Abstract
Background
Plasmodium vivax represents the most geographically widespread human malaria parasite affecting civilian and military populations in endemic areas. Targeting the pre-erythrocytic (PE) stage of the parasite life cycle is especially appealing for developing P. vivax vaccines as it would prevent disease and transmission. Here, naturally acquired immunity to a panel of P. vivax PE antigens was explored, which may facilitate vaccine development and lead to a better understanding of naturally acquired PE immunity.
Methods
Twelve P. vivax PE antigens orthologous to a panel of P. falciparum antigens previously identified as highly immunogenic in protected subjects after immunization with radiation attenuated sporozoites (RAS) were used for evaluation of humoral and cellular immunity by ELISA and IFN-γ ELISpot. Samples from P. vivax infected individuals (n = 76) from a low endemic malaria region in the Peruvian Amazon Basin were used.
Results
In those clinical samples, all PE antigens evaluated showed positive IgG antibody reactivity with a variable prevalence of 58–99% in recently P. vivax diagnosed patients. The magnitude of the IgG antibody response against PE antigens was lower compared with blood stage antigens MSP1 and DBP-II, although antibody levels persisted better for PE antigens (average decrease of 6% for PE antigens and 43% for MSP1, p < 0.05). Higher IgG antibodies was associated with one or more previous malaria episodes only for blood stage antigens (p < 0.001). High IgG responders across PE and blood stage antigens showed significantly lower parasitaemia compared to low IgG responders (median 1,921 vs 4,663 par/µl, p < 0.05). In a subgroup of volunteers (n = 17),positive IFN-γ T cell response by ELISPOT was observed in 35% vs 9–35% against blood stage MSP1 and PE antigens, respectively, but no correlation with IgG responses.
Conclusions
These results demonstrate clear humoral and T cell responses against P. vivax PE antigens in individuals naturally infected with P. vivax. These data identify novel attractive PE antigens suitable for use in the potential development and selection of new malaria vaccine candidates which can be used as a part of malaria prevention strategies in civilian and military populations living in P. vivax endemic areas.
Similar content being viewed by others
Background
Plasmodium spp. are the causative agent of malaria, one of the world’s deadliest infectious diseases. In 2022, about 249 million cases of malaria were reported by WHO with approximately 608,000 deaths, particularly in young children and pregnant women in sub-Saharan Africa [1]. While Plasmodium falciparum is the most prevalent malaria parasite on the African continent and responsible for most observed infections and deaths, P. vivax represents the most widely distributed human malaria parasite worldwide. Outside of Africa, P. vivax is the dominant cause of malaria with over 3 billion people living within its transmission limits [1]. Globally, several million clinical cases of P. vivax malaria are detected each year with a relevant portion in South East Asia and Latin America, where P. vivax is responsible for 50% and 70–85% of all malaria cases, respectively [1]. Traditionally, P. vivax has been considered to cause a “benign” form of malaria but is now recognized as a significant cause of morbidity and mortality due to increasing evidence of severe cases with a possible fatal outcome [2].
Malaria elimination efforts have resulted in a substantial decline in the global malaria burden in the past two decades. It is estimated that these efforts have resulted in a reduction of global malaria infections by 29% and mortality caused by malaria by 60% between 2000 and 2018 [1]. However, from 2014 to 2018 this downward trend has slowed considerably and even reversed in some regions (e.g., Venezuela) [1, 3]. Plasmodium vivax represents a special challenge to control efforts due to its unique biological features, including low-density blood-stage infection, asymptomatic infections and the formation of hypnozoites—the dormant forms residing in hepatocytes that can cause relapses months or years after the initial infection. Hypnozoites are believed to be responsible for up to 80% of infections and there is currently no diagnostic tool available for their detection [4]. Importantly, activation of hypnozoites can represents a big challenge for malaria elimination, especially in naïve civilian and military populations which travel to these P. vivax endemics zones. Previous studies showed relapse episodes in U.S. military population deployed to Afghanistan, where 38 Army Rangers reported activation of hypnozoites within an average of 233 days after return from P. vivax endemic areas, highlighting the risk to military personnel during deployments [5]. Further progress towards malaria elimination will require additional tools including the development of an effective vaccine or intervention, which can prevent or eliminate these dormant stages.
For many years, the efforts to develop a PE malaria vaccine have mainly been focused on P. falciparum while only two P. vivax vaccine candidates have reached clinical trials, targeting blood stage proteins DBPII and MSP-1 [5]. The most advanced and recently approved vaccine by WHO is the P. falciparum subunit vaccine RTS,S-AS01, which targets the Circumsporozoite Protein (CSP) via neutralizing antibodies and has shown consistent but short-lived efficacy of 25–36% in children in phase III and IV clinical trials [6, 7]. A novel R21 vaccine candidate with a similar target and vaccine platform as RTS,S/AS01 but with a significant increased ratio of CSP protein in vaccine formulation showed higher efficacy of 67–78% over 6–18 months children in clinical Phase I-III [8,9,10]. In addition to CSP, other proteins have been under investigation as vaccine targets such as the PE antigen thrombospondin-related adhesive protein (TRAP) and the highly promising blood stage protein Reticulocyte-binding Protein Homolog 5 (Rh5) [11]. Both candidates were chosen at least in part based on data showing that responses were associated with protection in naturally infected persons, currently Rh5 is progressing into phase I and II clinical trials after promising preclinical results in non-human primates [12,13,14].
