Abstract
Background
Over the past decade, the incidence of malaria has steadily declined in Myanmar, with Plasmodium vivax becoming predominant. The resilience of P. vivax to malaria control is attributed to the parasite’s ability to form hypnozoites in the host’s liver, which can cause relapse. Primaquine is used to eliminate hypnozoites but can cause haemolysis in glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals. It is thus necessary to estimate the frequency and variant types of G6PD deficiency in areas where primaquine will be widely used for P. vivax elimination.
Methods
In this study, a descriptive cross-sectional survey was conducted to determine the prevalence of G6PD deficiency in a population residing in Nay Pyi Taw, Myanmar, using a standard spectrophotometric assay, a rapid diagnostic test (RDT), Biosensor, and by genotyping G6PD variants.
Results
G6PD enzyme activity was determined from 772 leukocyte-depleted samples, with an adjusted male median G6PD activity value of 6.3 U/g haemoglobin. Using a cut-off value of 30% enzyme activity, the overall prevalence of G6PD deficiency was 10.8%. Genotyping of G6PD variants was performed for 536 samples, of which 131 contained mutations. The Mahidol variant comprised the majority, and males with the Mahidol variant showed lower G6PD enzyme activity. The G6PD Andalus variant, which has not been reported in Myanmar before, was also identified in this study.
Conclusion
This study provides a G6PD enzyme activity reference value for the Myanmar population and further information on the prevalence and variants of G6PD deficiency among the Myanmar population; it also evaluates the feasibility of G6PD deficiency tests.
Similar content being viewed by others
Background
The essential housekeeping enzyme, glucose-6-phosphate dehydrogenase (G6PD), plays an important role in the functioning of red blood cells (RBCs). It is located on the X chromosome with a high genetic diversity [1]. G6PD enzyme produces NADPH [a reduced form of nicotinamide adenine dinucleotide phosphate). Infections, such as hepatitis B, the consumption of some foods, such as fava beans, and the administration of some drugs, such as primaquine, can produce free radicals (reactive oxygen species (ROS)]. NADPH can compensate for these free radicals and protect RBCs from oxidative damage [2]. Thus, G6PD activity and the production of NADPH are vital for protecting cells from oxidative stress. While other cell types possess other enzymes producing NADPH, G6PD is the only enzyme in RBCs that produce NADPH. Therefore, RBCs cannot generate G6PD and G6PD deficiency in RBCs can lead to haemolysis [3]. Among the haematological diseases, G6PD deficiency is the most common disorder [4]. Over 400 million people suffer from G6PD deficiency worldwide, which is especially common in malaria-endemic regions [5]. In some parts of Myanmar, the prevalence of G6PD deficiency was 11.0%, according to a survey conducted in 1999–2003 [6]. By DNA analysis, people living in Shan State, Myanmar, had a high prevalence of G6PD deficiency, where 17.5% carried the G6PD Mahidol variant, with 11.8% in males and 21.0% in females [7]. Likewise, in Kachin State, G6PD deficiency was almost 20% [8]. To date, 185 G6PD single nucleotide polymorphism (SNP) sites have been described, with over 400 variants identified, most being single base changes [4]. These variants were mostly identified by biochemical analysis, based on the residual enzyme activity [2]. Males are hemizygous with only one X chromosome and can be either G6PD normal or deficient. However, a G6PD-deficient allele in females can be heterozygous and homozygous. Because of X chromosome inactivation, RBCs in a G6PD heterozygous female are mosaic, with one part G6PD-normal and the other G6PD-deficient, producing variable G6PD phenotypes. Generally, G6PD-heterozygous females exhibit less severe clinical symptoms than males [9].
G6PD deficiency can be diagnosed by qualitative, quantitative, and molecular methods. For a definitive diagnosis of G6PD deficiency, a quantitative analysis by a spectrophotometric assay is mandatory and recognized as the reference test [10]. However, this technique requires advanced laboratory settings and skillful technicians. In addition, the diagnosis of G6PD deficiency by spectrophotometry is poorly standardized and a definition of 100% activity needs to be established for each site and assayed again. Under field conditions, simple point-of-care tests that can qualitatively or quantitatively define the G6PD status of the patients, such as G6PD rapid diagnostic tests (RDTs) or biosensors, are desired. The CareStart™ G6PD RDT test showed good sensitivity and specificity when compared with the standard quantitative assay when diagnosing G6PD deficiency [11]. The CareStart™ G6PD Biosensor is a device that can measure the level of G6PD enzyme activity in U/dL. This hand-held device works like a glucometer and can identify heterozygous females with intermediate G6PD activity based on enzyme level. To obtain the appropriate G6PD enzyme activity in U/gHb, the G6PD activity obtained using the CareStart™ G6PD Biosensor requires further normalization with haemoglobin level. However, this device has not yet been validated with a haemoglobin reader in Myanmar.
