinking pattern than to the type of alcoholic drinks. Neth J Med. 2008;66:467–73.
Wang PL, Xiao FT, Gong BC, Liu FN. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget. 2017;8:99013–23.
Xu W, Wang H, Wan Y, Tan C, Li J, Tan L. Yu JT: alcohol consumption and dementia risk: a dose–response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:31–42.
Briggs NC, Levine RS, Bobo LD, Haliburton WP, Brann EA, Hennekens CH. Wine drinking and risk of non-Hodgkin’s lymphoma among men in the United States: a population-based case-control study. Am J Epidemiol. 2002;156:454–62.
Rodrigo R, Miranda A, Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta. 2011;412:410–24.
Cueva C, Gil-Sánchez I, Ayuda-Durán B, González-Manzano S, González-Paramás AM, Santos-Buelga C, Bartolomé B, Moreno-Arribas M. An integrated view of the effects of wine polyphenols and their relevant metabolites on gut and host health. Molecules. 2017;22:99.
Petersen K, Smith C. Ageing-associated oxidative stress and inflammation are alleviated by products from grapes. Oxidative Med Cell Longev. 2016;2016:1–12.
Rasines-Perea Z, Teissedre PL. Grape Polyphenols' effects in human cardiovascular diseases and diabetes. Molecules. 2017;22:1–19.
Zhang H, Liu S, Li L, Liu S, Liu S, Mi J, Tian G. The impact of grape seed extract treatment on blood pressure changes: a meta-analysis of 16 randomized controlled trials. Medicine. 2016;95:1–7.
Wightman JD, Heuberger RA. Effect of grape and other berries on cardiovascular health. J Sci Food Agric. 2015;95:1584–97.
Pérez-Jiménez J, Saura-Calixto F. Grape products and cardiovascular disease risk factors. Nutr Res Rev. 2008;21:158–73.
Di Lorenzo C, de Sousa LP, Pastor RF, Colombo F, Frigerio G, Restani P. Beneficial effects of non-alcoholic grape-derived products on human health: a literature review. In: BIO Web of Conferences. EDP Sciences; 2015. p. 04002.
Akaberi M, Hosseinzadeh H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother Res. 2016;30:540–56.
Zheng J, Zhou Y, Li S, Zhang P, Zhou T, Xu D-P, Li H-B. Effects and mechanisms of fruit and vegetable juices on cardiovascular diseases. Int J Mol Sci. 2017;18:555.
Alipour M, Davoudi P, Davoudi Z. Effects of unripe grape juice (verjuice) on plasma lipid profile, blood pressure, malondialdehyde and total antioxidant capacity in normal, hyperlipidemic and hyperlipidemic with hypertensive human volunteers. J Medicinal Plants Res. 2012;6:5677–83.
Apostolidou C, Adamopoulos K, Iliadis S, Kourtidou-Papadeli C. Alterations of antioxidant status in asymptomatic hypercholesterolemic individuals after resveratrol intake. Int J Food Sci Nutr. 2016;67:541–52.
Barona J, Blesso CN, Andersen CJ, Park Y, Lee J, Fernandez ML. Grape consumption increases anti-inflammatory markers and upregulates peripheral nitric oxide synthase in the absence of dyslipidemias in men with metabolic syndrome. Nutrients. 2012;4:1945–57.
Copetti C, Franco FW, EdR M, Soquetta MB, Quatrin A, VdM R, JCF M, Emanuelli T, Sautter CK, Penna NG. Acute consumption of bordo grape juice and wine improves serum antioxidant status in healthy individuals and inhibits reactive oxygen species production in human neuron-like cells. J nutri metabol. 2018;2018:1–11.
de Rijke YB, Demacker P, Assen NA, Sloots LM, Katan MB, Stalenhoef A. Red wine consumption does not affect oxidizability of low-density lipoproteins in volunteers. Am J Clin Nutr. 1996;63:329–34.
