Abstract
Background
Attractive targeted sugar baits (ATSBs) control sugar-feeding mosquitoes with oral toxicants, and may effectively complement core malaria interventions, such as insecticide-treated nets even where pyrethroid-resistance is widespread. The technology is particularly efficacious in arid and semi-arid areas. However, their performance remains poorly-understood in tropical areas with year-round malaria transmission, and where the abundant vegetation constitutes competitive sugar sources for mosquitoes. This study compared the efficacies of ATSBs (active ingredient: 2% boric acid) in controlled settings with different vegetation densities.
Methods
Potted mosquito-friendly plants were introduced inside semi-field chambers (9.6 m by 9.6 m) to simulate densely-vegetated, sparsely-vegetated, and bare sites without any vegetation (two chambers/category). All chambers had volunteer-occupied huts. Laboratory-reared Anopheles arabiensis were released nightly (200/chamber) and host-seeking females recaptured using human landing catches outdoors (8.00 p.m.–9.00 p.m.) and CDC-light traps indoors (9.00 p.m.–6.00 a.m.). Additionally, resting mosquitoes were collected indoors and outdoors each morning using Prokopack aspirators. The experiments included a “before-and-after” set-up (with pre-ATSBs, ATSBs and post-ATSBs phases per chamber), and a “treatment vs. control” set-up (where similar chambers had ATSBs or no ATSBs). The experiments lasted 84 trap-nights.
Results
In the initial tests when all chambers had no vegetation, the ATSBs reduced outdoor-biting by 69.7%, indoor-biting by 79.8% and resting mosquitoes by 92.8%. In tests evaluating impact of vegetation, the efficacy of ATSBs against host-seeking mosquitoes was high in bare chambers (outdoors: 64.1% reduction; indoors: 46.8%) but modest or low in sparsely-vegetated (outdoors: 34.5%; indoors: 26.2%) and densely-vegetated chambers (outdoors: 25.4%; indoors: 16.1%). Against resting mosquitoes, the ATSBs performed modestly across settings (non-vegetated chambers: 37.5% outdoors and 38.7% indoors; sparsely-vegetated: 42.9% outdoors and 37.5% indoors; densely-vegetated: 45.5% outdoors and 37.5% indoors). Vegetation significantly reduced the ATSBs efficacies against outdoor-biting and indoor-biting mosquitoes but not resting mosquitoes.
Conclusion
While vegetation can influence the performance of ATSBs, the devices remain modestly efficacious in both sparsely-vegetated and densely-vegetated settings. Higher efficacies may occur in places with minimal or completely no vegetation, but such environments are naturally unlikely to sustain Anopheles populations or malaria transmission in the first place. Field studies therefore remain necessary to validate the efficacies of ATSBs in the tropics.
Similar content being viewed by others
Background
The scale-up of effective vector control tools, notably insecticide-treated nets (ITNs) and indoor residual spraying (IRS), coupled with effective case management and other measures have contributed to significant reductions of malaria burden in sub-Saharan Africa [1, 2]. Unfortunately, malaria control appears to be stagnating as many high-burden countries are reporting increased cases and deaths [3]. Many malaria programmes are recording diminishing returns amid a growing set of challenges, such as insecticide resistance, drug resistance and high commodity prices [4].
For vector control, there are also concerns of behavioral adaptations of mosquitoes, such as outdoor-biting and day-time biting [4,5,6]. These mosquito behaviours can overlap with human behaviours and activities to create new prevention gaps not effectively targetable with ITNs and IRS [7,8,9,10]. Continued progress towards malaria elimination in Africa, therefore, requires fundamental changes to current strategies, backed by improved financing and resource allocation as well as enhanced research into new transformative technologies [4, 11]. In particular, there is an urgent need for tools that remain effective against insecticide-resistant, outdoor-biting and day-time biting mosquitoes [12].
Attractive targeted sugar baits (ATSBs) are a promising new technology that is increasingly being investigated for expanding the malaria prevention toolbox [13,14,15,16,17]. The technology exploits the natural sugar-feeding behaviours essential for mosquito survival [18,19,20,21,22]. This means sugar-based solutions can be used to target mosquitoes by adding orally-ingested toxicants, which may include common pesticides, boric acid, and nucleic acids [23,24,25,26,27,28]. Other candidate toxicants include mosquitocidal drugs, such as ivermectin [29]. With any of the toxicants, ATSBs effectively target sugar-seeking male and female mosquitoes indoors or outdoors, thereby complementing the current primary interventions, such as ITNs even in areas where malaria vectors are resistant to pyrethroids [13]. The ATSBs could also be used to attract mosquitoes in order to feed them with peptides, drugs or micro-organisms that can block the development of parasites or viruses within mosquitoes to achieve paratransgenesis or refractoriness against pathogens [30, 31].