Myanmar carries the heaviest malaria burden in the Greater Mekong Subregion (GMS) and plans to eliminate malaria by 2030 [12]. The predominance of Plasmodium vivax malaria presents a major challenge to malaria elimination. Primaquine mass drug administration (MDA) has been a strategy successfully deployed in vivax-endemic areas [13]. However, this strategy requires a good understanding of the G6PD deficiency status in the target region. A critical issue for malaria control in Myanmar is that of mobile and remote populations, such as military personnel, who are exposed to a higher risk of malaria infection. Therefore, this study aimed to determine the prevalence of G6PD deficiency among military populations and their families. This study also aimed to appraise the efficacy of both the qualitative CareStart™ G6PD RDT and the quantitative Biosensor, with a standard spectrophotometry assay used as a comparative measure. The prevalence of the common G6PD variants in the study population was also assessed using a molecular technique.
Methods
Population and sample collection
A cross-sectional survey was conducted in Nay Pyi Taw, Myanmar. A total of 772 blood samples were collected from military personnel and their families residing in military camps in Tatkon (160 samples), Ottara Thiri (332 samples), and Zayar Thiri (280 samples) (Fig. 1). The Human Subjects protocol for this study was approved by the Ethical Review Committee of the Faculty of Tropical Medicine, Mahidol University, Thailand (MUTM2016-036-01), and the Institutional Review Board of the Defence Services Medical Research Centre (DSMRC), Myanmar (IRB/2016/60). Written informed consent was obtained before blood collection. Three millilitres of blood were obtained by venipuncture and collected in an EDTA tube. Blood was stored in a cooler box with ice packs during transportation from the sites to the DSMRC laboratory. Samples were processed for G6PD testing within 24 h after blood collection. An aliquot of the blood was spotted on Whatman 903 paper for later analysis of G6PD mutations.
Determination of G6PD enzyme activity
The G6PD enzyme activity was determined using two quantitative assays (a spectrophotometry assay and a Biosensor assay). The qualitative G6PD test was performed with the CareStart™ G6PD RDT (Access Bio).
Spectrophotometry
G6PD enzyme activity was determined by Randox Glucose-6-Phosphate Dehydrogenase-PD410 reagent kit (Randox Laboratories Ltd., UK) according to the manufacturer’s protocol with some modifications, and analysed by Microlab 300 semi-automatic biochemistry analyzer (Randox Monza, Ireland). Briefly, whole blood was centrifuged at 1000 x g for 10 min to remove plasma. Packed blood was washed 3 times with 2 ml of cold 0.9% NaCl solution to remove the buffy coat. One hundred microlitres of packed blood were mixed with 0.5 ml lysis buffer and incubated at 4 °C for 15 min. The sample was centrifuged at 1000 x g for 10 min to collect the haemolysate. The assay was performed by mixing 50 µl of haemolysate with the reaction solution (3 ml of R1 and 100 µl of R2 solution) with incubation at 37 °C for 5 min. Immediately after adding 50 µl of substrate solution (R3 solution), initial optical density (OD) was read at 340 nm at 37 °C, with three repeated measurements at 1 min intervals. Two control samples, normal and deficient (Randox), were analysed before running the test samples and repeated every 50 tests. The results were considered valid if the control values fell within the range provided by the company. Haemoglobin (Hb) concentration was determined using a haematology analyzer (ABX Micros ES 60, France). Enzyme activity (U/L) was adjusted by the haemoglobin value to generate the standard unit of G6PD activity (Unit per gram of Hb, U/g Hb).
Biosensor
A CareStart™ G6PD analyzer (Access Bio, Korea) was used to quantify G6PD activity. After insertion of the test strip into the G6PD analyzer, the test strip window was filled with 10 µl of whole blood. Enzyme activity (U/dl) was adjusted by the Hb value obtained from the haematology analyzer to generate G6PD enzyme activity (U/g Hb).
