Abstract
Background
In Africa, the relationship between childhood nutritional status and malaria remains complex and difficult to interpret. Understanding it is important in the improvement of malaria control strategies. This study aimed to assess the influence of nutritional status on the occurrence of multiple malaria episodes in children aged 6 to 59 months between 2013 and 2017 living in the village of Dangassa, Mali.
Methods
A community-based longitudinal study was conducted using cross-sectional surveys (CSSs) at the beginning (June) and end (November) of the malaria transmission season associated with passive case detection (PCD) at the Dangassa Community Health Centre. Children with asymptomatic malaria infection during cross-sectional surveys were selected and their malaria episodes followed by PCD. Malaria indicators in person-months were estimated using an ordinal-logistic model repeated on subjects during follow-up periods.
Results
The incidence rate (IR) during the period of high transmission (June to October), for 1 episode and for 2 + episodes peaked in 2013 with 65 children (IR = 95.73 per 1000 person-months) and 24 cases (IR = 35.35 per 1000 person-months), respectively. As expected, the risk of multiple episodes occurring during the period of high transmission was 3.23 compared to the period of low transmission after adjusting for other model parameters (95% CI [2.45–4.26], p = 0.000). Children with anaemia were at high risk of having multiple episodes (OR = 1.6, 95% CI [1.12–2.30], p = 0.011). However, the risk of having 2 + episodes for anemic children was higher during the period of low transmission (RR = 1.67, 95% CI [1.15–2.42], p = 0.007) compared to the period of high transmission (RR = 1.58, 95% CI [1.09–2.29], p = 0.016). The trend indicated that anemic and underweight children were significantly associated with multiple malaria episodes during the period of low transmission (p < 0.001).
Conclusion
Results show that multiple episodes of malaria are significantly related to the nutritional status (anaemia and underweight) of the child during the two transmission seasons and more pronounced during the dry season (period of low transmission). Further research including other malnutrition parameters will be needed to confirm these findings.
Similar content being viewed by others
Background
Malaria, anaemia, and malnutrition are responsible for more than 50% of child mortality in the world, particularly among children under 5 years of age [1,2,3]. Malaria remains the leading cause of morbidity and mortality in Malian children under 5 years of age, with an overall prevalence of 19%, according to the malaria indicator survey (MIS) of 2021 [4]. The Koulikoro district is one of the third with a prevalence above the country mean prevalence (23%), following Mopti (27%), and Sikasso (26%) [4].
In Mali, besides malaria, malnutrition remains a major cause of morbidity and mortality among children under 5 years of age. Micronutrient deficiencies, known as the main cause of malnutrition, is highly prevalent during the malaria transmission season which last from June to October each year [5]. Most of the death from undernourished children, are due to common infections associated with immunodeficiency [6]. In Mali, it affects 27% of children under 5 years of age in chronic form, 19% of whom are underweight (“low weight for age”) [7]. Being a composite indicator, underweight reflects both stunting and wasting, with no differentiation between them. Therefore, children presenting a low weight for their age are both growths stunted and wasted [8].
As one of one of the most common public health problems in the world, and especially one of the complications of malaria infection in endemic regions, anaemia plays a major role in malaria morbidity and mortality [9, 10]. It is multifactorial, affecting 40% of children under 5 years of age, and half of these cases are attributed to nutritional iron deficiency [11]. More than 82% of children under 5 years in Mali suffer from anaemia, with 25% suffering from mild anaemia, 51% from moderate, and 6% from severe anaemia [7].
Children’s nutritional status is a sensitive indicator of community health. The effects of malnutrition in children are long-lasting and extend decades beyond childhood [8]. However, the relationship between nutritional status and clinical malaria, on the one hand, and anaemia on the other, remains complex and difficult to interpret. Anaemia has not been found associated with the frequency of malaria episodes, but it has been demonstrated to be significantly associated with malnutrition [12]. In contrast, other studies have shown that malnutrition and anaemia are associated with a higher risk of Plasmodium infection and infectious episodes [13, 14].