Di Renzo L, Carraro A, Valente R, Iacopino L, Colica C, De Lorenzo A. Intake of red wine in different meals modulates oxidized LDL level, oxidative and inflammatory gene expression in healthy people: a randomized crossover trial. Oxidative Med Cell Longev. 2014:2014:1–9.
Fuhrman B, Lavy A, Aviram M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am J Clin Nutr. 1995;61:549–54.
Kalfin R, Righi A, Del Rosso A, Bagchi D, Generini S, Guiducci S, Matucci Cerinic M, Das DK. Activin, a grape seed-derived proanthocyanidin extract, reduces plasma levels of oxidative stress and adhesion molecules (ICAM-1, VCAM-1 and E-selectin) in systemic sclerosis. Free Radic Res. 2002;36:819–25.
Micallef M, Lexis L, Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr J. 2007;6:27.
Neto MM, da Silva TF, de Lima FF, Siqueira TM, Toscano LT, de Moura SK, Silva AS. Whole red grape juice reduces blood pressure at rest and increases post-exercise hypotension. J Am Coll Nutr. 2017;36:533–40.
Paller CJ, Zhou XC, Heath EI, Taplin M-E, Mayer T, Stein MN, Bubley GJ, Pili R, Hudson T, Kakarla R. Muscadine grape skin extract (MPX) in men with biochemically recurrent prostate cancer: a randomized, multicenter, placebo-controlled clinical trial. Clin Cancer Res. 2018;24:306–15.
Safaei N, Babaei H, Azarfarin R, Jodati A-R, Yaghoubi A, Sheikhalizadeh M-A. Comparative effect of grape seed extract (Vitis vinifera) and ascorbic acid in oxidative stress induced by on-pump coronary artery bypass surgery. Ann Card Anaesth. 2017;20:45.
Toaldo IM, Cruz FA, da Silva EL, Bordignon-Luiz MT. Acute consumption of organic and conventional tropical grape juices (Vitis labrusca L.) increases antioxidants in plasma and erythrocytes, but not glucose and uric acid levels, in healthy individuals. Nutr Res. 2016;36:808–17.
Toscano LT, Silva AS, Toscano LT, Tavares RL, Biasoto ACT, de Camargo AC, da Silva CSO. Gonçalves MdCR, Shahidi F: Phenolics from purple grape juice increase serum antioxidant status and improve lipid profile and blood pressure in healthy adults under intense physical training. J Funct Foods. 2017;33:419–24.
Toscano LT, Tavares RL, Toscano LT, Silva CSO, AEMd A, ACT B, MdCR G, Silva AS. Potential ergogenic activity of grape juice in runners. Appl Physiol Nutr Metab. 2015;40:899–906.
Turki K, Charradi K, Boukhalfa H, Belhaj M, Limam F, Aouani E. Grape seed powder improves renal failure of chronic kidney disease patients. EXCLI J. 2016;15:424.
Yubero N, Sanz-Buenhombre M, Guadarrama A, Villanueva S, Carrion JM, Larrarte E, Moro C. LDL cholesterol-lowering effects of grape extract used as a dietary supplement on healthy volunteers. Int J Food Sci Nutr. 2013;64:400–6.
Durak İ, Burak Cimen M, Büyükkogak S, Kaçpmaz M, Serdar Öztürk H. The effect of red wine on blood antioxidant potential. Curr Med Res Opin. 1999;15:208–13.
Agte VV, Pathare P, Nilegaonkar S, Tupe R, Adesara K, Mali A, Padwal M, Melinkeri R. Effect of phytonutrient rich juice blends on antioxidant status and lipid profile in Young adults: a randomised trial. J Clin Diagnostic Res. 2018;12:6–10.
Díaz-Rubio ME, Pérez-Jiménez J, Martínez-Bartolomé MÁ, Álvarez I, Saura-Calixto F. Regular consumption of an antioxidant-rich juice improves oxidative status and causes metabolome changes in healthy adults. Plant Foods Hum Nutr. 2015;70:9–14.