G6PD RDT
The CareStart™ G6PD RDT (Access Bio, Korea) was used to analyze G6PD activity qualitatively according to the manufacturer’s procedure. In brief, 10 µl of whole blood sample was added to the sample well, followed by two drops of assay buffer in the buffer well. The result was read in 10 min when a distinct purple colour appeared in the result window indicating normal G6PD, or very faint or no colour indicating G6PD deficiency.
Determination of G6PD mutations by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP)
DNA extraction was performed using the Chelex method according to the manufacturer’s procedure. Hb was released by incubating the blood filter with 1 ml of 0.5% saponin-PBS solution in a 1.5 ml microcentrifuge tube overnight at room temperature. After removing the brownish-red solution, the blood filter was washed once with 1 ml of 0.5% saponin-PBS solution. After discarding the supernatant, 200 µl of hot Chelex solution was added. The mixture was vortexed immediately and boiled for 5 min. The supernatant containing DNA was collected in a clean new tube.
The common G6PD mutations, including G6PD Mahidol, Viangchan, Chinese-4, Chinese-5, Kaiping, Canton, Union, and Mediterranean variants, were genotyped according to the established PCR-RFLP method [14]. In a 25 µl reaction, 10 µl of extracted DNA sample was mixed with 12.5 µl of GoTagGreen Master Mix and 1 µl of 10 µM forward and reverse primers. Amplification was performed on a Mastercycler nexus GSX1 (Eppendorf), with first denaturation at 95 ℃ for 5 min, followed by the PCR cycling conditions described in Additional file 1: Table S1. The final extension was performed at 72 ℃ for 10 min. Ten microlitres of the PCR product were digested at 37 ℃ with 10 units of appropriate restriction enzymes (New England Biolabs) in a 25 µl reaction for 2 h. The DNA bands were separated and visualized on 3% agarose gel.
DNA sequencing
Direct sequencing of the PCR product was performed for G6PD-deficient samples whose G6PD mutations could not be identified using the PCR-RFLP procedure. The exon 2 to exon 13 fragments of the G6PD gene were amplified using the appropriate primer sets (Additional file 2: Table S2) [15, 16]. The amplicon was used as the template for DNA sequencing on a 3730xl DNA Analyzer (Thermo Fisher Scientific) using the same primer sets.
Statistical analysis
The G6PD spectrophotometry assay was used as the reference assay. The adjusted median was calculated on males, as previously published [10]. The adjusted male median (AMM) was computed twice using both spectrophotometry and biosensor techniques. Subsequently, the samples were categories twice based on the AMM, and their performance was evaluated by comparing the resulting categories. The diagnostic performance (sensitivity, specificity, positive predictive value, negative predictive value, accuracy) of the G6PD biosensor at different cut-off values (30%, 70%, and 80%) was calculated. Bland-Altman plot and receiver operating characteristic curve were used to identify the appropriate enzyme activity cut-off for the CareStart™ G6PD biosensor. The enzyme activity cut-off from the CareStart™ G6PD biosensor assay corresponding to the 30% G6PD activity from the spectrometry assay was selected based on the Youden Index. The 30% G6PD activity from the standard spectrophotometry assay was used as a cut-off to identify G6PD deficiency. All statistical analyses were conducted in GraphPad Prism 9.0 (GraphPad Software, USA).
Results
Quantitative analysis of G6PD enzyme activity
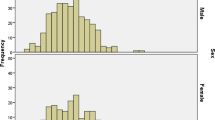
Quantitative measurement of G6PD activity using a standard spectrophotometry assay [17, 17] was performed on 772 blood samples collected from Tatkon, Ottara Thiri, and Zayar Thiri townships (579 males and 193 females). The majority of ethnic of participants were Myanmar (87.7%). Other ethnicities represented were Rakhine (3.8%), Kayin (3.8%), Shan (2.1%), Chin (1%), Kachin (0.6%), mixed (0.9%), and Mon (0.1%), respectively. To minimize the impact of severely deficient individuals on reference enzyme activity, the adjusted male median (AMM) was used to identify the reference value for the G6PD activity [10]. The overall AMM was determined to be 6.30 U/gHb and was used as the reference for 100% G6PD activity. The classical distribution of G6PD activity among females and males is shown in Fig. 2. The enzyme activity cut-off at 30% and 70% AMM was 1.89 U/g Hb and 4.41 U/g Hb, respectively. Based on the criteria for identification of deficient (< 30%), intermediate (30–<70%), and normal levels of G6PD activity (≥70%) [1], 85.9% (663/772) of the population had normal levels of G6PD activity (≥4.41 U/gHb). The intermediate G6PD activity level (1.89–4.41 U/g Hb) accounted for 3.2% of females. Even though the intermediate G6PD activity level was used mainly for females, 7.6% (44/579) of the male population also had G6PD levels in this category. The overall prevalence of G6PD deficiency in this population at a 30% cut-off (< 1.89 U/g Hb) was 10.9% (84/772). The prevalence of G6PD deficiency in males (12.4%, 72/579) was higher than in females (6.2%, 12/193) (Table 1).