Only a few such studies of naturally acquired immunity to P. vivax have been conducted, largely with a focus on antibodies to erythrocytic antigens. In these studies, reticulocyte-binding proteins critical for merozoite invasion have been associated with protective immunity (e.g. PvDBP-II and PvRBP2b) [15,16,17,18]. Large-scale screenings have identified antibodies to other blood-stage antigens associated with protection particularly when seen in combinations rather than with any single antigen alone [19, 20]. However, even fewer studies have explored the natural immunogenicity of P. vivax PE antigens [21] despite the fact that the PE stages of Plasmodium can be targeted with both antibodies and T cells [22, 23]. For P. falciparum, a partially protective PE intervention that only results in a reduction of liver stage parasites will have no perceptible benefit to the person as even a small number of breakthrough parasites can yield fulminant blood stage disease. However, for P. vivax, preventing a portion liver stages would still not yield sterilizing protection against primary infection but would have proportional effects on elimination since the number of hypnozoites would be reduced, resulting in the reduced number of relapses that are the main driver of disease [4, 24]
Taken together, this suggests that antibodies and T cells targeting multiple PE P. vivax proteins could be an integral part of developing the first effective P. vivax vaccine. The almost exclusive focus on the blood stage has resulted in a general lack of P. vivax PE candidate antigens and an even more opaque understanding of their role in natural immunity or even simply immunogenicity. This paucity of data around PE immunity in P. vivax limits the ability to take the first steps towards novel vaccine candidates. Here, naturally acquired immunity to a panel of P. vivax PE antigens was explored as a key step to facilitate vaccine development and to better understand naturally-acquired PE P. vivax immunity.
Methods
Human plasma and lymphocyte samples
Human samples were obtained between 2012 and 2017 from three passive surveillance protocols approved by the Institutional Review Board of the U.S. Naval Medical Research Unit SOUTH (NAMRU SOUTH) in compliance with all applicable federal regulations governing the protection of human subjects (NMRCD.2007.0004, NMRCD.2010.0002 and NAMRU6.2012.0006).
Protocols NMRCD.2007.0004 and NAMRU6.2012.0006 were conducted in Iquitos, the largest city in the department of Loreto located in Peruvian Amazon Basin. Samples were collected from the Hospital Regional de Loreto (HRL, n = 41) and Hospital de Apoyo de Iquitos (HAI, n = 35). HRL is a specialized health centre for complex diseases whereas HAI attends patients with less complex symptomatology, giving basic treatments and rapid diagnostics for transmissible and non-transmissible diseases. Subjects presenting malaria symptoms (fever, headache, chills) at both health centres were invited to participate in the study and blood samples were collected after providing their consent. All subjects enrolled (n = 76) were diagnosed by microscopy using thick blood smear (counting number of parasites vs number white blood cells to determine parasite per μL of blood) and confirmed by PCR [25] for P. vivax mono-infection. Loreto is described as hypoendemic malaria area with co-endemicity of P. vivax and P. falciparum. Plasmodium vivax represents 80—90% of total malaria cases with an annual average incidence of 42,164 ± 7,779 cases during 2012–2017. In addition, P. vivax transmission is sustained and heterogeneous along the year [26], resulting in underestimated malaria prevalence due to low- parasitaemia and/or asymptomatic cases.
In addition, a set of twenty human P. vivax negative control blood samples obtained from individuals living in the department of Piura was included, located in the North Coast of Peru (NMRCD.2010.0002), which is a very low incidence region for P. vivax (0.01 per 1000 inhabitants) during 2014 (Fig. 1).
Selection of P. vivax PE antigens for immunological assessments
The 12 P. vivax PE antigens are orthologs to a panel of P. falciparum antigens previously identified as PE vaccine targets [27]. These P. falciparum antigens were selected from a larger set of 131 P. falciparum recombinant proteins based on their reactivity against sera samples from sterilely protected subjects who underwent immunization with radiation attenuated sporozoites (RAS) followed by challenge with the bites of viable P. falciparum -infected Anopheles mosquitoes [28, 29]. This approach has been partially validated for three Plasmodium yoelii orthologs from this list of candidates: a putative cysteine protease inhibitor (Falstatin), the gamete egress and sporozoite traversal (GEST) and the early transcribed membrane protein (ETRAMP) which induced up to 60% protective efficacy when used in combination using the DNA and vaccinia virus prime-boost immunization approach [30]. In addition, this subset of P. vivax proteins have been verified as expressed in the P. vivax proteome [31].
Proteins were expressed using the full open reading frame according to Plasmo DB using the P. vivax Sal-I reference genome, with predicted amino acid lengths between 90 and 500. Proteins were used if the predicted protein size between 12 and 65 kiloDaltons (kDa) was observed. These proteins were successfully expressed by the cell-free wheat germ expression system [32] using the bi-layer method (small-scale) with a yield between ~ 300-1200 μg of protein per reaction. In addition, well-known antigens MSP-1 (PVX_099980) and DBP-II (Sal-I) expressed at blood stage and CSP VK-210 (PVX_119355) expressed at PE stage were used to compare immunogenicity levels with novel PE orthologs.
