Abstract
Background
Continuous vector surveillance and sustainable interventions are mandatory in order to prevent anopheline proliferation (or spread to new areas) and interrupt malaria transmission. Anopheline abundance and richness were evaluated in urban and peri-urban malaria foci at a medium-sized city in the Brazilian Amazon, comparing the protected human landing catch technique (PHLC) and alternative sampling methods over different seasonal periods. Additional information was assessed for female feeding behaviour and faunal composition.
Methods
Anophelines were sampled bimonthly in four urban and peri-urban sites in the city of Porto Velho, state of Rondônia, Brazil. The average number of captured mosquitoes was compared between an PHLC (gold standard), a tent trap (Gazetrap), and a barrier screen by means of generalized linear mixed models (GLMM), which also included season and environment (peri-urban/urban) as predictors.
Results
Overall, 2962 Anopheles individuals belonging to 12 species and one complex were caught; Anopheles darlingi represented 86% of the individuals. More mosquitoes were captured in the peri-urban setting, and the urban setting was more diverse. The model estimates that significantly more anophelines were collected by PHLC than by the Screen method, and Gazetrap captured fewer individuals. However, the Screen technique yielded more blood-engorged females. The peak hours of biting activity were from 6 to 7 p.m. in urban areas and from 7 to 8 p.m. in peri-urban areas.
Conclusions
Although peri-urban settings presented a greater abundance of anophelines, Shannon and Simpson diversities were higher in urban sites. Each technique proved to be useful, depending on the purpose: PHLC was more effective in capturing the highest anopheline densities, Gazetrap caught the greatest number of species, and the barrier screen technique captured more engorged individuals. There was no seasonal effect on Anopheles assemblage structure; however, a more diverse fauna was caught in the transitional season. Biting activity was more intense from 6 to 8 p.m., with a predominance of An. darlingi.
Similar content being viewed by others
Background
Malaria is an infectious disease caused by protozoa of the genus Plasmodium [1]. In 2020, about 241 million malaria cases were reported worldwide, with the African continent accounting for 95% of cases [2]. In Brazil, over 145,000 cases were recorded in the same year, 98.7% of which were exclusively in the Amazon region [3]. Five species of Plasmodium can cause human malaria; Plasmodium vivax and Plasmodium falciparum are the main species that burden the public health system in the Brazilian Amazon, where P. vivax is responsible for approximately 86% of confirmed cases [2].
Mosquitoes of the Anopheles genus are responsible for transmission of Plasmodium spp. In Brazil, about 67 species of anopheline mosquitoes have been described, and 49 species have been recorded in the Brazilian Legal Amazon region. Anopheles albitarsis, Anopheles aquasalis, Anopheles benarrochi, Anopheles braziliensis, Anopheles darlingi, Anopheles deaneorum, Anopheles janconnae, Anopheles marajoara, Anopheles nunesztovari sensu lato (s.l.), Anopheles oswaldoi s.l., Anopheles rangeli, Anopheles rondoni, Anopheles strodei, Anopheles triannulatus s.l., Anopheles intermedius, Anopheles mattogrossensis, Anopheles mediopunctatus and Anopheles peryassui have been found naturally infected with malaria parasites in this region [4].
The main malaria vector in northern Brazil is An. darlingi, which presents a high anthropophily and can be found feeding both indoors and outdoors, especially during morning and evening twilight hours [4,5,6,7]. Anopheles (Nyssorhynchus) darlingi is widely distributed in areas of low altitude in South America; this species is absent in the Northeast dry areas of Brazil, in the far South of the country, and in areas of high altitude [5]. Some conditions have challenged malaria elimination programmes such as anthropogenic processes (mining and deforestation), asymptomatic parasite carriers, recurrent P. vivax relapses, anti-malarial drug resistance, vector control limitations due to widespread insecticide resistance, in addition to the occurrence of particular epidemiological scenarios including urban, indigenous, or border malaria [8,9,10]. In urban settings, evidence of local malaria transmission has proven uncommon, and there are many unanswered questions regarding its entomological parameters, such as natural infection rates, identification of Anopheles species, and their temporal/spatial distribution [10].