The relationship between nutritional status, anaemia, and malaria episodes among children under the age of five is not well documented in Mali. This study aimed to investigate longitudinally the influence of nutritional status on multiple episodes of clinical malaria in children aged 6–59 months asymptomatic to malaria in Dangassa, health district of Kati, from 2013 to 2017.
Methods
Study area
The study was conducted in Dangassa (8° 12' 37.253'' W and 12° 8' 46.279' N) which is located 75 km southwest of Bamako in the health district of Ouéléssebougou, Koulikoro region (Fig. 1). This is a Sudan savannah eco-geographical zone with a rainy season lasting from June to October and a dry season from November to May. The dominant winds are the monsoon (rainy season) and the harmattan (dry season). Malaria transmission is seasonal in Dangassa, with 6 to 7 months of transmission per year (June to December). In Dangassa at the start of malaria transmission season (June-July), the prevalence of Plasmodium falciparum for children under 5 years of age was respectively 59.4%, 32.9% and 19.1% in 2014, 2015 and 2016 [15].
Study design
Cross-sectional surveys
Asymptomatic children were selected from the cross-sectional survey (CSSs) data. The selected children were used as a baseline for the follow-up of malaria cases at the community health center. Seven CSSs were performed: in June (at the start of the rainy season) and in October/November (at the end of the rainy season). However, in 2013 the only CSSs was performed in February, which was utilized as baseline for June and October for PCD. During each survey, demographic and clinical data were collected from each participant. Microscopy (smear) was used to assess the P. falciparum infection, while HemoCue® Hb 301 was used to measure haemoglobin levels. The Weight was measured using a weigh scale. During the CSSs, only participants with fever (defined as axillary temperature ≥ 37.5 °C or fever within the previous 48 h) were tested for malaria using malaria rapid diagnostic test (RDT). Participants tested positive for malaria were provided treatment according to the policies of the Malian National Malaria Control Programme (NMCP).
Passive case detection (PCD)
Asymptomatic children identified during CSSs were followed up by passive case detection (PCD). Malaria incidence was determined after each CSSs (Fig. 2). From February 2013 to March 2017, a physician and a biologist followed cohort participants for malaria incidence through community health-based passive surveillance. Therefore, head and mothers of children selected for the study were informed to visit the health centre if they developed fever or other symptoms of malaria. Study participants with fever (suspected malaria cases) are tested using RDT and microscopy. All confirmed cases (RDT positive cases) were treated free of charge in accordance with national recommendations for malaria treatment. In addition, all severe cases were referred to the district health centre at the project's expense. During this follow-up, data on anaemia and malnutrition were collected in the same way as during the cross-sectional surveys.
Operational definitions
-
Malaria episode: Malaria episode for a participant was defined as new one only when time between two subsequent infections was greater than 20 days. The number of episodes per child was classified into three categories 0, 1 and 2 or more.
-
Malnutrition indicator: ENA For Smart software was used to calculate scores (z-scores) based on the national results at the World Health Organization (WHO) reference population. Malnutrition indicators were determined using a standard deviation of −2 standard deviations or Z-score. The weight-for-age (WAZ) of children was classified in two categories, Z-score according to WHO growth standards references for children aged zero to 5 years 16. Score ≤ −2 standard deviation (SD) were defined as underweight, while score > −2 standard were defined as normal from the reference mean.
-
Anaemia: anaemia was defined as a haemoglobin level < 11 g/dl (16). It was classified into two categories: anaemia: Hb level < 11 g/dl; no anaemia: Hb level ≥ 11 g/dl 18.
Laboratory methods
Blood films were stained with 10% Giemsa and examined under a 100X oil immersion objective lens of a light microscope. Asexual parasites were estimated based on the number of parasitized red blood cells per 300 white blood cells multiplied by 25, assuming an average white blood cell count of 7500/µl. A minimum of 100 fields were examined before a thick drop was considered negative by two different experienced microscopists having read each smear separately. RDTs (BIOLANE®) for P. falciparum infection were performed according to the manufacturer's instructions. RDTs used were those currently recommended by the NMCP of Mali.
