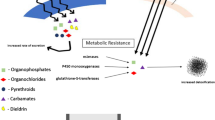
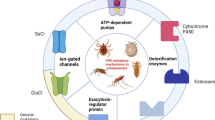
Abstract
Background
Knowing the species composition and insecticide resistance status of the target vector population is important to guide malaria vector control. The aim of this study was to characterize the malaria vector population in terms of species composition, insecticide susceptibility status and potential underlying resistance mechanisms in Ellibou, southern Côte d’Ivoire.
Methods
A 1-year longitudinal entomological survey was conducted using light traps and pyrethroid spray catches to sample adult mosquitoes in combination with larval sampling. The susceptibility status of Anopheles gambiae sensu lato (s.l.) to bendiocarb, deltamethrin, DDT and malathion was assessed using the World Health Organization insecticide susceptibility test. Additionally, An. gambiae specimens were screened for knockdown (kdr) and acetylcholineesterase (ace1) target site resistance alleles, and the expression levels of eight metabolic resistance genes, including seven cytochrome P450 monooxygenases (P450s) and one glutathione S-transferase (GST), measured with reverse transcription quantitative real-time polymerase chain reaction (qPCR).
Results
Overall, 2383 adult mosquitoes from 12 different taxa were collected with Culex quinquefasciatus and An. gambiae being the predominant taxa. Molecular identification of An. gambiae s.l. revealed the presence of Anopheles arabiensis, Anopheles coluzzii, An. gambiae sensu stricto (s.s.) and Anopheles coluzzii/An. gambiae s.s. hybrids. Anopheles gambiae mosquitoes were resistant to all insecticides except malathion. PCR diagnostics revealed the presence of ace1-G280S and the kdr L995F, L995S and N1570Y target-site mutations. Additionally, several genes were upregulated, including five P450s (i.e., CYP6P3, CYP6M2, CYP9K1, CYP6Z1, CYP6P1) and GSTE2.
Conclusion
This is the first documented presence of An. arabiensis in Côte d’Ivoire. Its detection – together with a recent finding further north of the country – confirms its existence in the country, which is an early warning sign, as An. arabiensis shows a different biology than the currently documented malaria vectors. Because the local An. gambiae population was still susceptible to malathion, upregulation of P450s, conferring insecticide resistance to pyrethroids, together with the presence of ace1, suggest negative cross-resistance. Therefore, organophosphates could be an alternative insecticide class for indoor residual spraying in the Ellibou area, while additional tools against the outdoor biting An. arabiensis will have to be considered.
Similar content being viewed by others
Background
Malaria is a major public health problem in sub-Saharan Africa. In Côte d’Ivoire, malaria is the leading cause of mortality and hospitalization in children under the age of 5 years [1]. Across sub-Saharan Africa, the key malaria vectors are Anopheles gambiae sensu stricto (s.s.), Anopheles coluzzii, Anopheles arabiensis and Anopheles funestus [2]. In Côte d’Ivoire, An. gambiae s.s., An. coluzii, Anopheles nili and An. funestus are known vectors driving malaria transmission [3] with An. gambiae s.s. and An. coluzzii being the key vectors [4]. Recently, the presence of An. arabiensis, another important malaria vector, has been reported from Bouaké in the central part of Côte d’Ivoire, an observation that might be linked to the urbanization process [5].
The control of malaria vectors primarily relies on indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) [6] with pyrethroids being the main class of insecticides for bed nets. However, susceptibility to pyrethroids has generally declined in Anopheles mosquitoes [4], including in Côte d’Ivoire, where insecticide resistance is widespread [4], representing a threat to the success of existing malaria control and elimination programmes as well as for the control of other vector-borne diseases.
Several molecular mechanisms are involved in conferring insecticide resistance, while the key mechanisms are metabolic resistance and target-site insensitivity. The most studied target-site insensitivity loci in An. gambiae sensu lato (s.l.) are point mutations in the voltage-gated sodium channel, conferring cross-resistance to pyrethroids and dichlorodiphenyltrichloroethane (DDT), including the L995F (formerly known as L1014F or kdr ‘west’) and L995S (formerly known as L1014S or kdr ‘east’) at the same codon position [7, 8]. Both kdr point mutations are present in An. gambiae across most of Africa and may co-occur in the same individual [9,10,11,12,13]. While L995F has been first detected in Côte d’Ivoire [14] and is now widely present, the L995S kdr has only recently been detected in Côte d’Ivoire in a single hybrid mosquito carrying both kdr mutations [15]. An additional kdr mutation—N1570Y (formerly known as N1575Y), the so called “super kdr”—occurs with and intensifies the effect of the L995F-mediated pyrethroid resistance and has recently also been detected in southern Côte d’Ivoire [16, 17]. Another key point mutation is the acetylcholinesterase ace1-G280S (formerly known as G119S) mutation and is associated with resistance to carbamates and organophosphates [18, 19]. Both kdr and ace1-G280S mutation were present in central and northern Côte d’Ivoire [20,21,22]. In contrast to target-site insensitivity, metabolic resistance is linked to overexpression of specific enzymes that break down, export or sequester insecticides. The three major enzyme families associated with metabolic insecticide resistance are carboxylesterases (COE), glutathione S-transferases (GSTs) and cytochrome P450 monooxygenases (P450s) [23].
Knowing the species composition and insecticide resistance status of the target vector population is important to guide malaria vector control. While several studies were conducted on malaria transmission, vector population and insecticide resistance in Côte d’Ivoire, highlighting the impact of irrigated rice farming on malaria transmission [22, 24,25,26,27], the picture across the country is still very patchy. This motivated the study reported here, carried out in the southern part of Côte d’Ivoire in the village of Ellibou, situated in a forest and ground swamp area suitable for malaria vectors. The village belongs to the region of Agneby-Tiassa and is located along the main motorway connecting Abidjan with the capital Yamoussoukro. Along this route, mosquitoes may be displaced through motorized vehicles. Thus far, Ellibou has not been the subject of entomological surveys. Given the behavioural and ecological differences among malaria vectors and the spread of resistance to public health insecticides across the country, a deeper understanding of local species composition and an accurate and updated insecticide resistance profile are central to the success of vector control. Hence, the overarching aim of this study was to characterize the local malaria vector population in Ellibou in terms of species composition, sporozoite rates, insecticide susceptibility status and potential underlying resistance mechanisms.