Distribution of G6PD activity (spectrophotometry and biosensor) among all samples. A Distribution of G6PD activity among all subjects, B distribution of G6PD activity among females, and C distribution of G6PD activity among all males. The adjusted male median (100% G6PD activity), 30%, and 70% reference G6PD activity are indicated in the blue dashed line
The CareStart™ G6PD biosensor was used to assess the enzyme activity of 150 blood samples collected from Ottara Thiri. The Bland-Altman plot showed that the CareStart™ G6PD biosensor often underestimated G6PD enzyme activity, especially when the G6PD level was above the 30% cut-off (Fig. 3). The assay-specific AMM of the CareStart™ G6PD biosensor was 2.58 U/gHb. The diagnostic performance of the CareStart™ G6PD biosensor is shown in Table 2. The ROC analysis between the CareStart™ G6PD biosensor and spectrometry assays was evaluated at 30% (Fig. 4A), 70% (Fig. 4B), and 80% (Fig. 4C) of the G6PD activity cut-off from the spectrometry assay. The area under the curve (AUC) was 0.8238, 0.7581, and 0.7374 using the 30%, 70%, and 80% G6PD activity cut-offs, respectively. The performance of the CareStart™ G6PD in identifying G6PD deficiency at 30% assay-specific G6PD activity cut-off (0.77) U/g Hb is shown in Table 2 with 63% sensitivity, 95% specificity, 57% positive predictive value, 96% negative predictive value, and 92% accuracy. The prevalence of G6PD deficiency as defined by this assay was 12.67%.
Qualitative analysis of G6PD activity
The CareStart™ G6PD RDT was used to analyse all 772 samples. The overall prevalence of G6PD deficiency across the study sites was 10.5% (81/772), 12.1% (70/579) in males, and 5.7% (11/193) in females. The distribution of G6PD enzyme activity among G6PD-normal and G6PD-deficient individuals is shown in Fig. 5. G6PD deficiency was clearly separated from G6PD-normal at 30% G6PD enzyme activity. The diagnostic performance of the CareStart™ G6PD RDT compared with the spectrometry assay is shown in Table 3. The sensitivity and specificity were 93% and 98%, respectively. The CareStart™ G6PD RDT performed well in discriminating G6PD deficiency with 88% PPV and 99% NPV. Six males were G6PD-normal by the RDT but showed enzyme activity below the 30% cut-off.
G6PD genetic variants
G6PD genotyping was performed on all 193 females and 183 males, including all with G6PD deficiency (75 samples) defined by either spectrometry or RDT methods, and 108 G6PD-normal were selected randomly. G6PD mutations were found in 43.2% (79/183) of males and 19.2% (37/193) of females. The majority G6PD variant was the Mahidol variant (86.1% in males and 91.9% in females). 46% (46.0%; 47/102) of the samples with the Mahidol variant showed G6PD activity below the 10% cut-off value defined by the spectrometry method (< 0.63 U/g Hb), including one homozygous female (Table 4). The Andalus variant, not previously reported in Myanmar, was identified by sequencing G6PD from one hemizygous male.