ELISA—human samples
Briefly, thirteen P. vivax PE antigens and two blood stage antigens were used to determine antibody prevalence and relative immunogenicity. ELISA plates were coated with recombinant proteins at concentration of 2–4 μg/ml to high-binding 96-well microplates (Nunc Maxisorp) overnight at room temperature and then washed with T-PBS1X (PBS1X/0.05%Tween20) five times. Microplates were blocked for 1 h with TBS1X-SM (TBS1X/Skim milk 5% buffer). After five washes with T-PBS1X, human plasma samples were added in duplicate at a 1:200 dilution in TBS1X-SM and incubated for 2 h at room temperature. After five washes with T-PBS1X, antibodies were detected using peroxidase-conjugated anti-human IgG monoclonal antibodies (Jackson Immunoresearch Cat#: 309–035-033, 1:6,000 dilution) and incubated for one hour at room temperature. After five washes with T-PBS1X, o-phenylenediamine (Sigma Aldrich Cat#: P3804) with hydrogen peroxide as a substrate was used and the reaction was stopped after 1 h of development with 50 μl of 3N HCl. Plates were read at 492 nm to determine optical density OD.
In order to assess variability between plates, a control on each plate a two-fold plasma serial dilution of a positive pool of 30 individuals with more than 10 P. vivax confirmed events was used. In addition, 20 plasma samples from individuals from malaria area with low incidence were used to calculate a positivity cut-off value for each protein using the average OD value plus 3 standard deviations.
ELISpot
A library of overlapping 15-mer synthetic peptides was obtained from Mimotopes Pty Ltd. The peptides overlapped by 9 aminoacids spanning the entire blood and PE proteins. Plasmodium vivax MSP-1 was used as a positive control of blood stage antigens, and seven pre-erythrocytic antigens were tested (CSP, CelTOS, Falstatin, ETRAMP, PVX_119755, GEST and HSP PVX_089585). These were resuspended according to the manufacturer’s instructions and each peptide pool used at 10 μg/ml. These peptides were used to determine the frequency of T-cell response producing IFN-γ to P. vivax PE proteins using Human IFN-γ ELISpot PRO (Cat.# 3420-2APW-10. Mabtech AB, Sweden) according to the manufacturer’s instructions.
Peripheral blood mononuclear cells (PBMCs) from P. vivax patients, who accepted large blood collection for immunological assays, were isolated from whole blood by density centrifugation using a Percoll gradient, counted and cryopreserved for storage in liquid nitrogen. Briefly, PBMCs were thawed in RPMI media with 10% FBS (SIGMA, Cat #: F4135), incubated overnight at 37 °C and then plated at 0.2 × 10^6 cells/well in duplicates. Peptides were added at 10 μg/ml to stimulate T cell response for 18 h, in parallel phorbol 12-myristate 13-acetate/ionomycin (PMA/ION) without peptides was used as a positive stimulation control. Spots were counted using the CTL IMUNOSPOT Analyzer and values as number of spots over media-only controls. Subjects were defined as positive IFN- γ response when the number of spots was higher than the negative control (volunteers without previous malaria infection) over 20%.
Statistical analysis
Variation of sample size per each analysis depended on the availability of samples, proteins quantity and data of epidemiological variables. Analysis was performed using STATA v16.0 statistical software (Stata Corp., College Station, TX, USA) and GraphPad Prism v9 (GraphPad Software, LLC). Differences among the frequency of epidemiological and immunological variables were analysed using Chi-square test for categorical variables and Mann–Whitney or Kruskal–Wallis test for numerical continuous variables to compare the median values and post-hoc Dunn’s test. Spearman rank correlation coefficient test was used to evaluate the correlation between epidemiological and immunological variables. To measure differences of antibodies level at different time points between groups (related samples) per each antigen, a paired Wilcoxon signed-rank test was used, the significance level for all statistical analysis performed was set at *p < 0.05 or **p < 0.001.
Results
Epidemiological variables
Epidemiological variables by site of enrolment showed significant differences only for weight and number of previous P. vivax episodes (Table 1). Both variables were higher for patients enroled at Hospital Regional de Loreto (HRL). Therefore, samples from each location were analysed as a single group.
Human plasma samples from P. vivax infected population and humoral response
In order to determine if antibodies recognizing P. vivax PE antigens are induced during naturally occurring P. vivax infections, plasma samples from 76 P. vivax positive subjects enroled at health centres of HRL and HAI were tested during 2012–2017 by ELISA.
This group of 76 P. vivax subjects showed positive antibody reactivity with high prevalence for blood stage antigens MSP1 (92%), DBP-II (86%) and the PE stage antigen CSP (99%) (Fig. 2). Twelve other P. vivax PE antigens showed positive antibody reactivity with variable prevalence in the range of 58–99%. Prevalence for each antigen was: TRAP PVX_082735 (99%), HSP PVX_089585 (93%), GAP40 PVX_080460 (93%), CELTOS PVX_123510 (91%), GEST PVX_118040 (89%), FALSTATIN PVX_099035 (88%), Hypothetical protein PVX_119755 (82%), Hypothetical protein PVX_094725 (75%), Hypothetical protein PVX_111090 (75%), ETRAMP (UIS3 ortholog) PVX_121950 (72%), SPECT1 PVX_083025 (62%), and Hypothetical protein PVX_093660 (58%) (Fig. 2).