ATSBs can be deployed in different ways, e.g. by spraying on vegetation [15, 25] or suspending inside dwellings and around eave spaces [32]. However, the dominant and most scalable approach currently appears to be specially-designed membranes affixed to vertical surfaces such as outside walls of dwellings. In a large field trial in Mali, such ATSBs were particularly effective against older females of the malaria vectors and significantly impacted on malaria transmission [16]. In that trial, the proportion of females that had undergone at least three gonotrophic cycles was reduced by 97–100%; while the number of sporozoite-infected females was reduced by ~ 98% in the intervention villages compared to control villages [16].
Available evidence suggests that current ATSBs have generally been highly efficacious in arid and semi-arid regions [15, 16, 33]. However, there are doubts whether they would remain equally efficacious in tropical areas with year-round transmission, and where the abundant vegetation might constitute highly competitive sugar sources for mosquitoes. Given the current efforts to develop tools against outdoor-biting risk [12, 34], this concern should be investigated to identify critical points of improvement and to evaluate the performance of the ATSBs in the tropics. The aim of this current study was therefore to assess and compare the efficacy of outdoor ATSBs in settings with varying vegetation densities so as to inform further development, field-testing and deployment of ATSBs in different ecological systems in sub-Saharan Africa.
Methods
Semi-field systems
This study was conducted inside the semi-field chambers at the Mosquito City facility of Ifakara Health Institute, in south-eastern Tanzania (8.10800° S, 36.66585° E) [35, 36]. Six chambers were used (Fig. 1). Since these enclosed systems experience high daytime temperatures, a layer of grass thatching was added to partially cover each chamber so as to prevent excess heat-related mortality of mosquitoes. Adjacent chambers were also separated using plastic sheetings to reduce the effects of microclimatic modification in one chamber from affecting the others.
Plants
Mosquito-friendly plants were identified based on published records of feeding and survival rates by malaria vectors [19]. Those that were available in the nearby areas of Ulanga and Kilombero districts, Tanzania, were selected for inclusion (Table 1). The selected plant species were potted and transplanted to the semi-field chambers, then allowed to flourish for at least 3 weeks before the trials began. Those that could not be potted were planted directly in the chambers (Fig. 2). Additionally, selected fresh fruits were sliced open and added at the start of the experiments as described below.
Vegetation densities in different semi-field chambers. Each of the densities, i.e. densely-vegetated, sparsely-vegetated and bare(no vegetation) environments were provided in duplicates (two chambers per category). In the bare chamber, soil-filled pots without plants were included so that the available resting surfaces for mosquitoes were similar in all chambers
Vegetation densities in semi-field chambers
Two vegetation densities were considered. These included dense vegetation cover in two chambers and sparse vegetation cover in two other chambers. Two other chambers were left bare to simulate settings completely devoid of plants. To achieve the densities of densely-vegetated, sparsely-vegetated and bare chambers, the plants were provided in ratios of 50:5:0 for potted plants, 6:1:0 for directly transplanted vegetation, and 6:3:1 for cut fruit (Fig. 2). To ensure an equal resting surface for mosquitoes, the total number of pots was maintained at 50 per chamber, but those in the bare chamber contained only soil and no plants.
Attractive targeted sugar baits (ATSBs)
The candidate ATSBs contained 2% boric acid (active ingredient) prepared in a 10% brown sugar solution. A green food dye was added to enable visual identification of mosquitoes that fed on the ATSBs. Preliminary tests comparing different ATSBs before selecting the test product are provided in Additional file 1. The ATSBs were prepared in locally-made receptacles made of laterally-sliced bamboo stems (Fig. 3). The ATSB solution was poured into these sliced containers, and cotton wool soaked into it. Five bait stations were placed around the hut to maximize the feeding success, this configuration was done by slightly adapting configurations described in Mali by Diarra et al. [37].
Mosquitoes
Laboratory-reared 3–5 day old nulliparous adult females of Anopheles arabiensis were used. The mosquitoes were aspirated into small cages and starved for 9 h before being released. The mosquitoes were obtained from a common insectary maintained within the vector biology laboratory, the VectorSphere, at Ifakara Health Institute. This insectary is maintained under controlled conditions of 25–27 °C and 80% relative humidity. The larvae were fed on Tetramin® fish food and adults provided with a 10% sugar solution. A total of 200 mosquitoes were released in each chamber each evening at 6 p.m. and allowed to acclimatize in the environment for 2 h prior to the start of any recapturing. This was done in all chambers, regardless of the presence or absence of ATSBs, by gently opening and shaking the release cages so that no mosquito remained in the cages.
Mosquito collections
In all tests, the process of recapturing host-seeking mosquitoes began 2 h after the starved mosquitoes had been released into the chambers. Human landing catches (HLC) were used to recapture outdoor-biting mosquitoes from 8.00 p.m. to 9.00 p.m. each night, after which the volunteers entered the huts inside each chamber to sleep under intact untreated nets. Thereafter, CDC light traps set beside the volunteer-occupied bed net was used to collect indoor host-seeking mosquitoes from 9.00 p.m. to 6.00 a.m. the following morning. Each morning at 6.00 a.m., Prokopack aspirators were used to collect resting mosquitoes inside and outside the huts for a fixed 20 min each morning. At the end of each test night, the Prokopack aspirators were used to remove any mosquitoes remaining in the chambers as exhaustively as possible, as the chambers prepared for the next trap-night.