Discussion
In Myanmar, a 14 day course of low-dose primaquine with a blood schizonticide drug (Chloroquine, 800 mg per day for 3 days) is provided to P. vivax and Plasmodium ovale patients to clear hypnozoites without testing G6PD status, putting G6PD-deficient patients at risk of life-threatening haemolysis [18]. Quantitative spectrophotometry is the standard method for testing G6PD enzyme activity and the definitive diagnosis of G6PD deficiency [2]. However, no internationally accepted threshold currently exists to define it [19]. Therefore, in the absence of a local G6PD reference value, G6PD deficiency cannot be determined accurately. Although Myanmar has a high prevalence of G6PD deficiency [8], local evidence-based data on quantitative enzyme activity are lacking, and no proposed cut-off value for G6PD deficiency specific to the Myanmar population exists. Different studies of G6PD deficiency have used different reference cut-off values [20,21,22]. Using the Trinity Biotech spectrophotometric assay, Oo et al. reported a higher adjusted male median G6PD activity of 8.28 U/gHb among adults in Myanmar compared with the 6.3 U/gHb in this study [7]. The Trinity Biotech G6PD reagent was discontinued during the time of this study. The reference range obtained from each commercial kit only reflects the age range, geographical location, and characteristics of each studied population [17, 23]. Harmonization of the assay protocol and reagents being used for the determination of G6PD activity would help limit variability in the nationwide-reference range. The reference values proposed by this study only reflect the G6PD activity determined by Randox spectrophotometric assay. In the present study, the prevalence of G6PD deficiency (10.9%) was comparable to the study conducted in different areas of Myanmar (Yangon, Mandalay, Sagaing, Pyin-Oo-Lwin, Sittwe, Kawthaung) [24].
In efforts to eliminate malaria, P. vivax and P. ovale are the most challenging among the human malaria parasites due to their formation of hypnozoites in the liver causing relapse without new infection. The 8-aminoquinoline drugs, primaquine and tafenoquine, are effective in eliminating hypnozoites, but prior G6PD testing is recommended for a safe radical cure. In countries where tafenoquine, a newly FDA-approved 8-aminoquinoline drug, is prescribed, quantitative analysis of G6PD activity is mandatory as the drug is only provided to patients who have enzyme levels of at least 70%. In the context of resource-constrained settings, point-of-care quantitative G6PD analysis is highly beneficial, as it can be readily used at the same health-facility level where malaria cases are routinely managed, for prompt and better-informed treatment options. The diagnostic performance of the CareStart™ G6PD biosensor, which was evaluated in this study and also in other countries, varied with some improvement over time, but still requires further improvement to meet the acceptable target product profile [10, 17, 25,26,27]. The need for an additional haemoglobin analyzer to obtain haemoglobin values for normalizing G6PD activity also complicates the usage of the machine, especially in a field setting. The widely well-accepted STANDARD G6PD biosensor has been validated across multiple countries [28,29,30] and can, therefore, be implemented without establishing a laboratory or country-specific threshold. Field evaluations by health staff using the STANDARD G6PD biosensor at the patient’s first contact point have shown reliable results and can be used as an alternative to the standard spectrophotometry assay [29, 31].
The CareStart G6PD RDT is a qualitative test requiring no equipment. According to the recommendation of the World Health Organization (WHO), the qualitative G6PD rapid test should have more than 95% sensitivity compared with the standard spectrophotometric assays and > 95% NPV at the G6PD enzyme activity cut-off value of ≤ 30%. Besides, the preferred product should be stable at temperatures of 30–40 °C found in tropical countries and have a visual readout that clearly differentiates between “deficient” and “normal” patients [32]. The performance of the G6PD rapid test in this study was among the preferred qualitative products recommended by the WHO, but it had 93% sensitivity, which was slightly lower than the preferred 95% sensitivity. Unfortunately, the product was no longer available in the market. This also urges the need for a new G6PD diagnosis.
In Myanmar, there are eight main ethnicities with 135 sub-ethnic groups. The dominant G6PD deficiency mutation in Myanmar is G6PD-Mahidol, accounting for 91.3% of G6PD variants among various ethnic groups [6, 33]. Other variants have also been reported, including Kaiping, Viangchan, Union, Canton, Chinese-4, Chinese-5, Mediterranean, Acores, Seattle, Jammu, Coimbra, Kerala-Kalyan, and Valladolid [6, 34,35,36]. The results in this study were consistent with these earlier findings. The Chinese-5, Chinese-4, Kaiping, and Union mutations found in this study were common variants in the Chinese population [37], suggesting a past spread to Southeast Asian countries from China [38]. The Andalus variant, first described in 1990 [39] and previously reported in a Malaysian population [40], was identified in the Myanmar population for the first time. Notably, almost 60% of males with the Mahidol mutation in this study had < 10% of the normal G6PD activity, within the Class II severe deficiency range, which concurs with an earlier study on the Thailand-Myanmar border [35].