Methods
Study site
The study was carried out in Ellibou (geographical coordinates: 5°40ʹ59.4ʺ N latitude, 4°30ʹ31.1ʺ W longitude; Fig. 1) from January to December 2015. Ellibou has a population of approximately 12,000 inhabitants according to “Recensement Géneral de la Population et de l’Habitat 2021” and belongs administratively to the Agneby-Tiassa region. The village is located in a forested area, some 60 km north-west of Abidjan with direct access to a national motorway. Agriculture and trade are the main economic activities in Ellibou. The village is surrounded by uneven ground leading to many small temporary ponds that serve as breeding sites for mosquitoes during the rainy season. At the time of the study, insecticide-treated nets (ITNs) were the only vector control intervention implemented in Ellibou.
Meteorological data
Meteorological data, including monthly average temperature and precipitation, were obtained from the local weather station of the Meteorological Department of the Society for Airport, Aeronautical and Meteorological Operations and Development in Côte d’Ivoire. The climate of Ellibou is characterized by four seasons: (i) a long rainy season from April to July; (ii) a short dry season in August and September; (iii) a short rainy season in October and November; and (iv) a long dry season from December to March.
Mosquito sampling and morphological identification
Mosquitoes were sampled as either adults or larvae. Adult mosquitoes were collected from houses using Centers for Disease Control and Prevention (CDC) light traps and pyrethroid spray catches (PSC) twice per week from January to December 2015. Both collection techniques were performed on the same days. In addition, the number of people that slept in the rooms the previous night was recorded.
The CDC light traps (John W. Hock Company; Gainesville, FL, USA) were placed in four arbitrarily chosen houses across four sectors given by the main road and the bridge, dividing Ellibou into four sectors (Fig. 1). In each sector, two houses were selected, one house for PSC and another one for CDC light traps with a distance of approximately 100 m between the houses. In each house, a CDC light trap was placed inside one bed room. Inside the bed room, the trap was mounted at 1.5 m above the floor and close to the bed of the study participants that slept under a long-lasting insecticidal net (LLIN). While all other lights in the room were switched off, mosquitoes that entered the room were attracted by the incandescent light of the trap and then aspirated into a collection bag by the trap’s fan. The traps ran for two consecutive days in each week from 20:00 to 06:00 h. Each morning, the mosquitoes caught in the traps were removed and transferred to the laboratory at Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (CSRS) in Abidjan for morphological identification. The mosquito species and sex, as well as the gonotrophic stage of the females (i.e. unfed, fed, semi-gravid or gravid) were recorded.
PSC were performed on two consecutive days per week in the mornings from 05:30 to 07:30 h in four additional, arbitrarily chosen houses by spraying a commercially available product “ORO” (Químicas ORO SA; San Antonio de Benagéber, Spain) containing pyrethrin and the synergist piperonyl butoxyde. Before spraying, a white sheet was placed on the floor, then the room was sprayed and any mosquitoes knocked down within 10–15 min after spraying were collected with forceps, put in Petri dishes and transferred to the entomology lab at CSRS for morphological identification.
For the insecticide susceptibility assays, the mosquitoes were collected as larvae during the long rainy season in June and July 2015. The larvae were sampled using a standardized dipper from breeding sites such as puddles on unpaved roads and around household courtyards within the village. Larvae that were morphologically identified as Anopheles sp. were transferred to the insectary at the CSRS and reared to adult stage. The larvae were fed ground Tetra MikroMin fish food (Tetra; Melle, Germany), while the emerging adult mosquitoes were provided with 10% honey solution ad libitum. All mosquito rearing was performed under controlled ambient environmental conditions with a temperature of 27 ± 2 °C, a relative humidity of 80 ± 4% and a 12:12 h light: dark photoperiod.
Sporozoite rate
In addition to species identification and insecticide resistance testing, the female Anopheles caught inside the houses with PSCs were assessed for their gonotrophic status and for the presence of Plasmodium falciparum sporozoites. For the presence of sporozoites, the mosquitoes were screened using the circumsporozoite protein-based enzyme-linked immunosorbent assay (ELISA) from heads and thoraces from all PSC-sampled Anopheles mosquitoes following the protocol by Wirtz et al. [28].
Insecticide susceptibility tests
The World Health Organization (WHO) insecticide susceptibility assays [29] were performed to assess the susceptibility of 3- to 5-day-old adult An. gambiae females reared from larval collections to diagnostic concentrations of deltamethrin (0.05%), DDT (4%), malathion (5%) and bendiocarb (0.1%). These insecticides were chosen because they represent the conventional insecticide classes for adult mosquitoes. The test papers were purchased from the official WHO provider, University Sains Malaysia.
In the experiments, batches of 20–25 mosquitoes per tube with four replicates (i.e. 80–100 mosquitoes) were exposed to each insecticide for 60 min and the number of specimens knocked down was recorded. Subsequently, the mosquitoes were transferred into holding tubes and supplied with 10% honey solution. After 24 h, the number of dead mosquitoes was recorded and insecticide resistance classification was performed based on WHO criteria [29]. The insecticide susceptible An. gambiae s.s. Kisumu strain was included in all assays as a control for the assay and for assessing the quality of the insecticide-impregnated test papers.