These mosquito collection procedures were maintained on all experimental nights and in all chambers regardless of presence or absence of ATSBs. To avoid biases in host-attractiveness, the volunteers were rotated between the experimental chambers each night. Each morning, all the recaptured mosquitoes (by HLC, CDC-light traps and Prokopack aspirators) were killed and their abdomen examined for evidence of having fed on ATSBs. In the initial tests, all the mosquitoes that had fed on the ATSBs died within 24 h (Additional file 1). Therefore all recaptured mosquitoes with ATSB markers in their abdomen were considered killed and out of circulation, thus included as dead.
Evaluating the efficacy of ATSBs
An initial test was conducted to evaluate the performance of ATSBs and to confirm their suitability for the main study. This experiment involved three chambers without vegetation and was conducted in two phases lasting a total of 24 consecutive nights, on a “before-and-after” set up. In the first phase, no ATSBs were used (16 nights; pre-intervention period), while in the second phase, the ATSBs were introduced in the same chambers (8 nights; intervention period).
The number of mosquitoes trapped by HLC (outdoor-biting densities), CDC-light traps (indoor-biting densities), Prokopack indoors (indoor-resting densities), and Prokopack outdoors (outdoor-resting densities) were compared between pre-intervention period (no ATSBs) and the intervention period (ATSBs added) to assess the efficacy of the ATSBs in each chamber.
Comparing the efficacy of ATSBs in chambers with dense, sparse and no vegetation
Six semi-field chambers were used, two with dense vegetation, two with sparse vegetation, and another two with no vegetation (Fig. 2). The experiment was conducted for 60 nights in six phases as illustrated in Fig. 4.
The first phase (pre-intervention phase) involved mosquito collections as described above but without any ATSBs in the chambers. This phase lasted 6 consecutive nights. In the second phase, ATSBs containing 2% borate were introduced into one of the two densely-vegetated chambers, one of the two sparsely vegetated chambers and one of the two bare chambers (five ATSBs per chamber, each suspended around the eave space as shown in Fig. 3). The other chambers were left without ATSBs to constitute contemporaneous controls (one densely-vegetated chamber, one sparsely vegetated chamber and one bare chamber). Mosquito collections with HLC, CDC light traps and Prokopack aspirators continued as above for 18 consecutive nights. The third phase was identical to the first phase since the ATSBs were removed from all chambers, and the mosquito collections continued for another six nights. In the fourth phase, for another six nights, ATSBs were added in all chambers so that there were no contemporaneous controls. A fifth phase was conducted for 18 nights consecutively and was the reverse of the second phase in that the chambers that had been assigned ATSBs became controls while those that had been controls now received ATSBs. In the final phase (sixth phase), the ATSBs were returned to all the chambers and the experiment continued for another six nights consecutively.
Based on this configuration, there were therefore 12 nights during which all the chambers were controls, 12 nights during which all the chambers had ATSBs and 36 nights during which half of the chambers (one densely-vegetated, one sparsely vegetated, and one bare) were controls while the other half had ATSBs. Therefore, in addition to assessing the impact of vegetation cover, the design also enabled valuation of the effects of introducing ATSBs into chambers that previously had none, as well as comparing similar chambers with and without the ATSBs.
Statistical analysis
Data analysis was done using the open-source statistical software, R version 3.6.2 [38]. The overall impact of ATSBs in chambers with different vegetation densities and chambers without vegetation was examined using data from each trapping method. This was done to understand effects on host-seeking mosquitoes outdoors (HLC catches), host-seeking mosquitoes indoors (CDC-Light trap catches), indoor-resting mosquitoes (Prokopack trap collections inside the huts) and outdoor-resting mosquitoes (Prokopack trap collections outside the huts). The analysis was done using generalized linear mixed effects models (GLMM), implemented using the lme4 package [39], following a binomial distribution. To assess effects of ATSBs, the number of mosquitoes recaptured was added as a response variable, while the intervention (ATSBs or no ATSBs) was added as a fixed factors. Volunteers ID, chamber ID, and experimental day were added as random terms in the models.
The efficacy of ATSBs was calculated as \(Efficacy=\frac{Control-Treatment}{Control}*100\), where “Control” was the number of mosquitoes recaptured in absence of ATSBs, and “Treatment” was number of mosquitoes recaptured in presence of ATSBs.
A separate set of GLMM models were used to compare performance of the ATSBs under different vegetation covers by considering data in the contemporaneous treatment and control chambers. Mosquito catches were used as response variable, while vegetation densities were added as fixed variable. Again, to account for any sampling biases and any unexplained variations between chambers and days, volunteers ID, chamber ID, and experimental day were added as random terms. Odds ratios and respective 95% confidence intervals were reported.