In this regard, several studies have brought to attention the need to resume investments in vector surveillance approaches and sustainable interventions, since the underestimation and weakening of these measures may provide an opportunity for vector population recovery in a given location or even colonization of a new area.
Training and maintaining entomologist teams, community participation, strengthening collaborations, and mixed control strategies must be encouraged; political support will be necessary as well. Many gaps in knowledge still exist in Brazil, and malaria surveillance should be improved with updated mapping of anopheline species, their vectorial competence, molecular taxonomic status, and breeding site records [11, 12]. Hence, vector surveillance is crucial to providing information on species composition, density, behaviour, biology, and natural infection by Plasmodium spp. [13]; this tool is the cornerstone to defining control and prevention strategies and investigating the effects of intervention efforts on malaria transmission [10,11,12].
The monitoring of adult anopheline mosquitoes can be based on entomological collections performed using a series of methods that aim to generate information about the vector mosquito and its relationship with the host [14]. Several techniques employ synthetic and/or physical stimuli in order to capture Anopheles spp. mosquitoes, such as BG-Malaria traps [15], BG-Sentinel traps [16], CDC traps (Centers for Disease Control and Prevention®), Shannon tents, screen traps [17, 18], and Mosquito Magnet® traps [19]. Entomological collection using humans as attractive bait is the most frequent and considered the gold standard mainly due to its effectiveness in capturing anthropophilic mosquitoes, which potentially infect humans. However, the Human Landing Catch technique (HLC) involves ethical issues similar to those raised by controlled infection studies in humans, due to the collector’s exposure, as well as the fact that the results are influenced by differences in attractiveness among operators [20]. In this context, alternative practices for capturing these vectors can help reduce the risks to humans [21], for instance tent traps baited with protected human/vertebrates which prevent mosquito bites [22, 23].
Several studies have sought to develop alternative methodologies/devices for vector sampling that take into account behavioural stimuli and biological traits, and specifically, do not constitute a risk to the collector’s health. The main outcomes assessed their efficiency in collecting mosquitoes in different environments and conditions as promising and feasible techniques that can be included in routine surveillance [24,25,26]. Thus, the present study aimed to compare anopheline diversity (abundance and richness) between protected HLC and alternative sampling techniques in urban and peri-urban malaria foci in a municipality within the Brazilian Amazon—Porto Velho—over different seasonal periods, providing additional information about female feeding behaviour and faunal composition. The municipality of Porto Velho accounted for 54% of the 14,412 autochthonous malaria cases recorded in the state of Rondônia in 2021, according to the federal government's Interactive Malaria Bulletin (https://public.tableau.com/app/profile/mal.ria.brasil#!/). Since urban cores comprise the largest human agglomerations, these populations may be at risk for the disease, and thus the elimination of malaria in these areas is a public health priority, as well as studies on vector species of Plasmodium spp.
Methods
Study areas
Sampling was carried out during the months of February, April, June, August, October, and December 2018. Four sampling sites were selected in the city of Porto Velho (Fig. 1), which is the capital of the state of Rondônia, corresponding to the following neighbourhoods/localities: Nova Esperança (NE), Bairro Novo (BN), Belmont (BE), and Colônia Viçosa (CV). Two sites were selected in urban settings (NE = 8°'32.18" S, 63° 52′ 5.96″ W and BN = 8° 48′ 8.74″ S, 63° 48′ 14.28″ W) and two different sites were selected in peri-urban settings (BE = 8° 39′ 30.60″ S, 63° 54′ 37.50″ W and CV = 8° 53′ 10.59″ S, 63° 49′ 55.81″ W).
Briefly, the sites selection criteria were the number of malaria cases, administrative urban boundaries, presence of vegetation and water courses, and anopheline occurrence. Epidemiological data were gathered from the Malaria Epidemiological Surveillance Information System (SIVEP-Malaria), stratified by probable place of infection in the city of Porto Velho, between 2015 and 2017, and neighbourhoods/localities were searched and plotted in the administrative maps of the city of Porto Velho. Thirteen locations with the highest number of malaria cases were listed (Additional file 1: Table S1, Fig. S1). Then, a survey of anophelines was carried out at twenty-five sites in order to verify the presence of Anopheles mosquitoes (Additional file 1: Table S2); vegetation cover and watercourses close to residential areas were also taken into account when selecting the four sites. Urban sites were located at the urban core and expansion area and peri-urban sites were peripheral, about 1–4 km from urban administrative boundaries (Additional file 1: Fig. S1); sites were about 5 km apart.