Nucleic acids extraction
Non-blood fed adult An. gambiae females, emerging from the larval collections that had not been exposed to insecticides, were stored in RNAlater (Ambion, Inc.; Austin, TX, USA). Nucleic acids were extracted from a subset of 141 specimens using MagaZorb® DNA Mini-Prep kits (Promega Corporation; Madison, WI, USA) with slight modifications of the manufacturer’s protocol. The MagaZorb® kit extracts both DNA and RNA simultaneously. In addition, nucleic acids were extracted from 2- to 3-day-old females of Kisumu (n = 50) and Ngousso strains (n = 50) and served as insecticide susceptible references.
Mosquitoes were ground on ice in 200 µl TE buffer using a plastic pestle in a 1.5 ml Eppendorf tube. Subsequently, 200 µl of lysis buffer was added and vortexed for 15 s. During a 10 min incubation period at room temperature (25 °C), the sample was repeatedly vortexed for 15 s every 2 min. To pellet the mosquito debris, the lysed sample was centrifuged for 2 min at 16,000 rcf and the clear supernatant was transferred into a 1.5 ml Eppendorf tube. Twenty microlitres of magnetic beads (MagaZorb® Reagent) and 500 µl of binding buffer were added to the supernatant and vortexed for 15 s. The sample was again incubated for 10 min at room temperature and repeatedly vortexed. After letting the magnetic beads sediment settle by placing the tube on a magnetic rack for 2 min, the supernatant was discarded. Subsequently, the magnetic beads were washed two times as follows: 200 µl of wash buffer was added, then the mixture was vortexed and incubated at room temperature for 1 min. After sedimentation of the beads on a magnetic rack for 2 min, the supernatant was discarded. Finally, 180 µl elution buffer was added, the mixture was vortexed and incubated for 10 min at 50 °C while repeatedly vortexing. The sample was vortexed again, spun down and placed on a magnetic rack for 2 min. On ice, a new 1.5 ml tube was prepared in which the supernatant containing the purified DNA and RNA was collected. The nucleic acid extracts were stored at − 80 °C until subjecting them to a series of quantitative PCR (qPCR) assays.
Molecular species identification
All qPCR reactions, including the species identification, target-site and metabolic resistance assays, were performed on a Bio-Rad CFX 96 real-time PCR machine (Bio-Rad; Hercules, CA, USA). In each reaction, 1 µl of the nucleic acids extract from individual mosquitoes was added to the one-step reverse transcription qPCR (RT-qPCR) master mix supplied by Fast Track Diagnostics (Esch-sur-Alzette, Luxembourg) together with the primers and probes at the concentrations described by Wipf et al. [30] to reach a total reaction volume of 10 µl. The following thermal cycle conditions were used for all assays: 50 °C for 15 min, 95 °C for 3 min, followed by 40 amplification cycles of 95 °C for 3 s and 60 °C for 30 s. The purified nucleic acids were subjected to two complementary TaqMan qPCR assays to identify the 141 specimens to species level within the An. gambiae complex with previously described adaptations to the original protocols put forth by Wipf et al. [30].
The first assay differentiated An. coluzzii/An. gambiae s.s. (Ag+) as a group from An. arabiensis (Aa+) and Anopheles bwambae/Anopheles melas/Anopheles merus/Anopheles quadirannulatus (Aq+) as a group [31]. The second assay distinguishes between An. gambiae s.s. (former molecular S-form) and An. coluzzii (former molecular M-form) in Ag + samples based on a short interspersed nuclear element (SINE) using common primers published by Santolamazza et al. [32] and probes by Wipf et al. [30].
For confirmation and in view of no prior records of An. arabiensis from Côte d’Ivoire at the time of this study in 2015, amplicons from a subset of 10 qPCR assays that identified An. arabiensis were repeated and their amplicons sequenced. The amplicons were sent to Microsynth AG (Balgach, Switzerland) for Sanger sequencing and searched using BLAST [33].
Insecticide target-site mutation assays
Previously published qPCR protocols were followed to identify the presence of insecticide resistance alleles, including the kdr mutations N1570Y [17], L995F and L995S [34, 35] and the ace1 mutation G280S [35].
Gene expression levels of metabolic resistance loci
The expression levels of eight detox genes previously associated with metabolic resistance in An. gambiae were measured in nucleic acids extracted from RNAlater-preserved, individual specimens that were raised from field-collected larvae. These genes included seven P450s (i.e. CYP4G16, CYP9K1, CYP6M2, CYP6P1, CYP6P3, CYP6P4 and CYP6Z1) and one GST (GSTE2). The expression levels were measured by RT-qPCR Taqman triplex assays, developed by Mavridis et al. [36], in which the transcripts of the ribosomal protein S7 (RPS7) serves as an internal reference to adjust for the overall expression level. Gene expression levels of field-collected specimens from Ellibou were calculated relative to two insecticide-susceptible laboratory strains of the same species. Anopheles gambiae s.s. from the field were compared to An. gambiae s.s. Kisumu, originating from Kisumu, Kenya, and An. coluzzii from the field were compared to An. coluzzii Ngousso, originating from Yaoundé, Cameroon. No gene expression data for An. coluzzii/An. gambiae s.s. hybrids and An. arabiensis were included in this study, because of the low number of field samples from Ellibou and because there was no susceptible laboratory reference stain of the same species available. Gene expression assays were ran for 50 individuals per species both from the field and laboratory reference strain, but only qPCR results that pass pre-set quality thresholds were included, e.g. individuals whose RPS7 quantification cycle (Cq) values were above 27.10 have been excluded from the analysis.
Statistical analysis
The monthly frequencies of mosquitoes collected from both CDC light traps and PSC collections were determined. Data were double entered into an Excel spreadsheet (Windows 10, Microsoft Corporation; Redmond, WA, USA).