Prevalence against P. vivax PE and blood stage antigens in P. vivax infected patients. Plasma samples from healthy controls (black circles, n = 20) and P. vivax patients (green squares, n = 76) were tested against 15 P. vivax antigens to determine seroprevalence by ELISA. Data shows individual values of IgG antibodies against each antigen measured by OD values. OD positivity cut-off (red line) value was defined as the average of low endemic control samples plus three standard deviations per each antigen. Black line represents the average value of OD values per each antigen
The magnitude of the antibody response as measured by OD values was higher for the blood stage MSP1 antigen with an OD average of 1.8 compared to the canonical PE antigen CSP with OD average of 0.8. The other 12 P. vivax PE antigens showed variable intensity with a mean OD range of 0.3–0.7 (Fig. 2). Overall, the antibody magnitude did not correlate with parasitaemia levels at the time of sampling except for a modest negative correlation in four PE antigens (Spearman RHO: -0.23 to -0.28, p < 0.05) (Table 2). This indicates that variations in OD intensity for each antigen are likely a result of the intrinsic immunogenicity of each antigen and inter-individual differences in responses rather than differences due to the parasitaemia at time of enrolment.
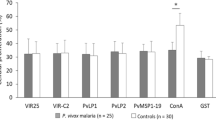
To determine if antibody magnitude for each individual antigen correlated with previous malaria exposure, a subgroup of 59 patients from the total of 76 was used for which data of self-reported previous malaria episodes was available. Individuals were stratified by no previous (n = 26), one previous (n = 16) and two or more previous (n = 17) P. vivax episodes. There was a significant higher IgG antibodies against only the blood stage antigens MSP1 and DBP in groups with one or more previous episodes as compared to the group with no previous P. vivax infection (p < 0.05) (Fig. 3). IgG antibodies against all P. vivax PE antigens showed similar IgG levels independent of the number of self-reported previous malaria episodes (Fig. 3). Together, these data reveal a broad and variable seropositivity to multiple P. vivax PE antigens during acute P. vivax infection that, unlike blood stage antigens, appear not to be boosted by multiple blood stage infections.
Boosting effect of blood and pre-erythrocytic P. vivax antigens in P. vivax infected patients. Plasma samples from zero previous (n = 26), one previous (n = 16) and two or more previous (n = 17) P. vivax episodes were used to determine boosting effect against each P. vivax antigen. Dot plots represent P. vivax episode groups with OD values of IgG antibodies against each antigen. Differences between groups per each antigen were assessed using the Kruskal–Wallis test with Dunn’s Post-hoc test. *p < 0.05, and **p < 0.001. Black, pink and green lines represent the average value of OD per each group of malaria episodes
Antibody breadth (number of antigens for which a volunteer was positive) also showed substantial variability between volunteers, especially amongst pre-erythrocytic antibodies (Fig. 4). Some volunteers responded broadly to nearly every antigen while others appeared to have weak antibody responses in terms of both breadth and individual magnitude. Indeed, a common predictor of antibody response to one PE antigen was a response to another (Fig. 5) suggesting that some volunteers naturally respond to infection with a greater antibody response. Those “high responders”, defined simply as those in the top 50% of total IgG magnitude across all antigens were evaluated against epidemiological variables. Significant differences between high and low responders were not observed by place of sample collection, sex, age, weight, temperature, and number of previous Pv episodes. However, high responders showed a significantly lower parasitaemia compared to low responders (median par/µl 1921 vs 4663, p < 0.05) at time of enrolment (Table 3). Interestingly, high and low antibody responders did not differ in parasitaemia levels after stratification by number of previous episodes (Fig. 6A, B). Volunteers are arranged in order of descending total antibody response across all antigens with the gap showing the division between “high responders” (1–38) and “low responders” (39–76).
Reactivity of antibodies against blood stage and pre-erythrocytic P. vivax antigens in P. vivax infected patients. Individual reactivity against each P. vivax antigen is shown by OD. Negative reactivity of antibodies is shown as 0 (box dark blue colours) and positive reactivity of antibodies with OD values between 0.1 and 3.5 (boxes blue to yellow colours)
Correlation matrix of antibodies against blood and pre-erythrocytic antigens in P. vivax infected patients. Plasma samples from P. vivax infected volunteers were used to represent the correlation of antibody response between P. vivax antigens. Pearson’s correlation coefficients are shown with values of -1.0 to 1.0 with red indicating negative correlation and blue a positive correlation. Significant correlations were represented by *p < 0.05 and **p < 0.001
Relationship between parasitaemia and number of previous malaria episodes amongst high and low antibody responders in P. vivax infected patients. Plasma samples from P. vivax infected patients with high (n = 38) and low (n = 38) antibody response against blood and pre-erythrocytic antigens were used to evaluate association with parasitaemia levels. (A) Dot plots represent parasitaemia levels by high (black circles) and low (pink circles) antibody responders. (B) Dot plots represent parasitaemia levels in high and low antibody responder groups stratified by 0 (black circles), 1 (green circles) and ≥ 2 (orange circles) previous P. vivax episodes. Black, pink, green and orange lines represent average values of parasitaemia per groups. Significant differences are shown by *p < 0.05 and **p < 0.01
Long-term analysis of a sub-group of 24 P. vivax patients for which samples were available six months after enrolment showed a significant decrease of OD values only for MSP1 (OD average decrease of -43%, Day 0: 1.71 vs Day 180: 0.97, p < 0.05) and CSP (OD average decrease of -28%, Day 0: 0.83 vs Day 180: 0.60, p < 0.001). Nine PE antigens showed more stable IgG levels with an average decrease of between antigens of 6% (range: -2 to -21 and SD ± 6%) (Fig. 7). These subjects did not experience a new malaria episode during six months of follow-up by monthly microscopy confirmation, indicating that some PE antibodies are maintained for a significant period after initial infection in the absence of boosting.