Sampling techniques
For adult mosquito capture, three sampling techniques were employed, based on the efficiency of anopheline capture reported in the literature, costs and benefits, logistical feasibility, and availability of materials. The protected human landing catch method—PHLC (Fig. 2a), and the alternative techniques, barrier screen and tent trap (Gazetrap) (Fig. 2b), were performed simultaneously for 6-h/night (from 6 to 12 pm). Techniques were performed exclusively in the outdoors, avoiding extradomiciliary habitat variability such as animal shelters, forest edges, movement of humans, etc., PHLC was considered the "gold standard" for anopheline collection, and it has been employed in several comparative and surveillance studies [27]. Briefly, PHLC consisted of capturing anopheline females that landed on human legs and feet protected by socks, before they started blood-feeding [28], with the aid of a manual suction aspirator and a flashlight with an LED light. PHLC was performed for 45 min every hour and the last 15 min were used for barrier screen sampling. The barrier screen (hereafter, referred to as “Screen”, 15 m width × 2 m height) (Fig. 2c) was built as described in Moreno et al. [17]; however, the Screen colour was changed from green to black. The resting mosquitoes were sampled on both sides of the Screen using a manual suction aspirator for 15 min during every hour of collection. The tent trap (hereafter, referred to as “Gazetrap”) incorporated components and materials based on two tent traps described in the literature. The Gazetrap was built from a gazebo canopy tent (3 × 3 m), and as proposed by Russell et al. [22] to a commercial tent, two sides were closed and two remained open; unlike these authors, only human bait was used. An inner chamber was included based on a Brazilian tent, Mosqtent®, to protect the humans from mosquito bites. Mosqtent® is also a gazebo-like tent, built with an elaborate and intricate double-chamber trap, based on human attractants [23]. After an interval of 45 min every hour, the sides were closed and mosquitoes inside the Gazetrap were collected using manual aspiration and electric suction for 15 min. Sampling techniques were placed from 1 to 30 m from the dwellings, always between the dwelling and the watercourse, which were about 5–90 m apart. Riparian habitats and outdoors were occupied by managed vegetation, with gardens, fruit orchards, and cassava plantations. Except for "Bairro Novo", all locations had free-ranging hens in the outdoor environment.
Study design
For each site, the anopheline collections were carried out over three consecutive days, bimonthly throughout the year 2018, totalling six sampling events, two during the rainy season (February and December), two during the dry season (June and August), and two events during transitional seasons (April and October). The three sampling techniques were employed simultaneously, with a minimum distance of 20 m between them, at each site. There was an hourly alternance of collectors between techniques in each night-sampling. Overall, 18 nights of sampling efforts were performed for each locality, and total effort of 432 h for Gazetrap, 324 h for PHLC, and 108 h for Screen. Mosquitoes were separated in plastic cages by site, collection hour, and capture technique and transported to the Entomology Laboratory at FIOCRUZ-RO. Mosquitoes were maintained alive and fed with cotton soaked in 10% sucrose solution until they were identified taxonomically. Adult mosquitoes were anesthetized with ethyl acetate PA and specimens were morphologically identified using stereo microscopes and dichotomous keys as proposed by Consoli and Lourenço-de-Oliveira [5] and Forattini [29]. The nomenclature of Anopheles mosquitoes followed the classification system proposed by Harbach [30]. After performing taxonomic delimitation, the anopheline species/taxa were counted and stored (by date, site, sampling type, collection time, and blood feeding status) in microtubes at − 20 °C for further molecular detection of Plasmodium spp. Whenever possible, two to four individuals of each anopheline species were mounted with an entomological pin to be deposited in the COLRO—the Entomological Collection of Fiocruz Rondônia and INCT-EpiAmO.