The level of significance was set at α = 0.05. Pearson product-moment correlation tests were performed in order to assess linear associations between the entomological parameters and meteorological data. The sporozoite rate was determined by calculating the proportion of mosquito found to be positive to the circumsporozoite protein. The 95% confidence interval (CI) for frequency was calculated by using the formula: \(\widehat{p}+/- z^{*} (\widehat{p}(1 - \widehat{p})/n)^{0.5}\), where z is 1.96. Data from the WHO insecticide susceptibility assays were interpreted according to WHO criteria [29] in the respective mosquito population. For all qPCR assays, the Cq values were determined from the measured amplification curves using the Bio-Rad CFX Maestro 1.0 software (Bio-Rad Laboratories; Hercules, CA, USA). For the target-site qPCR assays, the allele frequencies of each kdr and the ace1 mutation were calculated by using the Hardy-Weinberg formula: \(f\left( R \right)\, = \,\left( {2n.RR\, + \,n.RS} \right)/2N\) where n represents the number of mosquitoes of a given genotype and N the total number of mosquitoes analyzed. For the gene expression analysis, Cq values of the metabolic resistance genes were normalized against the Cq values of the ‘housekeeping’ gene RPS7 to account for total gene expression in each individual. Then, using the method of Pfaffl et al. [37] implemented the REST 2009 software version 2.0.13, the expression levels of the resistant An. gambiae s.s. and An. coluzzii field population were calculated relative to the susceptible Kisumu and Ngousso laboratory colonies, respectively.
Results
Mosquito species composition
During the 1-year study, 2383 adult female mosquitoes from 10 taxa were collected by CDC light traps and PSC. Almost two-thirds of all mosquitoes were caught in the PSCs (Table 1). About half of the mosquitoes were morphologically identified as Culex quinquefasciatus, constituting the most abundant mosquito species in Ellibou, followed by the malaria vector species of the Gambiae complex. In addition to An. gambiae s.l., the malaria vectors An. funestus s.l. and Anopheles pharoensis were present. Among Anopheles sp., members of the Gambiae complex were by far the most predominant taxon with higher proportions in the PSCs. Aedes, Culex and Mansonia were the other culicine genera collected in Ellibou, representing 57.8% and 72.0% of the total mosquito population using CDC light traps and PSCs, respectively.
The numbers of adult An. gambiae mosquitoes collected inside the houses increased considerably when precipitation was above 200 mm per month with about a 1-month lag. The monthly average temperature in Ellibou was 26 °C, ranging between 20 and 30 °C and was not correlated with mosquito abundance (Fig. 2).
Seasonal weather conditions and changes in mosquito densities, Ellibou, Côte d’Ivoire, in 2015. Curves showing monthly variation in An. gambiae s.l. caught by PSC and CDC light trap: A. Monthly temperature and precipitation; B. Monthly caught fed and unfed An. gambiae s.l. by PSC; C. Monthly caught fed and unfed An. gambiae s.l. by CDC light trap
A total of 141 An. gambiae s.l. female adults reared from larvae collected in Ellibou were subjected to molecular species identification assays. For 13 individuals the qPCR reaction did not yield a signal; hence, these samples were excluded from further analysis. The molecular diagnostics revealed the presence of An. arabiensis, An. coluzzii, An. gambiae s.s. and hybrids between An. coluzzii and An. gambiae s.s. Anopheles gambiae s.s. (38.3%) and An. coluzzii (39.0%) were almost equally represented and 4.7% were An. coluzzii/An. gambiae s.s. hybrids. As the An. arabiensis finding is the first in Côte d’Ivoire at the time of the study, the amplicons of 10 specimens identified as An. arabiensis were sequenced for confirmation. All 10 sequences were identical with the An. arabiensis isolate NMNH2 ribosomal RNA intergenic spacer (GenBank: EU091308.1).
Blood feeding and sporozoite rates
The number of blood-fed mosquitoes was linearly correlated with rainfall (r = 0.6, p < 0.05). Overall, 5.3% (95% CI 3.8–7.3%; n = 607) of tested blood-fed female An. gambiae were positive for sporozoites, while infective mosquitoes were detected throughout most of the year, except during the long dry season in January, February and December (Table 2).
Insecticide susceptibility tests
To assess the insecticide resistance status of An. gambiae in Ellibou, adult females were raised from larvae collected from across Ellibou and then exposed to discriminating concentrations of the four conventional classes of insecticides (i.e. organochlorines, pyrethroids, organophosphates and carbamates).
A sample of 87 − 100 mosquitoes was exposed to an insecticide representing each insecticide class. In the positive controls, all An. gambiae Kisumu tested exhibited 98–100% mortality to all insecticides, indicating a good quality of the insecticide-impregnated papers, while exposure to control papers without insecticide resulted in mortality rates below 5%. The local An. gambiae population in Ellibou showed resistance to all insecticides tested, except for malathion (Table 3).
Molecular resistance mechanisms
All four target-site resistance markers for which mosquito samples were screened were present, including the kdr alleles L995S, L995F and N1570Y, and the ace1-G280S mutation. The L995F kdr mutation was predominant across An. arabiensis, An. gambiae s.s., An. coluzzii and An. coluzzii/An. gambiae s.s. hybrids with allelic frequencies ranging from 23 to 100% (Table 4). The highest rate was found in An. gambiae s.s. (100%). In contrast to L995F kdr mutation, the L995S mutant allele was only detected in An. arabiensis–at both homozygous and heterozygous state–with an allelic frequency of 65%. In addition, 7 out of 23 An. arabiensis individuals were found carrying the double 995 F and 995 S mutant alleles simultaneously. In contrast, the N1570Y kdr mutation was only found in An. gambiae s.s. albeit only at low frequency (8%). The ace1-G280S mutation was found in An. arabiensis, An. coluzzii and An. gambiae s.s. as well the An. coluzzii/An. gambiae s.s. hybrids with frequencies between 6% and 50% (Table 4 and Additional file 1: Table S4).