All graphs were created using the ggplot2 package in R [40]. Lastly, Chi-Square test of proportions was used to assess whether there was any significant difference between the percentage reduction of mosquito catches in densely-vegetated chambers compared to sparsely-vegetated chambers and chambers devoid of any vegetation.
Model selection for the inclusion of the random effects (volunteers ID, chamber ID, and experimental day) was done using Akaike Information Criterion (AIC), the model with the lowest AIC value was considered as a best model.
Results
Efficacy of ATSBs
In the initial tests conducted to assess baseline performance of the ATSBs in bare un-vegetated chambers, introduction of the baits reduced the number of outdoor-biting An. arabiensis females (as measured using HLC) by 69.7% (95% CI 68.3–71.0%). In the same tests, the indoor-biting densities (as measured using CDC light traps) were reduced by 79.8% (78.9–81.1%) and the overall resting densities indoors and outdoors by 92.8% (91.6–94.6%). Full details of the findings are summarized in Table 2; Fig. 5.
Performance of the attractive targeted sugar bait (ATSBs), when tested in bare chambers devoid of any vegetation. The Figures show the number of mosquitoes recaptured with CDC light traps, human landing catches (HLC) and Prokopack aspirators with or without ATSBs. The red dots represents the mean and the black dots represents daily collections
Efficacy of ATSBs in semi-field chambers with dense, sparse and no vegetation
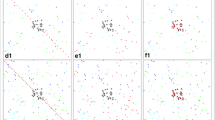
The introduction of the ATSBs significantly reduced the number of female An. arabiensis mosquitoes attempting to bite volunteers outdoors in all chambers, but mostly in the chambers with no vegetation. The catches were reduced by 25.4% (95% CI 24.2–26.6%) in densely-vegetated chambers by 34.5% (31.5–37.6%) in sparsely-vegetated chambers and by 64.1% (57.0–71.2%) in bare chambers without any vegetation (Fig. 6; Table 3). Chi-square tests conducted on the outdoor-biting data showed a significant difference in the impacts of ATSBs between chambers with the different vegetation densities (χ2 = 346.5, p < 0.001), suggesting that vegetation cover reduced the efficacies of ATSBs against mosquitoes biting outdoors (Table 4).
Comparative performance of the attractive targeted sugar baits (ATSBs) in chambers with dense, sparse, or bare (no vegetation). Figures show the number of mosquitoes recaptured with CDC light traps, human landing catches (HLC) and Prokopack aspirators with or without ATSBs. The red dots represent the mean and the black dots represents daily collections
The number of female An. arabiensis mosquitoes caught attempting to bite the volunteer while sleeping under the bed net indoors (i.e. indoor-biting risk as measured by CDC light traps) were reduced by 16.1% (95% CI 15.9–16.9%) in densely-vegetated chambers, 26.2% (24.5–28.5%) in sparsely-vegetated chambers and 46.8% (39.7–55.1%) in bare chambers with no vegetation (Fig. 6; Table 3). Here also, the Chi-square analysis showed that there was a significant difference of the impact of ATSBs between chambers with different vegetation densities (χ2 = 33.12, p < 0.001), again suggesting that vegetation cover reduced the efficacies of ATSBs against mosquitoes biting indoors (Table 4).
Lastly, the ATSBs also reduced the densities of resting mosquitoes (collected using Prokopack aspirators). The outdoor-resting densities were reduced by 45.5% (95% CI 43.9–45.0%) in the densely-vegetated chambers, by 42.9% (40.9–43.6%) in the sparsely vegetated chambers, and 37.5% (36.7–38.5%) in the chambers without any vegetation. Data obtained from the HLC catches, appeared to show a bimodal distribution though the differences between intervention and control arms were still readily detectable (Fig. 6; Table 3). However there was no difference in the efficacy of the ATSBs between the chambers with different vegetation densities (χ2 = 1.97, p = 0.37). Similarly, the indoor-resting densities were reduced, in this case by 37.5% (95% CI 36.0–38.7%) in chambers with dense vegetation, 37.5% (36.4–38.7%) in chambers with sparse vegetation, and 38.7% (36.4–41.2%) in bare chambers without vegetation. Here too, there was no differences in impact between the chambers (χ2 = 0.15, p = 0.93) (see Table 4). Vegetation did not affect the efficacy of ATSBs against resting mosquitoes, either indoors or outdoors (Table 4). Overall, the total number of mosquitoes collected while resting was 3.1-fold lower than the numbers caught while host-seeking (i.e. by HLC and CDC-light traps).