Data analysis
Generalized linear mixed models (GLMM) and generalized linear models (GLM) were used for abundance data analysis (Additional file 2: Appendix S1). Anopheline abundance was considered to be the number of individual mosquitoes recorded hourly for each three-day sampling event. The city setting (urban or peri-urban), technique (PHLC, Screen, or Gazetrap), and season (Rainy, Dry, or Transitional) were set as predictors. Since ecological data of vector counts are usually discrete variables and rarely assume normally distributed errors [31], models were carried out using negative binomial errors and “log” link (; glm.nb function—MASS package; nbinom2()—glmmTMB package). Due to the hierarchical/nested nature of study design, the variables “Site” (clustered by city setting), sampling “Day”, and “Sampling event” were set as random effects in the models, taking into account probable temporal and spatial autocorrelation in the outcomes. The proportion of estimated variance for model components (random and fixed factors) was examined by comparison of GLMM’ pseudo-R-squared (conditional and marginal R2). Model comparisons were carried out based on maximum likelihood, using SBC values (Schwarz's Bayesian criterion) for each fitted model (BIC function/stats). The models with the lowest SBC values were considered the best approximation models and compared with the null models. Model adjustment was analysed by means of diagnostic plots of the residual adequacy (normality, homoscedasticity, and outliers). Complementary analyses were performed to examine if the responses obtained on a given sampling day/event were related to previous sampling in time using testTemporalAutocorrelation() function from DHARMa package (Additional file 2: Appendix S1). Individual-based rarefaction curves were used to estimate and compare both the absolute species number (species richness) and the Anopheles species diversity between levels of predictors (settings, techniques, and seasons). Diversity estimates and its 95% confidence intervals were based on the Hill numbers: richness (q = 0), Shannon diversity (q = 1), and Simpson diversity (q = 2), using a matrix of abundance data for each anopheline species. Further, individual-based rarefaction (interpolation) and extrapolation curves of Hill diversity were plotted with 1000 bootstrap replications and 3500 individuals as endpoint (for graphical purposes), using the package iNEXT [32]. Individual-based rarefaction made it possible to estimate the expected richness in a small subset of “n” individuals drawn from the reference sample (observed abundance of the sampled species) [33]. Differences and similarities in the Anopheles species composition were tested by Permutational Multivariate Analysis of Variance (PERMANOVA) and Permutational Analysis of Multivariate Dispersions (PERMDISP). For this, Sørensen distance matrices were computed using presence/absence data and the multivariate analyses evaluate whether the within-group distances were different/similar from the between-group distances, take into account seasons and sampling techniques. Nonmetric multidimensional scaling (NMDS) plots were used to depict Anopheles assemblage dissimilarities among predictor levels. The R vegan, lattice, and permute packages were used in the compositional analyses. Mosquito biting behaviour (female engorgement and hourly activity) was graphically inspected in order to evaluate spatial and temporal patterns, and trap efficiency. The model estimate outputs were tabulated using sjPlot 2.8.4 and sjmisc 2.8.5. All graphs and analyses were performed in the R platform, version 3.6.0.
Results
Overall, 6136 dipterans were collected, belonging to nine Culicidae genera, biting midges (Ceratopogonidae: Culicoides spp.), and sandflies (Psychodidae: Phlebotominae) (Additional file 3: Tables S3 and S4). The genus Anopheles was the most abundant; 2962 (49.1%) individuals were identified, including 12 species and one complex (An. mediopunctatus/costai/forattini). Seventeen individuals were damaged and remained in the genus level delimitation (Additional file 4: Tables S5, S6, and S7). The statistical modelling approach with fixed (city setting, season, and sampling technique) and random effects (sampling day per event) revealed distinct patterns for the abundance estimates. The number of anopheline individuals varied strongly with city setting, sampling technique, and collection day in each sampling event, included as random factor. In turn, anopheline diversity was dependent on the Hill numbers, as result from the relative abundance of mosquito species, and Anopheles assemblage composition did not vary between seasons and sampling techniques.
Effects of city setting, season, and sampling technique on anopheline abundance
More anophelines were captured in the peri-urban sites (Fig. 3a), differing significantly from urban localities (z = − 8.61, p < 0.001), and in the months of June and August, during the dry season (Fig. 3c); however, no significant seasonal difference was observed on anopheline abundance (z = − 1.18; − 1.05; p > 0.05) when model was adjusted for collection day in each sampling event (see next section). The average number of Anopheles individuals also varied significantly among the three sampling techniques. As expected, PHLC was the most productive technique, capturing 1,662 anophelines (Fig. 3b) and anopheline abundance estimates for PHLC significantly differed from Gazetrap and Screen (z = 5.61, p < 0.001). There was no variation between estimates for Gazetrap and Screen in the fixed- and random-effects model (Additional file 4: Table S8).