Expression levels of the measured metabolic resistance genes are shown in Fig. 3. All genes included in the expression analysis were significantly different expressed in An. coluzzii from Ellibou when compared to the susceptible Ngousso laboratory strain. The most upregulated genes were CYP6P4 and CYP6M2, while CYP4G16 was downregulated in the An. coluzzii field population. In An. gambiae s.s. from Ellibou less genes were expressed. The other genes CYP6P4, CYP4G16 and GSTE2 were statistically not significantly different expressed in the An. gambiae s.s. comparison (Additional file 2).
Expression levels of eight metabolic resistance genes in An. coluzzii (blue) and An. gambiae s.s. (red) from Ellibou compared to insecticide susceptible laboratory strains of the same species: CYP4G16, CYP6M2, CYP6P1, CYP6P3, CYP6P4, CYP6Z1, CYP9K1 and GSTE2. Genes with a log2 fold change above the thick, gray horizontal line (log2FC > 0) were significantly higher expressed in the field population than in the reference lab populations, while genes below the horizontal line (log2FC < 0) were significantly lower expressed in the field than in the lab population; Levels of significance: *p-value ≤ 0.05; **p-value ≤ 0.01; and ***p-value ≤ 0.001, not plotted when p-value > 0.05
Discussion
Anopheles gambiae was the principal malaria vectors identified in Ellibou, southern Côte d’Ivoire, with P. falciparum-infected individuals present throughout the year except for the long dry season. Species identification of larval collections showed that An. coluzzii and An. gambiae s.s. constituted the major malaria vectors at almost equal proportions, while 4.7% of the An. gambiae s.l. population were An. coluzzii/An. gambiae s.s. hybrids.
Surprisingly, 18% of the An. gambiae population were An. arabiensis, this being the first documented finding of An. arabiensis in Côte d’Ivoire at the time this study was conducted in 2015.
Recently, a study from Bouaké in the central part of Côte d’Ivoire also reported the presence of An. arabiensis [5] albeit the finding was 5 years following the one made here. Moreover, An. arabiensis is found in Côte d’Ivoire’s neighbouring countries, including Burkina Faso, Mali and Ghana [38,39,40] and across the West Africa region in countries, such as, Benin [41], Cabo Verde [42], Gambia [43], Guinea Bissau [44], Mauritania [45], Nigeria [46], Senegal [47] and Togo [48]. Anopheles arabiensis generally breeds and predominates in semi-arid and savannah areas [49, 50]. However, An. arabiensis may also breed in similar breeding sites to those of An. gambiae such as small temporary, sunlit and clear freshwater pools [50] and is found in more polluted breeding sites which might be linked to increased expression of detoxifying enzymes and target-site resistant loci [51].
In 2002, Ellibou experienced a transformation of its environment, explained by a sudden increase in population due to the socio-political crisis in Côte d’Ivoire, which could have indirectly led to more suitable habitats for An. arabiensis. Moreover, the motorway connecting Abidjan with the country’s capital, Yamoussoukro, crosses Ellibou village that constitutes a checkpoint for the control of all transport vehicles and their passengers. Therefore, it is conceivable that An. arabiensis was introduced to Ellibou by road traffic from neighbouring countries while the species was already pre-adapted to the altered local environment.
The presence of An. arabiensis in Côte d’Ivoire, now confirmed by two independent studies, is worrying and suggests that this major malaria vector is spreading further into new areas in West Africa. Indeed, in several West African cities, An. arabiensis has already become a dominant malaria vector [51,52,53]. In view of An. arabiensis primarily feeding outdoors [54], the effectiveness of IRS and LLINs might diminish should An. arabiensis become more dominant, requiring alternative intervention strategies. Worryingly, several point mutations conferring insecticide resistance were also identified in the An. arabiensis population of Ellibou, further challenging its control using insecticides.
The co-occurrence of An. coluzzii and An. gambiae s.s. is on par with other observations made across Côte d’Ivoire [55,56,57]. The presence of both sibling species at almost equal proportions, together with the presence of hybrids, suggests that Ellibou is an area were hybridization of the two sibling species takes place. In this respect it is conspicuous that both sibling species show a rather similar insecticide resistance profile which could be a result of hybridization. This is on par with the hypothesis that hybridization between the two species might have implications for the introgression of insecticide-resistance loci and wider consequences for malaria transmission [58].
A weakness of the current study is that, in contrast to the specimens reared from larval collections, the adult An. gambiae specimens were not further identified to sibling species level. Moreover, the collection methods used for the adult mosquitoes, including the CDC light traps and PSC, are biased towards mosquitoes biting and resting indoors. Therefore, the extent to which each sibling species and their hybrids contribute to malaria transmission in the Ellibou region—and Côte d’Ivoire as a whole—requires further investigations.
The An. gambiae population of Ellibou showed phenotypic resistance to deltamethrin, DDT and bendiocarb, yet was still susceptible to malathion. Parallel to phenotypic insecticide resistance, several target-site resistance alleles were identified, including the kdr L995F, L995S and N1570Y as well as the ace1-G280S mutation, although the kdr allele L995S was only present in An. arabiensis, where it was present in both homozygous and heterozygous state. In addition to the target-site resistance alleles, overexpression of P450s genes (mainly CYP6M2, CYP6P3 and CYP9K1) confirm the observed pyrethroids resistance in the local malaria vectors. The phenotypic insecticide resistance data in the present study are similar to those reported from other settings in Côte d’Ivoire [4, 16, 30, 59] as is the high allelic frequency of the L995F kdr mutation in An. gambiae s.s. [59, 60]. The presence of the kdr N1570Y allele, in combination with the kdr L995F in An. gambiae s.s., may explain part of the resistance observed to deltamethrin in the Ellibou An. gambiae s.l. population [17]. With regards to An. arabiensis in Ellibou, similar results have been reported from Benin [61], Burkina Faso [62], Mauritania [45] and Senegal [63]. Another finding is that 7 An. arabiensis specimens possessed both the L995F and L995S kdr simultaneously. This genotype has already been reported in An. gambiae s.s. from Uganda [64] and Côte d’Ivoire [59], but the present study seems to be the first record of an An. arabiensis L995F/L995S hybrid from Côte d’Ivoire. In addition to the kdr mutations, the overexpression of the five elevated P450 metabolic genes might also add to the pyrethroid and DDT cross-resistance in An. gambiae s.l. from Ellibou [65].