Conclusion
Myanmar is a malaria-endemic country, and military personnel and people residing in remote border regions are at higher risk of infection. In Myanmar, G6PD testing is usually not done before administering primaquine, which carries a significant risk of clinically concerning haemolysis [41]. The quantitative or qualitative G6PD tests that are easy to use and at a cost comparable with the reference spectrophotometry assay or malaria RDT, respectively, should be deployed in remote endemic areas before radical treatment with primaquine or tafenoquine is prescribed. This study provided further information on the prevalence and variants of G6PD deficiency in the Myanmar population and evaluated the performance of the point-of-care test for the identification of G6PD deficiency.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AMM:
-
Adjusted male median
- AUC:
-
Area under the curve
- DSMRC:
-
Defense Sevices Medical Research Centre
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- Hb:
-
Haemoglobin
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NPV:
-
Negative predictive value
- OD:
-
Optical density
- PPV:
-
Positive predictive value
- PCR-RFLP:
-
Polymerase chain reaction-restriction fragment length polymorphism
- RDT:
-
Rapid diagnostic test
- ROC:
-
Receiver operating characteristic [curve]
- WHO:
-
World Health Organization
References
WHO. Glucose-6-phosphate dehydrogenase deficiency. Bull World Health Organ. 1989;67:601–11.
Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74.
Mehta A, Mason PJ, Vulliamy TJ. Glucose-6-phosphate dehydrogenase deficiency. Baillieres Best Pract Res Clin Haematol. 2000;13:21–38.
Mason PJ, Vulliamy TJ. Glucose-6-phosphate dehydrogenase (G6PD) deficiency: genetics. Hoboken: John Wiley & Sons, Ltd.; 2001.
Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9:e1001339.
Matsuoka H, Wang J, Hirai M, Arai M, Yoshida S, Kobayashi T, et al. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Myanmar: G6PD Mahidol (487G > A) is the most common variant in the Myanmar population. J Hum Genet. 2004;49:544–7.
Oo NN, Bancone G, Maw LZ, Chowwiwat N, Bansil P, Domingo GJ, et al. Validation of G6PD point-of-care tests among healthy volunteers in Yangon, Myanmar. PLoS ONE. 2016;11:e0152304.
Li Q, Yang F, Liu R, Luo L, Yang Y, Zhang L, et al. Prevalence and molecular characterization of glucose-6-phosphate dehydrogenase deficiency at the China-Myanmar border. PLoS ONE. 2015;10:e0134593.
Ley B, Luter N, Espino FE, Devine A, Kalnoky M, Lubell Y, et al. The challenges of introducing routine G6PD testing into radical cure: a workshop report. Malar J. 2015;14:377.
Domingo GJ, Satyagraha AW, Anvikar A, Baird K, Bancone G, Bansil P, et al. G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 2013;12:391.
Kim S, Nguon C, Guillard B, Duong S, Chy S, Sum S, et al. Performance of the CareStart G6PD deficiency screening test, a point-of-care diagnostic for primaquine therapy screening. PLoS ONE. 2011;6:e28357.
WHO Malaria Policy Advisory Committee and Secretariat. Malaria policy advisory committee to the WHO: conclusions and recommendations of eighth biannual meeting. Malar J. 2016;15:117.
Hsiang MS, Hwang J, Tao AR, Liu Y, Bennett A, Shanks GD, et al. Mass drug administration for the control and elimination of Plasmodium vivax malaria: an ecological study from Jiangsu province, China. Malar J. 2013;12:383.
Nuchprayoon I, Louicharoen C, Charoenvej W. Glucose-6-phosphate dehydrogenase mutations in Mon and burmese of southern Myanmar. J Hum Genet. 2008;53:48–54.
Nuchprayoon I, Sanpavat S, Nuchprayoon S. Glucose-6‐phosphate dehydrogenase (G6PD) mutations in Thailand: G6PD Viangchan (871G > A) is the most common deficiency variant in the thai population. Hum Mut. 2002;19:185.
Huang CS, Hung KL, Huang MJ, Li YC, Liu TH, Tang TK. Neonatal jaundice and molecular mutations in glucose-6‐phosphate dehydrogenase deficient newborn infants. Am J Hematol. 1996;51:19–25.