Long-term antibody analysis of blood and PE P. vivax antigens in P. vivax infected patients. Plasma samples from P. vivax infected patients (n = 24) were used to represent individual antibody variation measured by OD values after 6 months of P. vivax infection against each P. vivax antigen. Lines connect individual antibody variation between P. vivax infection Day 0 vs Day 180. Differences between time points per each antigen were assessed using Wilcoxon signed-rank test, *p < 0.05, and **p < 0.001
Human T cell responses
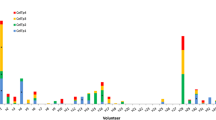
T cell responses were evaluated to eight antigens in a small set of individuals (n = 17) for which PMBCs were available. An ex vivo IFN-gamma response was detected in at least one subject for all the antigens tested. Positivity for IFN-gamma reactivity was the highest for the blood stage protein MSP1 and the pre-erythrocytic stage protein CSP, both at 35.3% (6/17 volunteers positive). From the remaining antigens tested, the highest percentage of positive responses was for ETRAMP at 33.3% (5/15), followed by Falstatin (25.0%, 4/16), CelTOS (23.5%, 4/17), the Hypothetical protein PVX_119755 (23.1%, 3/13) and HSP (18.2%, 2/11).
Across individuals, broad and variable T cell responses to PE antigens was observed (Fig. 8) with two subjects positive to CSP only, and six subjects positive to 2–4 PE antigens (in addition to or besides CSP). Variability between subjects in the number of SFU (spot forming units) per million cells was also seen, with two subjects highly reactive to multiple antigens. Additionally, two subjects were positive to only MSP-1 and no T cell response was found for seven subjects (41%).
Antibody response was tested in seven of the subjects for which both plasma and PBMCs were available. In this limited set, there was no apparent correlation between positive IgG response and ex vivo IFN-gamma response (Table 4). Together, these data indicate that T cell responses to both blood stage and PE antigens are also present but variable during acute P. vivax infection.
Discussion
Only a few P. vivax PE proteins such as CSP, TRAP and CelTOS have been thoroughly evaluated as vaccine candidates and still remain at the pre-clinical or early clinical stages [33]. More recently, have been seeing that natural acquired antibodies against P. vivax PE CelTOS, SPECT1, SSP3 and CSP proteins achieved significant inhibitory activity into formation of exoerythrocytic forms (EEFs) in in-vitro sporozoite-hepatocyte infection model, suggesting an important role of naturally acquired humoral immunity over the development of EEFs in P. vivax infected patients [34, 35].
New P. vivax PE candidates and, in particular, discovery of new antigens will be necessary for a better understanding of P. vivax PE immunity and to provide insight into their role in the prevention of both liver and blood stage infection. In the absence of well-established animal models of P. vivax infection, studying the acquisition of PE antibodies during natural infection is a logical starting point with the long-term goal of studying the role these PE antibodies play in functional immunity in order to justify integrating functional targets into novel vaccine candidates. In addition, as more candidates move into clinical trials in P. vivax-endemic areas, the level and impact of pre-existing immunity will be important to measure. However, to date, few studies of this kind been done for P. vivax PE antibodies.
A P. vivax cohort study (n = 31) in a low-transmission P. vivax area in western Thailand during 2013 showed prevalence of 76–86%, 18–21% and 0% for Pv CSP, CELTOS and TRAP antigens, respectively [36]. In addition, a cross-sectional study (n = 299) of naturally acquired immunity performed in Brazil during 2016 against whole protein and linear synthetic peptides of PE TRAP showed prevalence of 49% and 25–32%, respectively [37]. These results showed naturally acquired immunity against all 13 PE antigens tested in n = 76 P. vivax patients from a low endemic malaria region located in the Peruvian Amazon Basin was generally higher, with some individuals responding to all PE antigens (range of 58–99%). This variation could be related to the inherent characteristics of different circulating P. vivax parasites, differences in transmission dynamics or simply differences in protein production platforms as these previous studies used HEK-293 cell lines for production compared to wheat germ cell-free system.
Compared to the magnitude of P. vivax blood stage responses, the IgG response to PE antigens was lower. This is likely since boosting effect of anti-PE IgG with repeated infection was not observed. Rather, IgG responses to one antigen often correlated simply with a response to another antigen within individuals—leading to high and low antibody responders. Comparative analysis of high and low antibody responders against P. vivax PE and blood stage antigens vs. parasitaemia showed that high responders had lower parasitaemia levels. However, given the high correlation between antibody levels between both PE and blood stage antigens, correlations that may indicate if antibodies to any stage or target might be leading to protection against higher parasitaemia was not identified. These data suggest that high antibody levels may be a host-intrinsic factor, and this breadth of response—rather than a response to any single antigen—could be involved in the lower parasitaemia was observed. This aligns with published data demonstrating protection from P. vivax is associated with the acquisition of a multi-antigen antibody response at the blood stage [20]. However, low parasitaemia in high responders was seen irrespective of the number of previous P. vivax episodes, although these were not subsequent infections in the same individual. Thus, whether the lower parasitaemia is the result of a recently-acquired diverse antibody response better controlling subsequent infections or that the antibodies are a correlate of some other immune phenomenon that results in lower parasitaemia [38] will require investigation in well-designed longitudinal cohort studies with deeper immune phenotyping and active detection of new or relapse infections.