Intriguingly, despite the susceptibility of An. gambiae s.l. to malathion, ace1-G280S mutations conferring cross-resistance to carbamates and organophosphates were found across all three An. gambiae species from Ellibou. A possible explanation for this phenomenon is negative-cross resistance conferred by overexpression of P450s [30]. Indeed, several metabolic P450 enzymes, including CYP6M2, CYP6P1, CYP6P3, CYP6Z1 and CYP9K1, were found to be upregulated and might have increased the toxicity of malathion [66, 67].
Conclusion
The detection of An. arabiensis in southern Côte d’Ivoire, predating another finding further north of the country, confirms the presence of this malaria vector across the country. The presence of An. arabiensis is an early warning sign, as it shows a different behaviour and ecology than An. coluzzii and An. gambiae s.s., requiring different types of vector control interventions. It will be important to further investigate its spatial distribution, insecticide resistance status and contribution to malaria transmission for effective malaria control. Upregulation of P450s conferring insecticide resistance to pyrethroids together with the presence of ace1 suggests negative cross-resistance since the local An. gambiae s.l. population was susceptible to malathion. Therefore, organophosphates could be an alternative insecticide class for IRS in the Ellibou area of southern Côte d’Ivoire.
Availability data and materials
Data and materials of this study are included in this article and its additional files.
Abbreviations
- ace1 :
-
acetylcholinesterase
- CDC:
-
Centers for Disease Control and Prevention
- CI:
-
Confidence interval
- COE:
-
Carboxylesterases
- CSRS:
-
Centre Suisse de Recherches Scientifiques en Côte d’Ivoire
- DDT:
-
Dichlorodiphenyltrichloro ethane
- ELISA:
-
Enzyme-linked immunosorbent assay
- GST:
-
Glutathione S-transferase
- IRS:
-
Indoor residual spraying
- kdr :
-
Knockdown resistance
- LLIN:
-
Long-lasting insecticidal net
- PSC:
-
Pyrethroid spray catch
- RDT:
-
Rapid diagnostic test
- RT-qPCR:
-
Reverse transcription quantitative polymerase chain reaction
- SINE:
-
Short interspersed nuclear element
- SR:
-
Sporozoite rate
- WHO:
-
World Health Organization
References
CDC. Centers for Disease Control and Prevention in Côte d’lvoire. 2020. https://www.cdc.gov/globalhealth/countries/cote-d-ivoire/. Accessed 24 Feb 2023.
Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69.
Adja AM, N’Goran EK, Koudou BG, Dia I, Kengne P, Fontenille D, et al. Contribution of Anopheles funestus, an. gambiae and An. nili (Diptera: Culicidae) to the perennial malaria transmission in the southern and western forest areas of Côte d’Ivoire. Ann Trop Med Parasitol. 2011;105:13–24.
Camara S, Koffi AA, Ahoua Alou LP, Koffi K, Kabran JK, Koné A, et al. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasit Vectors. 2018;11:19.
Fournet F, Adja AM, Adou KA, Dahoui MMC, Coulibaly B, Assouho KF, et al. First detection of the malaria vector Anopheles arabiensis in Côte d’Ivoire: urbanization in question. Malar J. 2022;21:275.
WHO. World malaria report. Geneva: World Health Organization. 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed 6 Dec 2021.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7.
Chandre F, Manguin S, Brengues C, Dossou Yovo J, Darriet F, Diabate A, et al. Current distribution of a pyrethroid resistance gene (kdr) in Anopheles gambiae complex from west Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia. 1999;41:319–22.
Awolola TS, Oyewole IO, Amajoh CN, Idowu ET, Ajayi MB, Oduola A, et al. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Trop. 2005;95:204–9.
Dabire RK, Namountougou M, Diabate A, Soma DD, Bado J, Toe HK, et al. Distribution and frequency of kdr mutations within Anopheles gambiae s.l. populations and first report of the ace.1 G119S mutation in Anopheles arabiensis from Burkina Faso (West Africa). PLoS ONE. 2014;9:e101484.
Ochomo E, Subramaniam K, Kemei B, Rippon E, Bayoh NM, Kamau L, et al. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. Arabiensis in western Kenya. Parasit Vectors. 2015;8:616.
Djègbè I, Akoton R, Tchigossou G, Ahadji-Dabla KM, Atoyebi SM, Adéoti R, et al. First report of the presence of L1014S knockdown-resistance mutation in Anopheles gambiae s.s and Anopheles coluzzii from Togo, West Africa. Wellcome Open Res. 2018;3:30.
Elissa N, Mouchet J, Riviere F, Meunier JY, Yao K. Resistance of Anopheles gambiae s.s. to pyrethroids in Côte d’Ivoire. Ann Soc Belg Med Trop. 1993;73:291–4.
Chouaibou M, Kouadio FB, Tia E, Djogbenou L. First report of the east african kdr mutation in an Anopheles gambiae mosquito in Côte d’Ivoire. Wellcome Open Res. 2017;2:8.
Edi AVC, N’Dri BP, Chouaibou M, Kouadio FB, Pignatelli P, Raso G, et al. First detection of N1575Y mutation in pyrethroid resistant Anopheles gambiae in Southern Côte d’Ivoire. Wellcome Open Res. 2017;2:71.
Jones CM, Liyanapathirana M, Agossa FR, Weetman D, Ranson H, Donnelly MJ, et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci USA. 2012;109:6614–9.
Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, et al. Comparative genomics: insecticide resistance in mosquito vectors. Nature. 2003;423:136–7.
Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13:1–7.