Discussion
Mosquitoes feed primarily on natural sugar sources like plant leaves, nectaries, and honeydew, a behaviour that can be exploited to target both their male and female adults. The use of ATSBs has increasingly been demonstrated to effectively control malaria vectors, mostly in dry and semi-arid regions [15, 25, 32]. The technology has the added advantage that it can be used against both indoor-biting and outdoor-biting mosquitoes, including those that are active during the day. Moreover, by relying on oral insecticides, it offers a different mode of activity compared to currently approved vector control insecticides, many of which act on contact with insect cuticle [41]. Given these attributes, ATSBs could be highly effective in addressing current limitations of core malaria interventions such as ITNs and IRS even in areas where mosquitoes are resistant to pyrethroids, the commonest pesticide class used on ITNs.
This current study investigated the concern that this technology may not be equally efficacious in highly-vegetated settings where there would be numerous natural sugar sources, potentially competing with the ATSBs for the same mosquitoes. The large screened cages available at the Ifakara Health Institute’s Mosquito City facility [35, 36] provided a functional mesocosm to evaluate the effects of vegetation densities and compare the performance of ATSBs under controlled environments. Such an objective would otherwise require geographically-extensive and expensive trials to accomplish. Whereas such semi-field systems do not fully replace natural settings, they can enable direct and rapid assessments of important mosquito life cycle traits such as blood-feeding, sugar-feeding, mating and resting behaviours [35, 42, 43], as well as genetic and phenotypic variabilities [44]. These systems can also enable controlled assessments of the performance of new and existing vector control interventions. In previous studies, they have been used to test ITNs, zooprophylaxis, and endectocides [36], spatial repellents [45] and mosquito-assisted larviciding [46] and eave tubes [47].
In this study, the semi-field systems were used to simulate areas with varying vegetation densities, i.e. densely-vegetated, sparsely-vegetated and bare grounds with no vegetation, to enable comparison of the efficacy of ATSBs. The main findings were as follows: (i) the efficacy of ATSBs against indoor or outdoor-biting mosquitoes was highest in areas devoid of any vegetation; (ii) while there was a significant effect of varying vegetation densities on the performance of ATSBs, the devices still provided modest protection in both densely-vegetated and sparsely-vegetated settings; and (iii) the vegetation-dependent differences in the efficacy of ATSBs were observed for host-seeking mosquitoes (indoors and outdoors) but not resting mosquitoes due to low recaptures, against which the ATSBs performed modestly regardless of vegetation cover. Collectively, these findings suggest that ATSBs may remain modestly efficacious even in tropical countries where there is moderate to dense vegetation.
Mathematical evaluations of the potential impact of ATSBs have previously shown that even with modest mortality rates imparted by ATSBs on mosquito populations, the overall impact on malaria infections may be substantial [48, 49]. The modest efficacies observed in this study may therefore be indicative of greater potential and should be validated in field trials in different settings; for example through observational or randomized controlled trials. The observed differential efficacies in vegetated compared to non-vegetated settings suggest that natural sugar sources could indeed compete with the ATSBs, though such competition is unlikely to render the ATSBs fully ineffective.
Published evidence suggests that the modest efficacies observed for ATSBs are not uncommon. In the large scale Mali trial by Traore et al., the application of ATSBs was associated with 26.3% reduction in human-biting densities as measured by HLC, even though the baits appeared to preferentially kill older mosquitoes [16]. This trial also indicated that reduction of densities as measured by other trap types did not exceed 60% during the wet season. In the sub-tropical environments of Florida, Qualls et al. [50] evaluated ATSBs in a location surrounded by pine forests, wetlands and large ponds and observed more than 50% lower densities of Anopheles crucians for more 3 weeks. Similarly, Müller et al., showed in a small oasis full of vegetation and small freshwater springs in a desert area that ATSBs reduced the catches of Anopheles sergentii by 39% [51]. Interestingly, far higher efficacies, such as those observed in the initial experiments in this current study (Table 3; Fig. 5), have also been observed in field studies. For example, Beier et al. [33] applied ATSBs in areas with sugar-poor and sugar-rich oases and demonstrated that availability of local plants could delay but not compromise the technology; in this case they reduced the densities of An. sergentii over 95%.
Another finding from this study was that the reduction in mosquito densities was highest in non-vegetated settings (Table 1), a finding potentially attributed to limited sugar sources [33]. However, the high performance of ATSBs in chambers devoid of any vegetation may not be of any practical implications since such environments would generally be unlikely to sustain natural Anopheles populations nor malaria transmission; thus they may not be priority settings for the deployment of ATSBs. In the field studies in Mali by Traore et al. [16], the populations reduction, despite being modest in the wet seasons, exceeded 70% during the dry season for both male and female mosquitoes. Given that plant life is lower in dry seasons than wet seasons, this finding partially matches our observations that ATSBs were most efficacious in non-vegetated followed by sparsely-vegetated then densely-vegetated settings.