Previous studies have shown a correlation between antibody response and P. vivax parasitaemia levels in cross-sectional and cohort studies. This includes a large (n = 466) survey of the antibody response against blood stage protein vaccine target DBP-II which showed a negative correlation between P. vivax parasitaemia and antibodies in an area with hypo-endemic P. vivax transmission in Brazil [39]. As such, P. vivax DBP-II is the leading blood stage candidate for P. vivax blood stages. However, little is known about relationship between antibody response against PE antigens and parasitaemia in cross-sectional or cohort studies. Here, in a cross-sectional study in hypoendemic malaria area a modest negative correlation between parasitaemia and antibodies levels against 4 PE antigens was observed (Spearman RHO: -0.23 to -0.28, p < 0.05), suggesting these antigens should be included in further studies as functional antibody targets.
In addition, IgG antibodies at six months after P. vivax infection had variable stability, with a significant decrease in the most naturally immunogenic antibodies MSP-1(-43%) and CSP (-28%) antigens. However, nine other PE antigens tested showed relative stability (- 6% STD ± 6) as did the blood stage DBP antigen. This characteristic could be useful in defining serological markers of recent vs. historical exposure to use in malaria endemic areas near to effective malaria control or on way for malaria elimination process.
Even less well-studied than PE P. vivax antibodies are T cell responses to natural P. vivax infection. A few studies have assessed vaccine candidates eliciting CD8 + and/or CD4 + T cells that correlate with protection from liver-stage infection in mice [40, 41] and one clinical trial [42], but the mechanism that leads to T cell protection remains to be elucidated. Here, in a limited sample of volunteers, a positive T cell response in 35% vs 9–35% of tested individuals was observed against blood stage antigen MSP1 and PE antigens, respectively. From patients with a positive T cell response, 40% showed reactivity for both blood stage and PE antigens which did not appear to correlate with IgG responses. While the role of T cell-mediated protection against rodent malaria liver stages is robust [43, 44], the absence of appropriate animal models for P. vivax and, in particular, hypnozoites leaves this possibility unconfirmed. Interestingly, recent evidence demonstrated that CD8 + T cells can eliminate infected reticulocytes [45] opening the possibility of multi-stage CD8 + T cell vaccines to prevent or limit P. vivax infection. This limited data demonstrate that natural PE T cell immunity does exist and will need to be included in future studies with a larger population investigating the relationship between T cells and protection from infection at multiple stages.
Conclusion
In conclusion, here is presented the most antigenically diverse survey of PE immunity acquired during natural infection using samples from people living in an endemic area of the Peruvian Amazon Basin. These results demonstrate broad but variable reactivity across P. vivax PE antigens and between individuals. Together, these data clearly demonstrate a previously underappreciated prevalence of natural immunity to PE antigens which warrants specifically designed longitudinal cohorts where better correlations to transmission and protection from infection can be assessed. Such studies can add to a growing understanding of immunity to P. vivax infection in terms of both antigens and immune effector mechanisms with the goal of designing vaccines capable of preventing or reducing infection in civilian and deployed military populations.
Disclaimers
“The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government”
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- PE:
-
Pre-erythrocytic
- ELISA:
-
Enzyme-linked immunosorbent assay
- MSP1:
-
Merozoite surface protein-1
- DBP:
-
Duffy Binding Protein
- CSP:
-
Circumsporozoite protein
- WHO:
-
World Health Organization
- TRAP:
-
Thrombospondin-related adhesive protein
- RH5:
-
Reticulocyte-binding protein Homolog 5
- RAS:
-
Radiation attenuated sporozoites
- GEST:
-
Egress and sporozoite traversal
- ETRAMP:
-
Early transcribed membrane protein
- STD:
-
Standard deviation
- PCR:
-
Polymerase Chain Reaction
References
WHO. World Malaria Report 2023. Geneva: World Health Organization; 2023.
Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J. 2014;13:481.
Grillet ME, Hernandez-Villena JV, Llewellyn MS, Paniz-Mondolfi AE, Tami A, Vincenti-Gonzalez MF, et al. Venezuela’s humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. Lancet Infect Dis. 2019;19:e149–61.
White M, Amino R, Mueller I. Theoretical implications of a pre-erythrocytic Plasmodium vivax vaccine for preventing relapses. Trends Parasitol. 2017;33:260–3.
Tham WH, Beeson JG, Rayner JC. Plasmodium vivax vaccine research - we’ve only just begun. Int J Parasitol. 2017;47:111–8.
Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, et al. Fractional third and fourth dose of RTS, S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214:762–71.
Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-year efficacy of RTS, S/AS01 malaria vaccine among young African children. N Engl J Med. 2016;374:2519–29.