Haemoglobin concentration was measured in venous blood using HemoCue 301. Hemocue® 301 is an easy to use device for field workers with a long-life span of the batteries which is important in rural areas where electricity is not always available.
Statistical methods
Microsoft Access 2019 was used for database management and to track the malaria episode using PCD data between two CSSs. The data and graphical analysis were done using SAS version 9.2 and SPSS version 28. The person month incidence was adjusted for different time intervals: from June through October/November, from October/November through December and from December through June of the following year (2014–2016). In 2013, the times intervals were from February to June, then from June to October/November and from October/November to December. In 2017, the follow up time was from December 2016 to March 2017.
The yearly numbers of malaria episodes in person-months were adjusted using repeated ordinal-logistic regression model. The parameters were estimated using Generalized Estimating Equation (GEE) with the hybrid method (in which Fisher scoring iterations are performed before switching to the Newton–Raphson method). The maximum likelihood method was used for the algorithm convergence. The working correlation matrix has shown an exchangeable structure. The repeated subject effect was the children study ID with the intrasubject effect as the follow-up period. The p-value for analysis was set to 5%. The convergence criteria were satisfied, and the data fit well the model. The children exposure times within each transmission period were computed as the offset. The outcome variable for the model was the episodes numbers with three categories levels 0, 1 or 2 + episodes. Five risk factors: age groups, gender, anaemia status, weight for age (WAZ) and transmission period were used. From the model, the predict Incidence rate (IR), odd ratio (OR) and/or the relative risk (RR) for having higher malaria-episodes numbers were estimated.
Results
Seven CSSs, from 2013 (February), 2014 (June, October), 2015 (June, November) and 2016 (June, December), were carry-out during which 199,107,233,151,324,232 and 154 children were respectively enrolled at the beginning of each of the malaria-transmission-periods. The prevalence of clinical malaria was 15.6% in 2013, 15.0% vs 16.7%, 8.6% vs 13.6%, and 6.9% vs 13.6%, respectively at the start and end of transmission in 2014, 2015, and 2016 (Table 1).
Using the PCD data, the unadjusted comparison of no null percentage malaria episode numbers (0, 1 or 2 +) by age groups, gender, anaemia, and weight-for-age status for each transmission period indicated a significant difference in gender in 2013 (February to June), in WAZ status in 2014 (June to October) and in anaemia status in 2015 (June to November) (all p < 0.05). There was no significant difference elsewhere (Table 2).
The peak of incidence risk (IR) for one (IR = 95.73 in 1000 person-months, n = 65) and 2 + (IR = 35.35 in 1000 person-months, n = 24) episodes was observed in 2013 during the high transmission period (June to October). IR decreased to zero case at the end of the follow-up in March 2017. The lowest IR for 1 episode and 2 + episodes were observed in the second half time of the study follow-up period in 2016 (June–December) with 9 cases (IR = 6.78 in 1000 person-months) and 5 cases (IR = 3.77 in 1000 persons-months), respectively (Table 3).
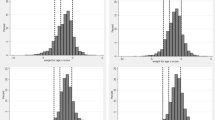
The univariate analysis of risk factors between age groups indicated that children 36–59 months were more likely to have higher numbers of malaria episodes than 6–35 months old. Males were more likely to experience a higher number of multiple malaria episodes as well as anaemia compared to females. However, none of the differences was significant (all p > 0.05) (Fig. 3a–d).
After adjusting for all other parameters, the odds ratio (OR) for having a higher number of malaria episodes during the high transmission period was higher than in the low transmission period [OR = 3.23, 95% CI (2.45–4.26), p = 0.000]. The children with anaemia were more likely to have higher numbers of malaria episodes [OR = 1.6, 95% CI (1.12–2.30), p = 0.011] than those with normal haemoglobin levels (Table 4).
