Abstract
Background
Malaria is a parasitic disease caused by various species of the blood parasite Plasmodium; of all the parasitic diseases, malaria has the highest prevalence and mortality with an estimated 247 million cases and 619,000 deaths recorded worldwide as of 2021. Malaria causes febrile illness with several changes in blood cell parameters. Some of these changes include leucopenia, thrombocytopenia, and anaemia. If these changes could be correlated with the degree of parasitaemia, it can serve as a guide to physicians when treating malaria. This study was therefore aimed at correlating haematological parameters with levels of parasitaemia during malaria infection.
Methods
The study was a cross-sectional study involving 89 malaria positive patients. About 5 ml of blood was collected from each participant who gave his or her informed consent to partake in the study. A full blood count was performed on their samples to determine their haematological parameters using a haematology auto-analyzer. A parasite count was also performed via microscopy to determine the degree of parasitaemia. The data obtained from the study was entered into a database and statistically analysed using Statistical Package for Social Sciences (SPSS) version 23 and Microsoft Excel 2016.
Results
The study comprised of 89 participants out of which 35 were males and 54 were females with the mean age of 26.15 years. Secondary education participants were the highest with quaternary education the lowest. The highest parasite count recorded was 398,174 parasites/µl of blood, lowest count was 101 with the average being 32,942.32584. There was also a significant positive Pearson’s correlation between total WBC and parasitaemia and with the WBC differentials, neutrophils, lymphocytes and monocytes had positive correlations while eosinophils and basophils had negative correlations. Furthermore, platelets, total RBC’s, haemoglobin, MCH, MCHC and Hct all showed negative correlations. Linear regression also showed a linear relationship between parasite density and the various haematological parameters.
Conclusion
The linear relationship (correlation) between WBC and MCH were the only significant ones at 95% and 99% confidence interval, respectively based on a two-tail t-test. Also, based on the regression analysis, the changes caused by WBC and PLT were the only significant changes at 95% confidence level in a two-tailed t-test.
Similar content being viewed by others
Background
Malaria is a life-threatening disease that is caused by parasites transmitted to people through the bites of infected female Anopheles mosquitoes [1]. The infection may result in a wide variety of symptoms, ranging from absent or very mild symptoms to severe disease and even death [1, 2]. There are five parasite species that cause malaria in humans, and two of these—Plasmodium falciparum and Plasmodium vivax—pose the greatest threat [1]. Malaria infections occur in five WHO regions and globally an estimated 3.4 billion people in 91 countries and territories are at risk of being infected with malaria and developing the disease with additional 1.1 billion at high risk [3]. In 2021, there were an estimated 247 million cases in malaria and 619,000 deaths worldwide [1]. Malaria is mainly endemic in tropical regions and developing countries due to the poor sanitation conditions in these developing countries [4]. In Ghana, 3.5 million cases of malaria are reported annually [5].
All of the clinical symptoms we see in malaria infection are caused by the asexual erythrocytic or blood stage parasites [6]. As the parasite develops in the erythrocyte, numerous waste substances that may be known or unknown, such as haemozoin pigment and other toxic factors accumulate in the infected erythrocyte [7]. These are released into the bloodstream when the infected cells lyses and releases merozoites that invade other red blood cells [7]. The haemozoin and the other toxic factors, such as glucose phosphate isomerase (GPI) stimulate macrophages as well as other cells to produce signaling molecules and other soluble factors which together act to produce fever and rigors and may even influence other severe pathophysiology associated with malaria [8, 9]. Furthermore, P. falciparum-infected erythrocytes, particularly those with mature trophozoites, adhere to the vascular endothelium of venular blood vessel walls and do not freely circulate in the blood [10]. When this sequestration of infected erythrocytes occurs in the vessels of the brain, it leads to cerebral malaria, which is associated with high mortality particularly in children [11].