Datoo MS, Natama MH, Some A, Traore O, Rouamba T, Bellamy D, et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet. 2021;397:1809–18.
Datoo MS, Natama HM, Some A, Bellamy D, Traore O, Rouamba T, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22:1728–36.
Datoo MS, Dicko A, Tinto H, Ouedraogo JB, Hamaluba M, Olotu A, et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet. 2024;403:533–44.
Sack B, Kappe SH, Sather DN. Towards functional antibody-based vaccines to prevent pre-erythrocytic malaria infection. Expert Rev Vaccines. 2017;16:403–14.
Douglas AD, Baldeviano GC, Jin J, Miura K, Diouf A, Zenonos ZA, et al. A defined mechanistic correlate of protection against Plasmodium falciparum malaria in non-human primates. Nat Commun. 2019;10:1953.
Douglas AD, Baldeviano GC, Lucas CM, Lugo-Roman LA, Crosnier C, Bartholdson SJ, et al. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe. 2015;17:130–9.
Minassian AM, Silk SE, Barrett JR, Nielsen CM, Miura K, Diouf A, et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med (N Y). 2021;2:701–19.
Franca CT, He WQ, Gruszczyk J, Lim NT, Lin E, Kiniboro B, et al. Plasmodium vivax reticulocyte binding proteins are key targets of naturally acquired immunity in young Papua New Guinean Children. PLoS Negl Trop Dis. 2016;10: e0005014.
King CL, Michon P, Shakri AR, Marcotty A, Stanisic D, Zimmerman PA, et al. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc Natl Acad Sci USA. 2008;105:8363–8.
Urusova D, Carias L, Huang Y, Nicolete VC, Popovici J, Roesch C, et al. Structural basis for neutralization of Plasmodium vivax by naturally acquired human antibodies that target DBP. Nat Microbiol. 2019;4:1486–96.
He WQ, Karl S, White MT, Nguitragool W, Monteiro W, Kuehn A, et al. Antibodies to Plasmodium vivax reticulocyte binding protein 2b are associated with protection against P. vivax malaria in populations living in low malaria transmission regions of Brazil and Thailand. PLoS Negl Trop Dis. 2019;13:e0007596.
Longley RJ, White MT, Takashima E, Morita M, Kanoi BN, Li Wai Suen CSN, et al. Naturally acquired antibody responses to more than 300 Plasmodium vivax proteins in three geographic regions. PLoS Negl Trop Dis. 2017;11:e0005888.
França CT, White MT, He WQ, Hostetler JB, Brewster J, Frato G, et al. Identification of highly-protective combinations of Plasmodium vivax recombinant proteins for vaccine development. Elife. 2017;6: e28673.
Molina DM, Finney OC, Arevalo-Herrera M, Herrera S, Felgner PL, Gardner MJ, et al. Plasmodium vivax pre-erythrocytic-stage antigen discovery: exploiting naturally acquired humoral responses. Am J Trop Med Hyg. 2012;87:460–9.
Yadava A, Hall CE, Sullivan JS, Nace D, Williams T, Collins WE, et al. Protective efficacy of a Plasmodium vivax circumsporozoite protein-based vaccine in Aotus nancymaae is associated with antibodies to the repeat region. PLoS Negl Trop Dis. 2014;8: e3268.
Boonhok R, Rachaphaew N, Duangmanee A, Chobson P, Pattaradilokrat S, Utaisincharoen P, et al. LAP-like process as an immune mechanism downstream of IFN-gamma in control of the human malaria Plasmodium vivax liver stage. Proc Natl Acad Sci USA. 2016;113:E3519–28.
Schafer C, Dambrauskas N, Reynolds LM, Trakhimets O, Raappana A, Flannery EL, et al. Partial protection against P. vivax infection diminishes hypnozoite burden and blood-stage relapses. Cell Host Microbe. 2021;29: 752–6.e4.
Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, Rosario VE do, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol.1993;61:315-20.
Carrasco-Escobar G, Gamboa D, Castro MC, Bangdiwala SI, Rodriguez H, Contreras-Mancilla J, et al. Micro-epidemiology and spatial heterogeneity of P. vivax parasitaemia in riverine communities of the Peruvian Amazon: a multilevel analysis. Sci Rep. 2017;7:8082.
Aguiar JC, Bolton J, Wanga J, Sacci JB, Iriko H, Mazeika JK, et al. Discovery of novel Plasmodium falciparum pre-erythrocytic antigens for vaccine development. PLoS ONE. 2015;10: e0136109.
Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight. 2017;2(1): e89154.
Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33:7452–61.
Limbach K, Aguiar J, Gowda K, Patterson N, Abot E, Sedegah M, et al. Identification of two new protective pre-erythrocytic malaria vaccine antigen candidates. Malar J. 2011;10:65.
Swearingen KE, Lindner SE, Flannery EL, Vaughan AM, Morrison RD, Patrapuvich R, et al. Proteogenomic analysis of the total and surface-exposed proteomes of Plasmodium vivax salivary gland sporozoites. PLoS Negl Trop Dis. 2017;11: e0005791.
Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun. 2008;76:1702–8.
Arévalo-Herrera M, Vásquez-Jiménez JM, Lopez-Perez M, Vallejo AF, Amado-Garavito AB, Céspedes N, et al. Protective efficacy of Plasmodium vivax radiation-attenuated sporozoites in Colombian volunteers: a randomized controlled trial. PLoS Negl Trop Dis. 2016;10: e0005070.