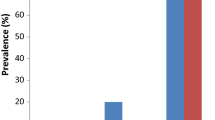
During low and high transmission periods, the trends of the predicted IR in person-months among the categories of each risk factor are shown in Fig. 4a–d. The trend indicated a significant difference between gender, WAZ, and anaemia status during the high transmission period. However, during the low transmission period, anaemic and underweight children were more likely to have 2 + malaria episodes (all p < = 0.001) except for underweight children in the follow-up period from October 2013 to June 2014, (p = 0.38).
In contrast, the relative risk of two or more episodes of anaemia in low transmission periods (RR = 1.67, 95%CI [1.15–2.42], p = 0.007) was slightly higher than in high transmission periods (RR = 1.58, 95%CI [1.09–2.29], p = 0.016) (Table 5).
Discussion
In endemic countries, malaria is a major public health problem, particularly in low-income countries [16]. Despite the complexity of the interaction between malaria, anaemia, and nutritional status [1], understanding their relationship is vital for the development of strategies that will reduce child morbidity and mortality. Using repeated cross-sectional surveys and passive case detection at a community health centre for five successive years, the relationship between anaemia, underweight, and multiple malaria episodes among children under the age of five in Dangassa was assessed.
Based on the baseline descriptive analysis, symptomatic malaria cases varied between CSSs and years. The number of cases was higher at the end of the rainy season than at the beginning. In malaria-endemic areas, the parasite density is usually higher at the end than the start of the rainy season [17]. During this period, the entomological inoculation rate is particularly high [18]. Toure et al. in Sélingué, Mali [19] showed that malaria is seasonal in Mali, with a peak at the end of the rainy season. Although the survey was conducted during the dry season, the prevalence of symptomatic malaria was compared to the prevalence at the beginning of the transmission season. As Dangassa is a riverine village where transmission is particularly prolonged (5 to 6 months), high spatial and temporal clustering of P. falciparum infection has been demonstrated during the dry season [20].
The significant difference between the percentage of malaria episodes numbers by anaemia status in June to November 2015 indicated a high percentage among no anaemia children. However, anaemia is frequently associated with clinical malaria particularly the severe form in area where the disease is endemic [21]. The introduction of both seasonal malaria chemoprevention (SMC) and LLINs universal coverage in Dangassa for children less than 5 years could explained this change in the risk of clinical malaria with respect to anaemia status [22]. Indeed, studies have shown a positive impact of SMC on the reduction of malaria transmission and malaria indicators such as anaemia in children in West Sahelian Africa [23, 24].
The peak of malaria incidence was observed in 2013 during the period of high transmission (June to October), with respectively 65 children ((IR = 95.73 in 1000 person-months) for multiple episodes and 24 cases (IR = 35.35 in 1000 person-months) for those with 1 episode. Most malaria cases detected by PCD in Dangassa are symptomatic and occur during the five- to six-month transmission season (June to November) [25]. Also, Ateba et al. reported a higher number of cases during the period when SMC was not distributed compared with the period when SMC was implemented in the same area [26].
However, the frequency of malaria episodes decreased significantly between 2013 and 2017 within the cohort. As a result, (i) the intensification of malaria control strategies in Dangassa, including the distribution of LLINs and the introduction of SMC, have significantly reduced the burden of malaria in Dangassa [27]; (ii) Community sensitization and participation in the study; (iii) Qualified staff deployed within the framework of the International Centre of Excellence for Malaria Research in West Africa (ICEMR-1) project; (iv) Health centre use by residents.
According to these results, underweight is associated with a higher number of episodes compared to normal children 10% error [OR = 1.46, 95% CI (0.95–2.23), p = 0.084]. Almeida et al. [28], in a rural community in the Amazon region, demonstrated that malaria-induced anorexia and vomiting during the acute phase of the disease, associated with insufficient micronutrients, in children under 5 years of age in a specific endemic context, could lead to a delay in the child's physical development after several episodes of malaria.