Malaria causes acute febrile illness and is often difficult to differentiate from other febrile illnesses such as leptospirosis and arboviral infection hence it might not be detected early in patients resulting in several complications [12]. Symptoms associated with malaria include diarrhoea, vomiting, myalgia and abdominal pain. However, life-threatening complications such as kidney and liver failure, anaemia, low blood sugar, cerebral malaria and pulmonary oedema may also occur [13]. It must also be mentioned that, the severity of the disease is related to the level of parasitaemia, with high levels of parasitaemia resulting in severe malaria. Consequently, a sample of blood with 5–10,000 parasites/µl could be considered as having a low parasitaemia resulting in mild malaria, and one with 10,000–100,000 parasites/µl could be considered as having intermediate parasitaemia resulting in moderate malaria, whilst parasitaemia above 100,000 parasites/µl is said to be hyperparasitaemia and may result in severe malaria that may further result in death [14]. Generally, the severity of disease and risk of mortality in malaria would be expected to be dependent on the degree of parasitaemia [15].
Furthermore, when one is infected with malaria, various haematological alterations such as anaemia, thrombocytopenia, leukocytosis or leukopenia do occur [16, 17]. Lymphocytopenia has also frequently been described in patients with malaria, but studies on its association with disease severity have yielded conflicting results [18]. It has been postulated that the variation in parasitaemia levels may cause similar variation in haematological parameters [15]. That is to say, the changes in the haematological parameters may vary in relation with the levels of parasitaemia. Parasitaemia is highest in P. falciparum infections since the parasite invades red blood cells of all ages hence causing the most severe infections [19]. Due to this and since Ghana has more P. falciparum infections, it would be vital if a correlation between haematological parameters and parasitaemia could be established as it will enable physicians to determine how to proceed with early treatment and management of malaria.
Methods
Aim, design and setting of study
The aim of this study was to correlate changes in blood cell parameters with levels of parasitaemia in malaria infection. This study was a cross sectional study conducted from January 2018 to December 2018 at the Mamprobi polyclinic in Accra, Ghana.
Participants
The study comprised of eighty-nine (89) male and female patients attending the polyclinic and diagnosed with malaria infection and who gave their written informed consent.
Data/sample collection and laboratory analysis
Three to five millilitres (3–5 ml) of blood samples were collected from a prominent vein of each participant by venipuncture into lavender-topped tubes containing Ethylene-Diamine-Tetra-Acetic acid (EDTA) and thoroughly mixed to prevent them from clotting. The patients' unique identification numbers on consent forms were written on each tube to ensure confidentiality. A haematology analyzer was then used to perform a full blood count on the patients' blood samples for their haematological parameters. Thick and thin blood films were also prepared using the same patients' blood samples. The thick films were stained with Giemsa and the thin films with Leishman stain. The thick films were then examined under × 100 magnification for malaria parasites identification and the thin films for parasite specie identification. To determine the parasite density, two hundred (200) leukocytes were first counted whilst observing the malaria parasites present. If less than 10 parasites were observed after counting 200 leukocytes the count was continued till 500 leukocytes were counted. The parasite density was then calculated using the formula below.
This gave the number of parasites/µl of blood.
Data analysis
Data obtained were analyzed using Microsoft Excel 2016 and Statistical Package for Social Sciences (SPSS) software Version 23 (SPSS Inc., Chicago, USA). Descriptive statistics, Correlation analysis and linear regression analysis were carried out on the data.
Results
Demographics of participants
There were eighty-nine (89) participants in the study out of which 35 were males and 54 were females representing 39% and 61%, respectively. The oldest participant was 78 years while the youngest was 9 months. The mean age recorded was 26.15 years. With regards to educational background, 13 participants had none, 15 had primary, 43 had secondary, 16 had tertiary and 2 had quaternary education (Table 1).
Parasite indentification and density measurement from participants
Only P. falciparum species were identified. In terms of the parasite count, the highest parasite count was 398,174 parasites/µl whilst the lowest was 101 parasites/µl and the average parasite count recorded was 32,942.32584 parasites/µl. Also, in terms of gender distribution, males had the highest average parasite count whilst females had the lowest as seen in Table 2.
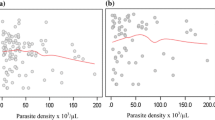
The mean parasite density were also compared with age of participants and the outcome is shown in Fig. 1.