Ntumngia FB, Kolli SK, Annamalai Subramani P, Barnes SJ, Nicholas J, Ogbondah MM, et al. Naturally acquired antibodies against Plasmodium vivax pre-erythrocytic stage vaccine antigens inhibit sporozoite invasion of human hepatocytes in vitro. Sci Rep. 2024;14:1260.
Nicholas J, De SL, Thawornpan P, Brashear AM, Kolli SK, Subramani PA, et al. Preliminary characterization of Plasmodium vivax sporozoite antigens as pre-erythrocytic vaccine candidates. PLoS Negl Trop Dis. 2023;17: e0011598.
Longley RJ, Reyes-Sandoval A, Montoya-Diaz E, Dunachie S, Kumpitak C, Nguitragool W, et al. Acquisition and longevity of antibodies to Plasmodium vivax preerythrocytic antigens in Western Thailand. Clin Vaccine Immunol. 2016;23:117–24.
Matos ADS, Rodrigues-da-Silva RN, Soares IF, Baptista BO, de Souza RM, Bitencourt-Chaves L, et al. Antibody responses against Plasmodium vivax TRAP recombinant and synthetic antigens in naturally exposed individuals from the Brazilian Amazon. Front Immunol. 2019;10:2230.
Kotliarov Y, Sparks R, Martins AJ, Mule MP, Lu Y, Goswami M, et al. Broad immune activation underlies shared set point signatures for vaccine responsiveness in healthy individuals and disease activity in patients with lupus. Nat Med. 2020;26:618–29.
Nicolete VC, Frischmann S, Barbosa S, King CL, Ferreira MU. Naturally acquired binding-inhibitory antibodies to Plasmodium vivax Duffy Binding Protein and clinical immunity to malaria in rural Amazonians. J Infect Dis. 2016;214:1539–46.
Bauza K, Malinauskas T, Pfander C, Anar B, Jones EY, Billker O, et al. Efficacy of a Plasmodium vivax malaria vaccine using ChAd63 and modified vaccinia Ankara expressing thrombospondin-related anonymous protein as assessed with transgenic Plasmodium berghei parasites. Infect Immun. 2014;82:1277–86.
Atcheson E, Bauza K, Salman AM, Alves E, Blight J, Viveros-Sandoval ME, et al. Tailoring a Plasmodium vivax vaccine to enhance efficacy through a combination of a CSP virus-like particle and TRAP viral vectors. Infect Immun. 2018;86:e00114-e118.
Bennett JW, Yadava A, Tosh D, Sattabongkot J, Komisar J, Ware LA, et al. Phase 1/2a trial of Plasmodium vivax malaria vaccine candidate VMP001/AS01B in malaria-naive adults: safety, immunogenicity, and efficacy. PLoS Negl Trop Dis. 2016;10: e0004423.
Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6: e1000998.
Parmar R, Patel H, Yadav N, Parikh R, Patel K, Mohankrishnan A, et al. Infectious sporozoites of Plasmodium berghei effectively activate liver CD8alpha(+) dendritic cells. Front Immunol. 2018;9:192.
Junqueira C, Barbosa CRR, Costa PAC, Teixeira-Carvalho A, Castro G, Sen Santara S, et al. Cytotoxic CD8(+) T cells recognize and kill Plasmodium vivax-infected reticulocytes. Nat Med. 2018;24:1330–6.
Acknowledgements
The authors are grateful to Dr. Hugo Valdivia, Dr. Andres G. Lescano, Dr. Danielle Pannebaker, Dr. Danett Bishop, Dr. Christie Joya, and Dr. Lisa Sperling for their support and critical review during the development of this work at NAMRU SOUTH. Julio A. Ventocilla is a master’s/doctoral student on Epidemiological Research at Universidad Peruana Cayetano Heredia supported by a scholarship from Emerge, the Emerging Diseases Epidemiology Research Training D43 TW007393 training grant awarded by the Fogarty International Center of the US National Institutes of Health.
Funding
This work was supported by the Military Infectious Disease Research Program Grants # F0398_14_LI, F0521_17 and F0578_19 awarded to Dr. G. Christian Baldeviano.
Author information
Authors and Affiliations
Contributions
GCB, JCA and BKW conceived, designed, and supervised the project studies. JVA, LLT, RP, AF and ML performed experiments of protein production and/or evaluation of immunogenicity by ELISA or ELISPOT. JVA, LLT and BKW performed the data and statistical analysis. JVA, LLT and BKW wrote the first draft of the manuscript. JVA, LLT and BKW wrote the final version of the paper. All authors reviewed, edited and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The human study protocols NMRCD.2007.0004, NMRCD.2010.0002 and NAMRU6.2012.0006 were approved by the U.S. Naval Medical Research Unit SOUTH Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects.
Consent for publication
Not applicable. No personal identifiable data was used in the study. All authors have provided consent to publish the findings in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ventocilla, J.A., Tapia, L.L., Ponce, R. et al. Evaluation of naturally acquired immune responses against novel pre-erythrocytic Plasmodium vivax proteins in a low endemic malaria population located in the Peruvian Amazon Basin. Malar J 23, 163 (2024). https://doi.org/10.1186/s12936-024-04978-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-04978-z