The adjusted model showed a significant association between multiple episodes in anaemic and normal children [OR = 1.6, 95%CI (1.12–2.30), p = 0.011]. Thus, in high transmission areas such as Dangassa, where the disease is prevalent, many young children are anaemic, and people infected with the disease may receive a single bite each day, exposing them to repeated episodes of the disease. Consequently, in these contexts, severe malarial anaemia (haemoglobin < 7 g/dl) [29], in young children, a blood transfusion is required, which characterizes a compromised rapid recovery of the anaemia, since malaria infection causes haemolysis of parasitized and non-parasitized erythrocytes, and dyserythropoiesis of the bone marrow [30].
The occurrence of multiple episodes was significantly associated with anaemic and underweight children during the low transmission period. Anaemia has a complex aetiology, including single- and multifactorial causality [31]. Thus, the high occurrence of malaria in Dangassa during the low transmission period in anaemic and underweight children is associated probably through protein-energy and vitamin deficiencies. In 2018, Aimée et al., from the Democratic Republic of Congo, reported that iron deficiency can negatively affect children's weight growth [32]. Mariken et al., measuring the association between nutritional status and malaria incidence in young children before the malaria transmission season, found that, underweight was associated with a higher incidence of clinical malaria in Burkina Faso [33].
The risk of having multiple malaria episodes during the high transmission period was 3.23 times higher than during the period of low transmission [CI (2.45–4.26); p = 0.000]. Although the number of multiple malaria episodes increase following exposure to malaria transmission, the rainfall, which is low during periods of low transmission and high during periods of high transmission, is an important factor in the dynamics of malaria transmission. Toure et al. [19] reported the same results and showed a clear seasonal pattern, with a reduced below 50% malaria incidence during the dry season.
The study has the limitation that, because the data were analysed secondary, some anthropometric parameters needed, such as height and the upper arm circumference to determine children’s nutritional status, were not collected. Consequently, some variables related to these indices could not be incorporated into these analysis.
Conclusion
The presence of multiple malaria episodes was significantly associated with nutritional status (anaemia and low weight-for-age) in children during both high and low transmission periods. However, this relationship was more pronounced during the dry season (“low transmission” period). A further study including more parameters are necessary to support this hypothesis.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confident interval
- CSSs:
-
Cross sectional surveys
- GEE:
-
Generalized estimating equation
- Hb:
-
Haemoglobin
- HTP:
-
High transmission period
- ICEMR-WA:
-
International Centre of Excellence for Malaria Research in West Africa
- IR:
-
Incidence rate
- LLINs:
-
Insecticide-impregnated mosquito nets
- LTP:
-
Low transmission period
- MIS:
-
Malaria Indicator Survey
- NMCP:
-
National Malaria Control Program
- OR:
-
Odd ratio
- PCD:
-
Passive case detection
- RDT:
-
Rapid diagnostic test
- RR:
-
Relative risk
- SD:
-
Standard deviation
- SMC:
-
Seasonal Malaria Chemoprevention
- WAZ:
-
Weight-for-age
- WHO:
-
World Health Organization
References
Das D, Grais RF, Okiro EA, Stepniewska K, Mansoor R, van der Kam S, et al. Complex interactions between malaria and malnutrition: a systematic literature review. BMC Med. 2018;16:186.
Ehrhardt S, Burchard GD, Mantel C, Cramer JP, Kaiser S, Kubo M, et al. Malaria, anemia, and malnutrition in african children–defining intervention priorities. J Infect Dis. 2006;194:108–14.
Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, et al. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS ONE. 2013;8: e64636.