Correlation between parasitaemia and various haematological parameters
Pearson’s correlation was run between parasitaemia and various haematological parameters and significant positive correlation was found between total WBC and parasitaemia. On the part of the WBC differentials, neutrophils, lymphocytes and monocytes had positive correlations while eosinophils and basophils had negative correlations. Specifically, it was seen that for 1 unit (parasites/µl) rise in parasite density there was 0.300 units (WBC × 109/l) rise in WBC and of the five white cells, the neutrophils were the most responsible for this rise by 0.297 units (Neu × 109/l) followed by monocytes and lymphocytes with a rise of 0.053 and 0.014 units respectively. Eosinophils and basophils fell by 0.021 and 0.025 units, respectively anytime parasitaemia rose by 1 unit (Table 3).
Also, Haemoglobin and RBC both had negative correlations and reduced by 0.205 and 0.062 units respectively per unit increase in parasitaemia. Haematocrit also had a negative correlation thus dropping by 0.142 units for every unit increase in parasite density. MCH and MCHC also had negative correlations of − 0.240 and − 0.040, respectively. There was also a 0.142 reduction in platelet levels per every unit increase in parasite density (Table 4).
Linear regression between parasitaemia and haematological parameters
A regression analysis was performed to ascertain the relationship between selected haematological parameters (WBC, Hgb, HCT, MCH, MCHC, Platelet and RBC) and parasitaemia. The model had parasitaemia as the outcome variable and the various haematological parameters as predictor variables. A multiple R of 0.44 (correlation between the outcome and predictor variables) was obtained. This suggests a moderate degree of association. The R-squared of 0.195 implies that approximately 19.5% of variations in parasitaemia (outcome variable) can be explained by the selected Haematological parameters (predictor variables). However, the model’s adjusted R-squared value which explains variation in outcome variables caused by predictor variables while adjusting for the number of predictor variables was relatively low (0.125). This indicates that other unmeasured factors might also be influencing the outcome variable (Fig. 2).
An ANOVA was also performed to further understand the model’s fit and significance. The results of the test inferred that the regression model as a whole was statistically significant as evidenced by the F-statistic of 2.797 (p-value = 0.1117). This indicated that the predictor variables had a significant influence on predicting the outcome variable. The significant F-statistic and corresponding p-value supported the model's overall fit. Notwithstanding, only WBC and platelet counts showed statistically significant relationships with the outcome variable. The positive coefficient for WBC indicates that an elevation in WBC is associated with an increase in the outcome variable. In contrast, the negative Platelet coefficient indicates that a decrease in Platelet count is associated with a decline in the outcome variable. All the other predictor variables (Hgb, HCT, MCH, MCHC, and RBC) did not show statistically significant associations with parasitaemia.
Discussion
In this study eighty-nine malaria patients were recruited, and the majority of participants (61%) were females, and the rest (39%) were males. The average parasite count of the participants was 32,942.32584 parasites/µl with the lowest being 101 parasites/µl and the highest of 398,174 parasites/µl. The average parasite count of the males (42,000 parasites/µl) was 50% higher than that of their female counterparts (28,000 parasites/µl) although, the female participants outnumbered the males in the study. The age range of the participants was between 9 months and 78 years with the mean age being 26.15 years. The age with the highest parasitaemia was 12 while that with the lowest was 62. Also, most of the WBC counts were either within the normal range or lower than the reference but there were few cases of leukocytosis. The haemoglobin, platelet and other RBC indices also showed variations as parasitaemia increased. This clearly demonstrates that there is a relationship between the degree of parasitaemia and various haematological parameters.
After the analysis, correlations were established between parasitaemia and various haematological parameters. There was a positive correlation between parasitaemia and WBC count.
Eosinophils and basophils are the least numerous WBCs in circulation and often associated with allergic reactions thus this might be a reason for their negative correlation. Also, haemoglobin and RBC both had negative correlations as parasitaemia increased. This could be due to the fact that as the Plasmodium parasite resides in RBCs, their activities results in the lyses of the cells thus reducing their numbers leading to haemolytic anaemia in most malaria infection cases [20].
MCH and MCHC, which are dependent on RBC, Hb and Hct values also showed negative correlations. There was also a reduction in platelet levels per every unit increase in parasite density, which could be due to the immune and non-immune destruction of platelets during malaria infection. The exact course of platelet destruction is not known but some of the assumed mechanisms may include coagulation disturbances, sequestration in spleen, antibody mediated platelet destruction, oxidative stress, and the role of platelets as cofactors in triggering severe malaria [21].