Ley B, Alam MS, O’Donnell JJ, Hossain MS, Kibria MG, Jahan N, et al. A comparison of three quantitative methods to estimate g6pd activity in the Chittagong Hill Tracts, Bangladesh. PLoS ONE. 2017;12:e0169930.
Chen X, He Y, Miao Y, Yang Z, Cui L. A young man with severe acute haemolytic anaemia. BMJ. 2017;59:j4263.
Pfeffer DA, Ley B, Howes RE, Adu P, Alam MS, Bansil P, et al. Quantification of glucose-6-phosphate dehydrogenase activity by spectrophotometry: a systematic review and meta-analysis. PLoS Med. 2020;17:e1003084.
Oo WM, Pyar KP, Tun AM, Mya TM, Nyunt KM, Hlaing TM. Study glucose-6-phosphate dehydrogenase deficiency in Myanmar military personnel. In: Myanmar Military Medical Conference. Yangon, 2015.
Yang Y, Zhu Y, Li D, Li Z, Lu H, Wu J, et al. Characterization of glucose-6-phosphate dehydrogenase deficiency and identification of a novel haplotype 487G > A/IVS5-612(G > C) in the Achang population of Southwestern China. Sci China C Life Sci. 2007;50:479–85.
Azma RZ, Hidayati N, Farisah NR, Hamidah NH, Ainoon O. G6PD enzyme activity in normal term malaysian neonates and adults using a OSMMR2000-D kit with hb normalization. Southeast Asian J Trop Med Public Health. 2010;41:982–8.
Pfeffer DA, Satyagraha AW, Sadhewa A, Alam MS, Bancone G, Boum Y, et al. Genetic variants of glucose-6-phosphate dehydrogenase and their associated enzyme activity: a systematic review and meta-analysis. Pathogens. 2022;11:1045.
Jalloh A, Tantular IS, Pusarawati S, Kawilarang AP, Kerong H, Lin K, et al. Rapid epidemiologic assessment of glucose-6-phosphate dehydrogenase deficiency in malaria-endemic areas in Southeast Asia using a novel diagnostic kit. Trop Med Int Health. 2004;9:615–23.
Weppelmann TA, von Fricken ME, Wilfong TD, Aguenza E, Philippe TT, Okech BA. Field trial of the CareStart biosensor analyzer for the determination of glucose-6-phosphate dehydrogenase activity in Haiti. Am J Trop Med Hyg. 2017;97:1262–70.
Pengboon P, Thamwarokun A, Changsri K, Kaset C, Chomean S. Evaluation of quantitative biosensor for glucose-6-phosphate dehydrogenase activity detection. PLoS ONE. 2019;14:e0226927.
Djigo OKM, Ould Khalef Y, Ould Ahmedou Salem MS, Gomez N, Basco L, Briolant S, et al. Assessment of CareStart G6PD rapid diagnostic test and CareStart G6PD biosensor in Mauritania. Infect Dis Poverty. 2021;10:105.
Zobrist S, Brito M, Garbin E, Monteiro WM, Clementino Freitas S, Macedo M, et al. Evaluation of a point-of-care diagnostic to identify glucose-6-phosphate dehydrogenase deficiency in Brazil. PLoS Negl Trop Dis. 2021;15:e0009649.
Bancone G, Gilder ME, Win E, Gornsawun G, Penpitchaporn P, Moo PK, et al. Technical evaluation and usability of a quantitative G6PD POC test in cord blood: a mixed-methods study in a low-resource setting. BMJ Open. 2022;12:e066529.
Pal S, Bansil P, Bancone G, Hrutkay S, Kahn M, Gornsawun G, et al. Evaluation of a novel quantitative test for glucose-6-phosphate dehydrogenase deficiency: bringing quantitative testing for glucose-6-phosphate dehydrogenase deficiency closer to the patient. Am J Trop Med Hyg. 2019;100:213–21.
Adhikari B, Tripura R, Dysoley L, Callery JJ, Peto TJ, Heng C, et al. Glucose 6 phosphate dehydrogenase (G6PD) quantitation using biosensors at the point of first contact: a mixed method study in Cambodia. Malar J. 2022;21:282.