Novembrino C, Cighetti G, De Giuseppe R, Vigna L, de Liso F, Pellegatta M, Gregori D, Maiavacca R, Bamonti F. Effects of encapsulated fruit and vegetable juice powder concentrates on oxidative status in heavy smokers. J Am Coll Nutr. 2011;30:49–56.
Spormann TM, Albert FW, Rath T, Dietrich H, Will F, Stockis J-P, Eisenbrand G, Janzowski C. Anthocyanin/polyphenolic–rich fruit juice reduces oxidative cell damage in an intervention study with patients on hemodialysis. Cancer Epidemiology and Prevention. Biomarkers. 2008;17:3372–80.
Urquiaga I, Troncoso D, Mackenna MJ, Urzúa C, Pérez D, Dicenta S, De la Cerda PM, Amigo L, Carreño JC, Echeverría G. The consumption of beef burgers prepared with wine grape pomace flour improves fasting glucose, plasma antioxidant levels, and oxidative damage markers in humans: a controlled trial. Nutrients. 2018;10:1388.
Yuan L, Meng L, Ma W, Xiao Z, Zhu X, Feng JF, Yu H, Xiao R. Impact of apple and grape juice consumption on the antioxidant status in healthy subjects. Int J Food Sci Nutr. 2011;62:844–50.
Sattarinezhad A, Roozbeh J, Yeganeh BS, Omrani G, Shams M. Resveratrol reduces albuminuria in diabetic nephropathy: a randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45:53–9.
Avellone G, Di Garbo V, Campisi D, De Simone R, Raneli G, Scaglione R, Licata G. Effects of moderate Sicilian red wine consumption on inflammatory biomarkers of atherosclerosis. Eur J Clin Nutr. 2006;60:41–7.
Estruch R, Sacanella E, Mota F, Chiva-Blanch G, Antúnez E, Casals E, Deulofeu R, Rotilio D, Andres-Lacueva C, Lamuela-Raventos R. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: a randomised cross-over trial. Nutr Metab Cardiovasc Dis. 2011;21:46–53.
Lafay S, Jan C, Nardon K, Lemaire B, Ibarra A, Roller M, Houvenaeghel M, Juhel C, Cara L. Grape extract improves antioxidant status and physical performance in elite male athletes. J Sports Sci Med. 2009;8:468.
Noguer M, Cerezo AB, Navarro ED, Garcia-Parrilla M. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol Res. 2012;65:609–14.
Torres A, Cachofeiro V, Millán J, Lahera V, Nieto M, Martín R, Bello E, Alvarez-Sala L. Red wine intake but not other alcoholic beverages increases total antioxidant capacity and improves pro-inflammatory profile after an oral fat diet in healthy volunteers. Revista Clinica Espanola. 2015;215:486–94.
Mellen PB, Daniel KR, Brosnihan KB, Hansen KJ, Herrington DM. Effect of muscadine grape seed supplementation on vascular function in subjects with or at risk for cardiovascular disease: a randomized crossover trial. J Am Coll Nutr. 2010;29:469–75.
Chuang C-C, McIntosh MK. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr. 2011;31:155–76.
Woerdeman J, Van Poelgeest E, Ket J, Eringa E, Serné E, Smulders Y. Do grape polyphenols improve metabolic syndrome components? A systematic review. Eur J Clin Nutri. 2017;71:1381–92.
Feringa HH, Laskey DA, Dickson JE, Coleman CI. The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. J Am Diet Assoc. 2011;111:1173–81.
Vaisman N, Niv E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: a double blind, placebo-controlled, randomized study. Int J food Sci Nutri. 2015;66:342–9.
Kubyshkin A, Avidzba A, Borisyuk V, Stoyanov V, Fomochkina I, Ogai YA, Chernousova I, Zaitsev G, Guguchkina T, Markosov V. Polyphenols of red grapes in wine and concentrates for use in rehabilitation technologies. Agricultural Biol. 2017;52:622–30.
Castilla P, Dávalos A, Teruel JL, Cerrato F, Fernández-Lucas M, Merino JL, Sánchez-Martín CC, Ortuño J, Lasunción MA. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am J Clin Nutr. 2008;87:1053–61.
Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, Teruel JL, Lucas MF, Gómez-Coronado D, Ortuño J, Lasunción MA. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr. 2006;84:252–62.
Razavi S-M, Gholamin S, Eskandari A, Mohsenian N, Ghorbanihaghjo A, Delazar A, Rashtchizadeh N, Keshtkar-Jahromi M, Argani H. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J Med Food. 2013;16:255–8.
Bo S, Ciccone G, Castiglione A, Gambino R, De Michieli F, Villois P, Durazzo M, Cavallo-Perin P, Cassader M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–31.
Park YK, Lee SH, Park E, Kim J-S, Kang M-H. Changes in antioxidant status, blood pressure, and lymphocyte DNA damage from grape juice supplementation. Ann New York Acad Sci. 2009;1171:385.
Bloomer R, Davis PG, Consitt L, Wideman L. Plasma protein carbonyl response to increasing exercise duration in aerobically trained men and women. Int J Sports Med. 2007;28:21–5.
Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–5.
Smoliga JM, Colombo ES, Campen MJ. A healthier approach to clinical trials evaluating resveratrol for primary prevention of age-related diseases in healthy populations. Aging (Albany NY). 2013;5:495.
Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81:313S–6S.
Meyer AS, Yi O-S, Pearson DA, Waterhouse AL, Frankel EN. Inhibition of human low-density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis vinifera). J Agric Food Chem. 1997;45:1638–43.
Sato M, Ramarathnam N, Suzuki Y, Ohkubo T, Takeuchi M, Ochi H. Varietal differences in the phenolic content and superoxide radical scavenging potential of wines from different sources. J Agric Food Chem. 1996;44:37–41.
Dohadwala MM, Vita JA. Grapes and cardiovascular disease. J Nutr. 2009;139:1788S–93S.
Folts JD. Potential health benefits from the flavonoids in grape products on vascular disease. In: Flavonoids in cell function: Springer; 2002. p. 95–111.
Reinisalo M, Kårlund A, Koskela A, Kaarniranta K, Karjalainen RO. Polyphenol stilbenes: molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxidative Med Cell Longev. 2015;2015:1–24.
Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52:S84–94.
Natella F, Belelli F, Gentili V, Ursini F, Scaccini C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J Agric Food Chem. 2002;50:7720–5.
Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–90.
Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243S–55S.
Dani C, Oliboni LS, Vanderlinde R, Pra D, Dias JF, Yoneama ML, Bonatto D, Salvador M, Henriques JA. Antioxidant activity and phenolic and mineral content of rose grape juice. J Med Food. 2009;12:188–92.
Chiva-Blanch G, Urpi-Sarda M, Ros E, Valderas-Martinez P, Casas R, Arranz S, Guillén M, Lamuela-Raventós RM, Llorach R, Andres-Lacueva C. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clin Nutr. 2013;32:200–6.
Das S, Vasisht S, Das N, Srivastava L, Dubey K, Watal G. Correlation between total antioxidant status and lipid peroxidation in hypercholesterolemia. Current Sci-Bangalore. 2000;78:486.
Acknowledgements
The authors appreciate the Nutrition and Food Security research center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran to support this study.
Funding
This research was funded by Nutrition and Food Security research center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: SS-KH and MH designed the study. SS-KH and ZSS performed systematic research and study selection; SS-KH and MH independently evaluated the methodological quality of the included articles according to Cochrane risk of bias tools. The data collected and extracted by SS-KH and ZSS. SS-KH and MH performed the statistical analysis. SS-KH wrote the draft of the manuscript. MH critically revised the manuscript and approved the final version of manuscript to be submitted. All authors read and approved the final version of the article.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sarkhosh-Khorasani, S., Sangsefidi, Z.S. & Hosseinzadeh, M. The effect of grape products containing polyphenols on oxidative stress: a systematic review and meta-analysis of randomized clinical trials. Nutr J 20, 25 (2021). https://doi.org/10.1186/s12937-021-00686-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-021-00686-5