Again, the regression relationship between RBC, Hb and MCH with parasite density were negative while that of MCHC and Hct were positive. However, these relationships were insignificant based on a two-tail t-test performed at 95% confidence level. The findings of this study are similar to that of Wang and Xing who posited that interactions between Plasmodium infested RBCs and normal RBCs can result in fluctuations in Hct [22]. Similarly, Maina et al. found that platelets, lymphocytes, eosinophils, red blood cell count and haemoglobin were significantly lower in malaria-infected children [23]. Again, Akhtar et al. also carried out a study where they showed that 70% of the patients with malaria had thrombocytopenia, 94% anaemia, 12% lymphopenia and 17% monocytosis [24]. The current study is also in line with the work of Al-Salahy et al. who found that neutrophils levels were significantly higher in cases of falciparum malaria in comparison to healthy normal subjects [25].
It is worthy to mention that red blood cells infected with P. falciparum malaria parasite are known to adhere to endothelial cells lining blood vessels [26]. This phenomenon, also known as sequestration is associated with a number of features seen in severe malaria infection such as cerebral malaria and pregnancy-associated malaria [27]. This adherence of infected red blood cells, occurs in small capillaries and post-capillary venules of specific organs such as the brain and lungs [28]. Indeed the sequestration of malaria parasite infected RBCs has been correlated with mechanical obstruction of blood flow in small blood vessels and vascular endothelial cell activation, which may lead to serious pathology [29]. In terms of parasitaemia, the sequestration may affect the level negatively causing less number of parasites to be seen in peripheral blood as compare with the actual number in the individual. Sequestration may, therefore, results in low levels of parasitaemia. A limitation worthy of mention is that, not many malaria cases were seen at the clinic during the period of the study and some of the patients were also not willing to take part in the study. However, the number of participant needed for the study was obtained.
Conclusion
The outcome of the study indicates that there exists a correlation between the various haematological parameters and degree of parasitaemia. However, upon performing two-tail t-test to determine the significance of the correlation at 95% and 99% confidence level, it was concluded that only WBC and MCH had a significant linear relationship (correlation) with the degree of parasitaemia at 95% and 99% confidence level, respectively. Regression analysis also showed that the variation in parasitaemia (dependent variable) resulted in changes in the various haematological parameters (independent variables). Howbeit, only the changes caused by WBC and PLT were significant at 95% confidence level in a two-tailed t-test.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- WBC:
-
White blood cell
- PLT:
-
Platelet
- MCH:
-
Mean cell haemoglobin
- RBC:
-
Red blood cell
- MCHC:
-
Mean cell haemoglobin concentration
- Hct:
-
Haematocrit
- Hb:
-
Haemoglobin
- WHO:
-
World Health Organization
- GPI:
-
Glucose phosphate isomerase
References
WHO. Malaria key facts - newsroom. Geneva, World Health Organization, 2022. https://www.who.int/news-room/fact-sheets/detail/malaria.
Editors. Malaria. Encyclopedia Britannica. 2022.
WHO. ‘About malaria’. Geneva, World Health Organization, 2018.
Bloland PB, Williams HA. Malaria control during mass population movements and natural disasters. Washington: National Academies Press; 2002.
UNICEF. Ghana Fact Sheet: malaria. July 2007 situation’, UNICEF Ghana. 2007.
Crutcher JM, Hoffman SL. Malaria. In: Baron S (ed.). Medical Microbiology. 4th Edn. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 83.
Olivier M, Van Den Ham K, Shio MT, Kassa FA, Fougeray S. Malarial pigment hemozoin and the innate inflammatory response. Front Immunol. 2014;5:25.
Mawson AR. The pathogenesis of malaria: a new perspective. Pathog Glob Health. 2013;107:122–9.
Price RN, Simpson JA, Nosten F. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65:614–22.
Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;26:11–6. https://doi.org/10.1017/s1462399409001082.
Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68:267–74.
Lathia TB, Joshi R. Can hematological parameters discriminate malaria from nonmalarious acute febrile illness in the tropics? Indian J Med Sci. 2004;58:239–44.
Desruisseaux MS, Fabiana SM, Louis MW, Herbert BT, Linnie MG. Cerebral malaria: a vasculopathy. Am J Pathol. 2010;3:1075–8.
Wilairatana P, Tangpukdee N, Krudsood S. Definition of hyperparasitemia in severe falciparum malaria should be updated. Asian Pac J Trop Biomed. 2013;3:586.