It was also interesting to observe that the efficacy against resting mosquitoes was similar across all vegetation densities assessed, even though there had been clear differences when considering host-seeking mosquitoes. This lack of statistically-significant differences is partly due to the lower densities of resting mosquitoes collected compared to the densities caught by HLC and CDC light traps. Overall, there were fewer mosquitoes collected resting compared to those collected host-seeking indoors and outdoors. The observations of ATSBs retaining high efficacy despite competing plants and flowers have also previously been recorded in Mali in a semi arid region, where the relative abundance of both male and female Anopheles gambiae declined by 90% [15]. One question is whether the concentration of active ingredient can be varied to maintain high efficacies in highly-vegetated settings. Previous bioassays in mosquito cages, the density of An. gambiae killed by boric acid were observed to increase with increasing concentrations of boric acid [14]. Future studies should therefore also assess the possibility that any negative impacts of vegetation on the performance of ATSBs can be reversed by increasing the concentration of the active ingredient.
The application of ATSBs outdoors, as done in this study, appears to have had implications for both indoor and outdoor-densities of mosquitoes. Mosquito densities were also reduced indoors across all vegetation covers (Tables 2 and 3). In earlier studies by Müller et al. in Mali [25], where the toxic baits were deployed near larval habitats, the mosquito densities declined by up to 94% [25]. Similar impacts were observed in Tanzania where ATSBs were applied to places near rice fields [13]. Separately, in a field study in Mali covering fourteen villages, half of which received ATSBs outdoors, it was observed that mosquito densities indoors (measured by CDC light traps) also declined by 57% [49].
To the best of our knowledge, this is the first study to directly assess the impact of vegetation densities on the performance of ATSBs. While the objectives were broadly achieved, a number of limitations are noted. Notably, the vegetation categories and plant ratios (dense, sparse and bare) were broadly arbitrary and might not be fully representative of all malaria-endemic communities. Furthermore, the candidate plants used here were those which had been previously shown to be preferred by mosquitoes [19]. This study did not include any plants with repellent properties, which might also have had an influence on the efficacy of the ATSBs. Another limitation is that we used a fixed number of bait stations, given that increasing number of bait stations can increase the feeding rate [37], future studies and deployment may need to optimize the ratio of ATSBs/household or ATSBs for every given area to maximize potential.
There were some limitations regarding the methods of used. In particular, the vegetation densities were not rotated and instead this study combined a before-and-after design with a “intervention vs. control” comparison. The main reason for repeating the phases was to account for any variation caused by nightly weather changes, as random assignment in such a limited setting could potentially miss such variations and hinder the assessments of ATSBs efficacy. Besides, since some of the larger plants were actually planted in the chambers, rather than being potted, it was not possible to rotate the independent variable (vegetation densities). Instead, it became necessary to rotate the interventions between the chambers with similar vegetation covers. Moreover, since there were only six chambers available in total, the maximum number that could be assigned to any vegetation density was only two. To address these limitations, the study used multiple repeated measures in each semi-field chamber over a total of 84 nights, each time ensuring an appropriate set of controls. Broadly however, it should be noted that these systems remain limited and that such as study would be more realistic if they were: (a) conducted in real field settings in multiple villages having different vegetation densities over multiple seasons, (b) include a placebo treatment for the control arms, as well as appropriate blinding of volunteers to the study phases and interventions and (c) include enhanced statistical evaluations to better fit the data distribution, e.g. bimodal distributions in the HLC catches illustrated in Fig. 5.
Secondly, it is possible that ATSBs may affect the natural mosquito foraging behaviours, and change the proportions foraging outdoors or indoors. In this study, the ATSBs were located at the eaves level and may potentially have caused a shift between indoor to outdoor feeding proportions. Unfortunately, since there were no comparative trapping observations between the indoor and outdoor locations, it was not possible to evaluate such effects in this study. Lastly, since this study was conducted for only An. arabiensis, further studies might focus on other species, such as Anopheles funestus, An. gambiae and Anopheles coluzzii, which are also major vectors in Africa.
Conclusion
Attractive targeted sugar baits control sugar-seeking mosquitoes with oral toxicants and can be used both indoors or outdoors. This study has demonstrated that while vegetation densities can indeed influence the performance of ATSBs, the technology is likely to be at least modestly efficacious in sites with varying vegetation densities including sparsely-vegetated and densely-vegetated settings. It is likely that higher efficacies could be achieved in places with completely no vegetation, but such settings are naturally unlikely to sustain Anopheles mosquitoes nor malaria transmission locally. Additional field studies therefore remain necessary to validate performance of ATSBs in different settings in the tropics. Such studies should also consider investigating the efficacy ATSBs applied indoors and outdoors.
Availability of data and materials
Data are available at Ifakara Health Institute data for sharing upon request.