Baird JK. Point-of-care G6PD diagnostics for Plasmodium vivax malaria is a clinical and public health urgency. BMC Med. 2015;13:296.
Iwai K, Hirono A, Matsuoka H, Kawamoto F, Horie T, Lin K, et al. Distribution of glucose-6-phosphate dehydrogenase mutations in Southeast Asia. Hum Genet. 2001;108:445–9.
Phompradit P, Kuesap J, Chaijaroenkul W, Rueangweerayut R, Hongkaew Y, Yamnuan R, et al. Prevalence and distribution of glucose-6-phosphate dehydrogenase (G6PD) variants in Thai and burmese populations in malaria endemic areas of Thailand. Malar J. 2011;10:368.
Bancone G, Chu CS, Somsakchaicharoen R, Chowwiwat N, Parker DM, Charunwatthana P, et al. Characterization of G6PD genotypes and phenotypes on the northwestern Thailand-Myanmar border. PLoS ONE. 2014;9:e116063.
Reclos GJ, Hatzidakis CJ, Schulpis KH. Glucose-6-phosphate dehydrogenase deficiency neonatal screening: preliminary evidence that a high percentage of partially deficient female neonates are missed during routine screening. J Med Screen. 2000;7:46–51.
Jiang W, Yu G, Liu P, Geng Q, Chen L, Lin Q, et al. Structure and function of glucose-6-phosphate dehydrogenase-deficient variants in Chinese population. Hum Genet. 2006;119:463–78.
Wang J, Luo E, Hirai M, Arai M, Abdul-Manan E, Mohamed-Isa Z, et al. Nine different glucose-6-phosphate dehydrogenase (G6PD) variants in a malaysian population with malay, chinese, indian and Orang Asli (aboriginal malaysian) backgrounds. Acta Med Okayama. 2008;62:327–32.
Vives-Corrons JL, Kuhl W, Pujades MA, Beutler E. Molecular genetics of the glucose-6-phosphate dehydrogenase (G6PD) Mediterranean variant and description of a new G6PD mutant, G6PD Andalus1361A. Am J Hum Genet. 1990;47:575–9.
Ainoon O, Yu YH, Amir Muhriz AL, Boo NY, Cheong SK, Hamidah NH. Glucose-6-phosphate dehydrogenase (G6PD) variants in malaysian Malays. Hum Mutat. 2003;21:101.
Liu H, Zeng W, Malla P, Wang C, Lakshmi S, Kim K, et al. Risk of hemolysis in Plasmodium vivax malaria patients receiving standard primaquine treatment in a population with high prevalence of G6PD deficiency. Infection. 2023;51:213–22.
Acknowledgements
We would like to thank NIH and Mahidol Vivax Research Unit of the Faculty of Tropical Medicine, Mahidol University, for supporting this study. In addition, we would like to acknowledge all the individuals who participated in this study. We are very grateful to the staff at the Defence Services Medical Research Centre, Nay Pyi Taw, for their assistance and collaboration during the data collection and conduct of the experiments. We would like to thank Paul Adams from the Office of Research Services, Faculty of Tropical Medicine, Mahidol University for his English editing.
Funding
This study was supported by the Thailand Research Fund (TRG5880144), the National Institutes of Health (NIH; U19AI089672), and the Korean National Research Institute of Health-DMP2018 (4800-4837-340-01, 2018). THA was supported by an NIH Fogarty International Center fellowship (D43TW006571).
Author information
Authors and Affiliations
Contributions
THA, TMH, JS, LC, JK, and WR designed the study. THA, ZMT, CS, and WR performed the sample collection, laboratory experiments, and data analysis. TMH performed data collection. THA, WR, and JK did the statistical analysis. WR and THA drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Faculty of Tropical Medicine, Mahidol University (MUTM 2016-036-01) and the Institutional Review Board of the Defence Services Medical Research Centre (DSMRC), Myanmar (IRB/2016/60).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Primers and PCR-RFLP conditions for genotyping of G6PD variants.
Additional file 2: Table S2.
Primersset for amplification of different exons of G6PD encoding gene.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aung, T.H., Suansomjit, C., Tun, Z.M. et al. Prevalence of G6PD deficiency and diagnostic accuracy of a G6PD point-of-care test among a population at risk of malaria in Myanmar. Malar J 22, 143 (2023). https://doi.org/10.1186/s12936-023-04559-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04559-6