Bilal JA, Gasim GI, Karsani AH, Elbashir LM, Adam I. Malaria parasite density estimation using actual and assumed white blood cells count in children in Eastern Sudan. J Trop Pediatr. 2016;62:171–5.
Rodrigues-da-Silva RN, Lima-Junior JDC, Fonseca BDPF, Antas, PRZ, Baldez A, Storer FL, et al. Alterations in cytokines and haematological parameters during the acute and convalescent phases of Plasmodium falciparum and Plasmodium vivax infections. Mem Inst Oswaldo Cruz. 2014;154–62.
Kotepui M, Piwkham D, PhunPhuech B, Phiwklam N, Chupeerach C, Duangmano S. Effects of malaria parasite density on blood cell parameters. PLoS ONE. 2015;10: e0121057.
van Wolfswinkel ME, Vliegenthart-Jongbloed K, de Mendonça MM, Wever PC, McCall MB, Koelewijn R, et al. Predictive value of lymphocytopenia and the neutrophil-lymphocyte count ratio for severe imported malaria. Malar J. 2013;12:101.
Brooks GF, Carroll KC, Butel JS, Morse SA, Mietzner TA. Jawetz, Melnick &Adelberg’s Medical Microbiology, 26th Edn. New York, McGraw Hill Education,
Jandle JH. Hemolytic anemias caused by infection of red blood cells. In: Jandle JH (ed.). Blood: Textbook of Heamatology. Boston/Toronto. Little, Brown and Company. (2nd Edn). 1996:473–501.
Gupta NK, Bansal SB, Jain UC, Sahare K. Study of thrombocytopenia in patients of malaria. Trop Parasitol. 2013;3:58.
Wang T, Xing Z. Local hematocrit fluctuation induced by malaria-infected red blood cells and its effect on microflow. Biomed Res Int. 2018;2018:8065252.
Maina RN, Walsh D, Gaddy C, Hongo G, Waitumbi J, Otieno L, et al. Impact of Plasmodium falciparum infection on haematological parameters in children living in Western Kenya. Malar J. 2010;9(Suppl 3):S4.
Akhtar S, Gumashta R, Mahore S, Maimoon S. Hematological changes in malaria: a comparative study. IOSR-J Pharma Biol Sci. 2012;2:15–9.
Al-Salahy M, Shnawa B, Abed G, Mandour A, Al-Ezzi A. Parasitaemia and its relation to hematological parameters and liver function among patients malaria in Abs, Hajjah, Northwest Yemen. Interdiscip Perspect Infect Dis. 2016;2016:5954394.
Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–17.
Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–80.
Gamain B, Smith JD, Viebig NK, Gysin J, Scherf A. Pregnancy-associated malaria: parasite binding, natural immunity and vaccine development. Int J Parasitol. 2007;37:273–83.
Mishra SK, Newton CR. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009;5:189–98.
Acknowledgements
We are grateful to the directors and laboratory managers of the Mamprobi polyclinic for their assistance in carrying out this study.
Funding
The study was funded using the University of Ghana book and research allowances of the research team members. The University of Ghana did not play any role as far as the design of the study, collection, analysis, and interpretation of data as well as writing of the manuscript are concerned.
Author information
Authors and Affiliations
Contributions
SAB participated in the design, co-supervised the research, and drafted the manuscript. BTM participated in the design, co-supervised the work and proof read the manuscript. GJ participated in the design of the study and carried out the experimental work. DNOA carried out the data analysis and editing of the manuscript. SA-M/LA participated in the supervision of the work and proof reading of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was sought from the Ethics and Protocol Review Committee of the School of Biomedical and Allied Health Sciences, College of Health Sciences, University of Ghana (SBAHS-MLS/10575644/SA/2018-2019). The purpose of the study was thoroughly explained to participants, and it was made known to them that their information would be kept confidential, and they could withdraw their involvement in the study at any time. Only those who gave their informed consent were recruited. All information obtained was used for the purposes of this research only.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Antwi-Baffour, S., Mensah, B.T., Johnson, G. et al. Haematological parameters and their correlation with the degree of malaria parasitaemia among outpatients attending a polyclinic. Malar J 22, 281 (2023). https://doi.org/10.1186/s12936-023-04710-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04710-3