Institut National de la Statistique Institut, Programme National de Lutte contre le Paludisme, ICF. Malaria indicator survey in Mali 2021. Bamako, Mali, Rockville, USA; 2022
Teh RN, Sumbele IUN, Meduke DN, Ojong ST, Kimbi HK. Malaria parasitaemia, anaemia and malnutrition in children less than 15 years residing in different altitudes along the slope of mount cameroon: prevalence, intensity and risk factors. Malar J. 2018;17:336.
Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS ONE. 2014;9: e105017.
Institut National de la Statistique—INSTAT, Cellule de Planification et de Statistique Secteur Santé-Développement, ICF. Mali Demographic and Health Survey 2018. Bamako, Mali; 2019.
Nigatu G, Assefa Woreta S, Akalu TY, Yenit MK. Prevalence and associated factors of underweight among children 6–59 months of age in Takusa district. Northwest Ethiopia Int J Equity Health. 2018;17:106.
Green HK, Sousa-Figueiredo JC, Basanez MG, Betson M, Kabatereine NB, Fenwick A, et al. Anaemia in Ugandan preschool-aged children: the relative contribution of intestinal parasites and malaria. Parasitology. 2011;138:1534–45.
Morakinyo OM, Balogun FM, Fagbamigbe AF. Housing type and risk of malaria among under-five children in Nigeria: evidence from the malaria indicator survey. Malar J. 2018;17:311.
WHO. Anaemia. Geneva, World Health Organization, 2023. https://www.who.int/news-room/fact-sheets/detail/anaemia.
Muller O, Traore C, Jahn A, Becher H. Severe anaemia in west African children: malaria or malnutrition? Lancet. 2003;361:86–7.
Sumbele IU, Kimbi HK, Ndamukong-Nyanga JL, Nweboh M, Anchang-Kimbi JK, Lum E, et al. Malarial anaemia and anaemia severity in apparently healthy primary school children in urban and rural settings in the mount cameroon area: cross sectional survey. PLoS ONE. 2015;10: e0123549.
Yadav CP, Hussain SSA, Pasi S, Sharma S, Bharti PK, Rahi M, Sharma A. Linkages between malaria and malnutrition in co-endemic regions of India. BMJ Glob Health. 2023;8: e010781.
Toure M, Keita M, Kane F, Sanogo D, Kante S, Konate D, et al. Trends in malaria epidemiological factors following the implementation of current control strategies in dangassa. Mali Malar J. 2022;21:65.
WHO. World malaria Report 2023. Geneva, World Health Organization, 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023.
Mayengue PI, Kouhounina Batsimba D, Niama RF, Ibara Ottia R, Malonga-Massanga A, Fila-Fila GPU, et al. Variation of prevalence of malaria, parasite density and the multiplicity of Plasmodium falciparum infection throughout the year at three different health centers in Brazzaville. Republic Congo BMC Infect Dis. 2020;20:190.
PMI. Vectorlink project Mali: annual entomological monitoring report. 2019. https://pdf.usaid.gov/pdf_docs/PA00ZC4W.pdf
Toure M, Sanogo D, Dembele S, Diawara SI, Oppfeldt K, Schioler KL, et al. Seasonality and shift in age-specific malaria prevalence and incidence in binko and carriere villages close to the lake in selingue. Mali Malar J. 2016;15:219.
Shaffer JG, Toure MB, Sogoba N, Doumbia SO, Gomis JF, Ndiaye M, et al. Clustering of asymptomatic Plasmodium falciparum infection and the effectiveness of targeted malaria control measures. Malar J. 2020;19:33.
Shankar H, Singh MP, Hussain SSA, Phookan S, Singh K, Mishra N. Epidemiology of malaria and anemia in high and low malaria-endemic North-Eastern districts of India. Front Public Health. 2022;10: 940898.
Konate D, Diawara SI, Toure M, Diakite SAS, Guindo A, Traore K, et al. Effect of routine seasonal malaria chemoprevention on malaria trends in children under 5 years in dangassa. Mali Malar J. 2020;19:137.
Druetz T. Evaluation of direct and indirect effects of seasonal malaria chemoprevention in Mali. Sci Rep. 2018;8:8104.