Wolie RZ, Koffi AA, Ahoua Alou LP, Sternberg ED, N’Nan-Alla O, Dahounto A, et al. Evaluation of the interaction between insecticide resistance-associated genes and malaria transmission in Anopheles gambiae sensu lato in central Côte d’Ivoire. Parasit Vectors. 2021;14:581.
Zoh DD, Ahoua Alou LP, Toure M, Pennetier C, Camara S, Traore DF, et al. The current insecticide resistance status of Anopheles gambiae (s.l.) (Culicidae) in rural and urban areas of Bouaké, Côte d’Ivoire. Parasit Vectors. 2018;11:118.
Zogo B, Soma DD, Tchiekoi BN, Somé A, Ahoua Alou LP, Koffi AA, et al. Anopheles bionomics, insecticide resistance mechanisms, and malaria transmission in the Korhogo area, northern Côte d’Ivoire: a pre-intervention study. Parasite. 2019;26:40.
Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–59.
Koudou BG, Tano Y, Doumbia M, Nsanzabana C, Cisse G, Girardin O, et al. Malaria transmission dynamics in central Côte d’Ivoire: the influence of changing patterns of irrigated rice agriculture. Med Vet Entomol. 2005;19:27–37.
Konan YL, Koné AB, Doannio JM, Fofana D, Odehouri-Koudou P. Malaria transmission in Tiassalékro, a irrigated rice growing village situated in the south forest area of Côte d’Ivoire. Bull Soc Pathol Exot. 2009;102:26–30.
Fofana D, Konan K, Djohan V, Konan Y, Koné A, Doannio J, et al. Specific diversity and culicidian nuisance in the villages of N’gatty and Allaba in laguna area of Ivory Coast. Bull Soc Pathol Exot. 2010;103:333–9.
Yokoly FN, Zahouli JBZ, Small G, Ouattara AF, Opoku M, de Souza DK, et al. Assessing Anopheles vector species diversity and transmission of malaria in four health districts along the borders of Côte d’Ivoire. Malar J. 2021;20:409.
Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45.
WHO. Test procedure for insecicide restistance monitoring in malaria vectors. Geneva: World Health Organization; 2013.
Wipf NC, Duchemin W, Kouadio FA, Fodjo BK, Sadia CG, Mouhamadou CS, et al. Multi-insecticide resistant malaria vectors in the field remain susceptible to malathion, despite the presence of Ace1 point mutations. PLoS Genet. 2022;18:e1009963.
Bass C, Williamson MS, Field LM. Development of a multiplex real-time PCR assay for identification of members of the Anopheles gambiae species complex. Acta Trop. 2008;107:50–3.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111.
Mavridis K, Wipf N, Muller P, Traore MM, Muller G, Vontas J. Detection and monitoring of insecticide resistance mutations in Anopheles gambiae: individual vs pooled specimens. Genes (Basel). 2018;9:479.
Mavridis K, Wipf N, Medves S, Erquiaga I, Müller P, Vontas J. Rapid multiplex gene expression assays for monitoring metabolic resistance in the major malaria vector Anopheles gambiae. Parasit Vectors. 2019;12:9.
Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36.
Hunt RH, Fuseini G, Knowles S, Stiles-Ocran J, Verster R, Kaiser ML, et al. Insecticide resistance in malaria vector mosquitoes at four localities in Ghana, West Africa. Parasit Vectors. 2011;4:107.
Cissé MB, Keita C, Dicko A, Dengela D, Coleman J, Lucas B, et al. Characterizing the insecticide resistance of Anopheles gambiae in Mali. Malar J. 2015;14:327.
Epopa PS, Collins CM, North A, Millogo AA, Benedict MQ, Tripet F, et al. Seasonal malaria vector and transmission dynamics in western Burkina Faso. Malar J. 2019;18:113.
Koukpo CZ, Fassinou A, Ossè RA, Agossa FR, Sovi A, Sewadé WT, et al. The current distribution and characterization of the L1014F resistance allele of the kdr gene in three malaria vectors (Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis) in Benin (West Africa). Malar J. 2019;18:175.
da Cruz DL, Paiva MHS, Guedes DRD, de Souza Gomes EC, Pires SG, Gomez LF, et al. First report of the L1014F kdr mutation in wild populations of Anopheles arabiensis in Cabo Verde, West Africa. Parasit Vectors. 2021;14:582.
Opondo KO, Jawara M, Cham S, Jatta E, Jarju L, Camara M, et al. Status of insecticide resistance in Anopheles gambiae (s.l.) of the Gambia. Parasit Vectors. 2019;12:287.
Dabiré KR, Diabaté A, Agostinho F, Alves F, Manga L, Faye O, et al. Distribution of the members of Anopheles gambiae and pyrethroid knock-down resistance gene (kdr) in Guinea-bissau, West Africa. Bull Soc Pathol Exot. 2008;101:119–23.
Mint Mohamed Lemine A, Ould Lemrabott MA, Niang EHA, Basco LK, Bogreau H, Faye O, et al. Pyrethroid resistance in the major malaria vector Anopheles arabiensis in Nouakchott, Mauritania. Parasit Vectors. 2018;11:344.
Onyabe DY, Conn JE. The distribution of two major malaria vectors, Anopheles gambiae and Anopheles arabiensis, in Nigeria. Mem Inst Oswaldo Cruz. 2001;96:1081–4.
Ngom el HM, Faye ND, Talla C, Ndiaye el H, Ndione JA, Faye O, et al. Anopheles arabiensis seasonal densities and infection rates in relation to landscape classes and climatic parameters in a Sahelian area of Senegal. BMC Infect Dis. 2014;14:711.
Ahadji-Dabla KM, Romero-Alvarez D, Djègbè I, Amoudji AD, Apétogbo GY, Djouaka R, et al. Potential roles of environmental and socio-economic factors in the distribution of Insecticide Resistance in Anopheles gambiae sensu lato (Culicidae: Diptera) across Togo, West Africa. J Med Entomol. 2020;57:1168–75.