Abbreviations
- ATSBs:
-
Attractive targeted sugar baits
- IHI:
-
Ifakara Health Institute
- IRB:
-
Institutional Review Board
- ITNs:
-
Insecticide Treated Bed Nets
- IMR:
-
National Institute for Medical Research
- CDC:
-
Centre for disease control and prevention
- HLC:
-
Human landing catch
- IRS:
-
Indoor residual spraying
References
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Eisele TP, Larsen DA, Walker N, Cibulskis RE, Yukich JO, Zikusooka CM, et al. Estimates of child deaths prevented from malaria prevention scale-up in Africa 2001–2010. Malar J. 2012;11:93.
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
Cohen AJM, Okumu F, Moonen B. The malaria fight’s diminishing gains and growing challenges. Sci Transl Med. 2022;14:eabn3256.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80.
Sangbakembi-Ngounou C, Costantini C, Longo-Pendy NM, Ngoagouni C, Akone-Ella O, Rahola N, et al. Diurnal biting of malaria mosquitoes in the central African Republic indicates residual transmission may be “out of control.” Proc Natl Acad Sci USA. 2022;119:e2104282119.
Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–95.
Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE. 2019;14:e0217414.
Monroe A, Moore S, Koenker H, Lynch M, Ricotta E. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in sub-saharan Africa: a review of the published literature. Malar J. 2019;18:6.
Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes—new insights into Malar vectors. London: IntechOpen; 2013. p. 671–704.
Okumu F, Gyapong M, Casamitjana N, Castro MC, Itoe MA, Okonofua F, et al. What Africa can do to accelerate and sustain progress against malaria. PLoS Glob Public Health. 2022;2:e0000262.
Sougoufara S, Ottih EC, Tripet F. The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasites Vectors. 2020;13:295.
Stewart ZP, Oxborough RM, Tungu PK, Kirby MJ, Rowland MW, Irish SR. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS ONE. 2013;8:e84168.
Furnival-Adams JEC, Camara S, Rowland M, Koffi AA, Ahoua Alou LP, Oumbouke WA, et al. Indoor use of attractive toxic sugar bait in combination with long-lasting insecticidal net against pyrethroid-resistant Anopheles gambiae: an experimental hut trial in Mbé, central Côte d’Ivoire. Malar J. 2020;19:11.
Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 2010;9:210.
Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar J. 2020;19:72.
Williams YA, Tusting LS, Hocini S, Graves PM, Killeen GF, Kleinschmidt I, et al. Expanding the vector control toolbox for malaria elimination: a systematic review of the evidence. Adv Parasitol. 2018;99:345–79.
Gary RE, Foster WA. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Med Vet Entomol. 2004;18:102–7.
Impoinvil DE, Kongere JO, Foster WA, Njiru BN, Killeen GF, Githure JI, et al. Feeding and survival of the malaria vector gambiae on plants growing in Kenya. Med Vet Entomol. 2004;18:108–15.
Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443–74.
Manda H, Gouagna LC, Nyandat E, Kabiru EW, Jackson RR, Foster WA, et al. Discriminative feeding behaviour of Anopheles gambiae s.s on endemic plants in western Kenya. Med Vet Entomol. 2007;21:103–11.
Vrzal EM, Allan SA, Hahn DA. Amino acids in nectar enhance longevity of female Culex quinquefasciatus mosquitoes. J Insect Physiol. 2010;56:1659–64.
Schlein Y, Müller GC. An approach to mosquito control: using the dominant attraction of flowering Tamarix jordanis trees against Culex pipiens. J Med Entomol. 2008;45:384–90.
Müller GC, Junnila A, Qualls W, Revay EE, Kline DL, Allan S, et al. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. Med Vet Entomol. 2010;24:346–51.
Müller AGC, Junnila A, Schlein Y. Effective control of adult Culex pipiens by spraying an attractive toxic sugar bait solution in the vegetation near larval habitats. J Med Entomol. 2010;47:63–6.
Schlein Y, Müller GC. Diurnal resting behavior of adult Culex pipiens in an arid habitat in Israel and possible control measurements with toxic sugar baits. Acta Trop. 2012;124:48–53.
Junnila A, Revay EE, Müller GC, Kravchenko V, Qualls WA, de Xue R, et al. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015;152:195–200.
Revay EE, Schlein Y, Tsabari O, Kravchenko V, Qualls W, De-Xue R, et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA-exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta Trop. 2015;150:29–34.
Tenywa FC, Kambagha A, Saddler A, Maia MF. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. Malar J. 2017;16:338.
Bilgo E, Vantaux A, Sanon A, Ilboudo S, Dabiré RK, Jacobs-Lorena M, et al. Field assessment of potential sugar feeding stations for disseminating bacteria in a paratransgenic approach to control malaria. Malar J. 2018;17:367.
Riehle MA, Jacobs-Lorena M. Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochem Mol Biol. 2005;35:699–707.
Qualls WA, Müller GC, Traore SF, Traore MM, Arheart KL, Doumbia S, et al. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar J. 2015;14:301.
Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar J. 2012;11:31.
Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184.