Diawara F, Steinhardt LC, Mahamar A, Traore T, Kone DT, Diawara H, et al. Measuring the impact of seasonal malaria chemoprevention as part of routine malaria control in Kita. Mali Malar J. 2017;16:325.
Doumbia S, Toure M, Sogoba N, Alifrangis M, Diakite M, Diarra A, et al. The West Africa ICEMR partnerships for guiding policy to improve the malaria prevention and control. Am J Trop Med Hyg. 2022;107:84–9.
Ateba FF, Febrero-Bande M, Sagara I, Sogoba N, Toure M, Sanogo D, et al. predicting malaria transmission dynamics in dangassa, Mali: a novel approach using functional generalized additive models. Int J Environ Res Public Health. 2020;17:6339.
Doumbia S, Sogoba N, Diakite M, Toure M, Keita M, Konate D, et al. A decade of progress accelerating malaria control in Mali: evidence from the West Africa international center of excellence for malaria research. Am J Trop Med Hyg. 2022;107:75–83.
Alexandre MA, Benzecry SG, Siqueira AM, Vitor-Silva S, Melo GC, Monteiro WM, et al. The association between nutritional status and malaria in children from a rural community in the Amazonian region: a longitudinal study. PLoS Negl Trop Dis. 2015;9: e0003743.
Dejene BE, Abuhay TM, Bogale DS. Predicting the level of anemia among Ethiopian pregnant women using homogeneous ensemble machine learning algorithm. BMC Med Inform Decis Mak. 2022;22:247.
Mwaiswelo RO, Mmbando BP, Chacky F, Molteni F, Mohamed A, Lazaro S, et al. Malaria infection and anemia status in under-five children from Southern Tanzania where seasonal malaria chemoprevention is being implemented. PLoS ONE. 2021;16: e0260785.
Safiri S, Kolahi AA, Noori M, Nejadghaderi SA, Karamzad N, Bragazzi NL, et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. J Hematol Oncol. 2021;14:185.
Musimwa AM, Kitoko HT, Wakamb GK, Okitotsho SW, Numbi OL. [Serum iron concentration in malnourished children from an urban and rural area in democratic republic of the Congo] (in French). Pan Afr Med J. 2018;31:55.
de Wit M, Cairns M, Compaore YD, Sagara I, Kuepfer I, Zongo I, et al. Nutritional status in young children prior to the malaria transmission season in Burkina faso and Mali, and its impact on the incidence of clinical malaria. Malar J. 2021;20:274.
Acknowledgements
We thank the ICEMR program team, the community of Dangassa, and Mali’s NMCP.
Funding
This study was supported by the National Institutes of Health Cooperative Agreements U19AI089696 for the West African Center of Excellence for Malaria Research. We would like to thank the Fogarty International Center of the National Institutes of Health of the United States for supporting Soumba Keita under Grant D43TW008652.
Author information
Authors and Affiliations
Contributions
SK conceived the idea presented and drafted the manuscript with support from OT, MT, FK, MK, IS, NS, MD and SD. SK and OT developed the modelling and performed the analyses. DS, SD and DK were responsible for data collection in the field. HC was responsible for data management. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate.
Ethical approvals were obtained from the National Institutes of Health (NIAID), from both Institutional Review Boards (IRBs) of Tulane University (FWA00002055) and the University of Sciences, Techniques and Technology of Bamako in Mali (2011/77/FMPOS). Written informed consent was obtained from each participant or parent/legal guardian for children under 18 years of age enrolled in this study.
Consent for publication
Not applicable.
Competing interests
The authors have declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Keita, S., Thiero, O., Toure, M. et al. Prognostics of multiple malaria episodes and nutritional status in children aged 6 to 59 months from 2013 to 2017 in Dangassa, Koulikoro region, Mali. Malar J 23, 186 (2024). https://doi.org/10.1186/s12936-024-04999-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-04999-8