Coetzee M, Craig M, le Sueur D. Distribution of african malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000;16:74–7.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117.
Jones CM, Toé HK, Sanou A, Namountougou M, Hughes A, Diabaté A, et al. Additional selection for insecticide resistance in urban malaria vectors: DDT resistance in Anopheles arabiensis from Bobo-Dioulasso, Burkina Faso. PLoS One. 2012;7:e45995.
Kristan M, Fleischmann H, della Torre A, Stich A, Curtis CF. Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and An. gambiae s.s. malaria vectors in Nigeria and Ghana. Med Vet Entomol. 2003;17:326–32.
Fournet F, Cussac M, Ouari A, Meyer PE, Toé HK, Gouagna LC, et al. Diversity in anopheline larval habitats and adult composition during the dry and wet seasons in Ouagadougou (Burkina Faso). Malar J. 2010;9:78.
Limwagu AJ, Kaindoa EW, Ngowo HS, Hape E, Finda M, Mkandawile G, et al. Using a miniaturized double-net trap (DN-Mini) to assess relationships between indoor-outdoor biting preference and physiological ages of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Malar J. 2019;18:282.
Dossou-Yovo J, Ouattara A, Doannio JM, Riviere F, Chauvancy G, Meunier JY. Aspects du paludisme dans un village de savane humide de Côte d’Ivoire. Med Trop. 1994;54:331–6.
Adja AM, N’Goran KE, Kengne P, Koudou GB, Toure M, Koffi AA, et al. Transmission vectorielle du paludisme en savane arborée à Gansé en Côte d’Ivoire. Med Trop. 2006;66:449–55.
Diakite NR, Adja AM, von Stamm T, Utzinger J, N’Goran EK. Situation épidémiologique avant la mise en eau du barrage hydroagricole de cinq villages de Bouaké, centre Côte-d’Ivoire. Bull Soc Pathol Exot. 2010;103:22–8.
Caputo B, Tondossoma N, Virgillito C, Pichler V, Serini P, Calzetta M, et al. Is Côte D’Ivoire a new high hybridization zone for the two major malaria vectors, Anopheles coluzzii and An. gambiae (Diptera, Culicidae)? Infect Genet Evol. 2022;98:105215.
Fodjo BK, Koudou BG, Tia E, Saric J, N’Dri PB, Zoh MG, et al. Insecticides resistance status of an. gambiae in areas of varying agrochemical use in Côte d’Ivoire. Biomed Res Int. 2018;2018:2874160.
Tia E, Chouaibou M, Gbalegba CNG, Boby AMO, Kone M, Kadjo AK. Distribution of species and kdr gene frequency among Anopheles gambiae s.s. and Anopheles coluzzii populations in five agricultural sites in Côte d’Ivoire. Bull Soc Pathol Exot. 2017;110:130–4.
Djègbè I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, et al. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar J. 2011;10:261.
Badolo A, Traore A, Jones CM, Sanou A, Flood L, Guelbeogo WM, et al. Three years of insecticide resistance monitoring in Anopheles gambiae in Burkina Faso: resistance on the rise? Malar J. 2012;11:232.
Ndiath MO, Cailleau A, Orlandi-Pradines E, Bessell P, Pages F, Trape JF, et al. Emerging knock-down resistance in Anopheles arabiensis populations of Dakar, Senegal: first evidence of a high prevalence of kdr-e mutation in west african urban area. Malar J. 2015;14:364.
Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/melt curve analysis. Malar J. 2006;5:16.
Vontas J, Grigoraki L, Morgan J, Tsakireli D, Fuseini G, Segura L, et al. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc Natl Acad Sci USA. 2018;115:4619–24.
Yunta C, Hemmings K, Stevenson B, Koekemoer LL, Matambo T, Pignatelli P, et al. Cross-resistance profiles of malaria mosquito P450s associated with pyrethroid resistance against WHO insecticides. Pestic Biochem Physiol. 2019;161:61–7.
Adolfi A, Poulton B, Anthousi A, Macilwee S, Ranson H, Lycett GJ. Functional genetic validation of key genes conferring insecticide resistance in the major african malaria vector, Anopheles gambiae. Proc Natl Acad Sci USA. 2019;116:25764–72.
Acknowledgements
Thanks are addressed to the village chief of Ellibou, the president of youth Gbadja Beke Venance and Amani Assoumou Hervé for facilitating the activities in the study village. Additionally, all inhabitants of Ellibou are thanked for granting access to their homes. Particular thanks go to late Nestor Késsé and Junior Kouamé who assisted with the field sampling. Kouamé B. Roger, a geographical information system specialist is acknowledged for support in designing the map.
Funding
No external funding was received for this work.
Author information
Authors and Affiliations
Contributions
BPN, GR and CSM designed the study. BPN collected the data, pursued data analysis and wrote the first draft of the manuscript. BKF contributed to mosquito collection. BPN, NCW and PM performed the molecular analysis. GR, JU and CSM supervised the study. NCW, JS, GR, JU and PM provided scientific inputs to the manuscript. All authors read, revised and approved the final manuscript prior to submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received approval from the “Comité National d’Éthique des Sciences de la Vie et de la Santé de Côte d’Ivoire” (reference no. 054/MSHP/CNER-kp). Participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Genotype of each individual mosquito of all analyzed insecticide resistance loci.
Additional file 2: Table.
Genes expression analysis results between field strains and laboratory strains):Anopheles gambiae s.s. from the field were compared to An. gambiae s.s. Kisumu, originating fromKisumu, Kenya, and An. coluzzii from the field were compared to An. coluzzii Ngousso, originating fromYaoundé.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
N’Dri, B.P., Wipf, N.C., Saric, J. et al. Species composition and insecticide resistance in malaria vectors in Ellibou, southern Côte d’Ivoire and first finding of Anopheles arabiensis in Côte d’Ivoire. Malar J 22, 93 (2023). https://doi.org/10.1186/s12936-023-04456-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04456-y