Ferguson HM, Ng KR, Walder T, Kadungula D, Moore SJ, Lyimo I, et al. Establishment of a large semi-field system for experimental study of african malaria vector ecology and control in Tanzania. Malar J. 2008;7:158.
Ng’habi K, Viana M, Matthiopoulos J, Lyimo I, Killeen G, Ferguson HM. Mesocosm experiments reveal the impact of mosquito control measures on malaria vector life history and population dynamics. Sci Rep. 2018;8:13949.
Diarra RA, Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, et al. Testing configurations of attractive toxic sugar bait (ATSB) stations in Mali, West Africa, for improving the control of malaria parasite transmission by vector mosquitoes and minimizing their effect on non-target insects. Malar J. 2021;20:184.
R Core Team. R: a language and environment for statistical computing. Vienna, Australia; 2019.
Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2016.
WHO. Prequalification vector control: prequalified lists of vector control products. Geneva: World Health Organization; 2022. https://extranet.who.int/pqweb/vector-control-products.
Nambunga IH, Msugupakulya BJ, Hape EE, Mshani IH, Kahamba NF, Mkandawile G, et al. Wild populations of malaria vectors can mate both inside and outside human dwellings. Parasites Vectors. 2021;14:514.
Knols BGJ, Njiru BN, Mathenge EM, Mukabana WR, Beier JC, Killeen GF. MalariaSphere: a greenhouse-enclosed simulation of a natural Anopheles gambiae (Diptera: Culicidae) ecosystem in western Kenya. Malar J. 2002;1:19.
Ng’habi KR, Lee Y, Knols BGJ, Mwasheshi D, Lanzaro GC, Ferguson HM. Colonization of malaria vectors under semi-field conditions as a strategy for maintaining genetic and phenotypic similarity with wild populations. Malar J. 2015;14:10.
Mmbando AS, Ngowo H, Limwagu A, Kilalangongono M, Kifungo K, Okumu FO. Eave ribbons treated with the spatial repellent, transfluthrin, can effectively protect against indoor-biting and outdoor-biting malaria mosquitoes. Malar J. 2018;17:368.
Lwetoijera D, Kiware S, Okumu F, Devine GJ, Majambere S. Autodissemination of pyriproxyfen suppresses stable populations of Anopheles arabiensis under semi-controlled settings. Malar J. 2019;18:166.
Sternberg ED, Ng’Habi KR, Lyimo IN, Kessy ST, Farenhorst M, Thomas MB, et al. Eave tubes for malaria control in Africa: initial development and semi-field evaluations in Tanzania. Malar J. 2016;15:447.
Marshall JM, White MT, Ghani AC, Schlein Y, Muller GC, Beier JC. Quantifying the mosquito’s sweet tooth: modelling the effectiveness of attractive toxic sugar baits (ATSB) for malaria vector control. Malar J. 2013;12:291.
Fraser KJ, Mwandigha L, Traore SF, Traore MM, Doumbia S, Junnila A, et al. Estimating the potential impact of attractive targeted Sugar baits (ATSBs) as a new vector control tool for Plasmodium falciparum malaria. Malar J. 2021;20:151.
Qualls WA, Müller GC, Revay EE, Allan SA, Arheart KL, Beier JC, et al. Evaluation of attractive toxic sugar bait (ATSB)—barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in sub-tropical environments in Florida. Acta Trop. 2014;131:104–10.
Müller GC, Kravchenko VD, Schlein Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive toxic (spinosad) sugar bait stations in an oasis. J Am Mosq Control Assoc. 2008;24:147–9.
Acknowledgements
We are grateful for all volunteers who assisted in data collection for the whole duration of the study and the insectary staff who supported mosquito production.
Funding
This work was supported by Howard Hughes Medical Institute (HHMI)-Gates Foundation International Scholars Award (Grant: OPP1099295) and the Bill and Melinda Gates Foundation (Grant: INV-002138).
Author information
Authors and Affiliations
Contributions
LLM, FOO conceptualized the idea, designed the study, and supervised the experiments, LLM analyzed the data and drafted the manuscript; FCM, JBM, NFK, EWK, and HSN helped in data analysis and supervision. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by Ifakara Health Institute Review Board (Ref: IHI/IRB/No: 007-2018) and the Medical Research Coordinating Committee (MRCC) at the National Institute for Medical Research-NIMR (Ref: NIMR/HQ/R.8a/VOL IX/2895). Individual verbal and written consent was also obtained from volunteers.
Consent for publication
This manuscript has been approved for publication by the Institute for Medical Research of the United Republic of Tanzania (Ref. No: NIMR/HQ/P.12 VOL XXXV/37).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
A additional file to provide details on preliminary studies done prior to and in support of the main study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Muyaga, L.L., Meza, F.C., Kahamba, N.F. et al. Effects of vegetation densities on the performance of attractive targeted sugar baits (ATSBs) for malaria vector control: a semi-field study. Malar J 22, 190 (2023). https://doi.org/10.1186/s12936-023-04625-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04625-z