Temporal patterns in the abundance of Anopheles spp.
The best-fit model suggested no seasonal variation in the anopheline abundance when considering collection day per sampling event as a random effect, since consecutive daily samplings were considered the main source of serial dependence. Although there is no significant temporal autocorrelation (Additional file 2: Appendix S1), collection day explained part of variance in the model. The variance in the mean number of Anopheles spp. individuals was mainly due to between-group differences (τ00 = 0.70), i.e., between the bimonthly sampling events (Additional file 4: Table S8). Besides, random effect accounted for about half of the variance computed for the entire model (Conditional r-squared, R2c = 0.067) since pseudo-R2 estimated for fixed predictors was 0.031 (Marginal r-squared, R2m, Additional file 4: Table S8).
Effects of city setting, season, and sampling technique on anopheline diversity
The estimates for absolute number of anopheline species (Richness, q = 0) did not differ between urban and peri-urban settings; PHLC, Gazetrap, and Screen techniques; and Dry and Transitional seasons. There was a partial overlap in the 95% confidence, and a tendency of the lowest number of Anopheles species was observed in the Rainy season. However, the estimates of Shannon and Simpson diversity indices showed different patterns: urban setting, Gazetrap technique, and Transitional season presented a more diverse fauna of Anopheles species (Fig. 4).
Individual-based rarefaction (solid lines) and extrapolation curves (dashed lines) of Anopheles diversity from Porto Velho, in the Brazilian Amazon. Diversity based on Hill numbers (0, 1, 2) were estimated for city settings, sampling techniques, and seasons. Geometric shapes in the lines depict the reference sample for each comparison level
Proportion of Anopheles individuals per setting × technique and technique × hours
All techniques showed similar performance when comparing the proportion of specimens captured in urban versus peri-urban settings (Fig. 5a). Although the alternative Screen and Gazetrap methods captured fewer anophelines, there was a proportionality between PHLC and these techniques in terms of the number of individuals collected over the sampling hours (Fig. 5b).
Anopheles biting activity
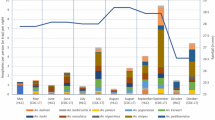
In general, anophelines were more frequently captured between 6 and 8 p.m., but there was a clear difference in the biting activity of anopheline mosquitoes between urban and peri-urban settings (Fig. 6). The first sampling hour (from 6 to 7 p.m.) was the main peak of activity in the urban sites (n = 185), when the highest number of Anopheles specimens were caught. However, anopheline catches were higher during the second hour (from 7 to 8 p.m.) in peri-urban areas, with an average slightly higher than in the urban sites (\(\overline{X }\)= 5.4 and \(\overline{X }\)= 5.7). After 8 p.m., both the absolute and average number of individuals decreased. Anopheline richness did not show clear patterns between the collection periods (Fig. 6).
Feeding status
Almost all of the mosquitoes captured were females (n = 2959), and about a third (33%, n = 952) were engorged with blood upon capture. The proportion of engorged and non-engorged females varied among the sampling techniques. As expected, more engorged mosquitoes (47.8%, n = 305) were captured relatively in the Screen than in PHLC (28.3%, n = 470) or Gazetrap (26.9%, n = 177) (Fig. 7).
Anopheles species composition
Anopheles darlingi was the predominant species in all sites and accounted for about 86% of the total individuals captured (n = 2563), followed by An. triannulatus (5.98%, n = 177), An. mattogrossensis (3.24%, n = 96), and An. konderi (1.49%, n = 44). An. strodei, An. nuneztovari, An. benarrochi, An. minor, An. argyritarsis, An. deaneorum, An. mediopunctatus/costai/forattini, An. braziliensis and An. peryassui were represented by less than 1% of the individuals. The species An. benarrochi, An. braziliensis and the An. mediopunctatus/costai/forattini complex were found only in urban sites (Fig. 8). In addition, the alternative techniques were exclusively responsible for their captures. Anopheles braziliensis was captured only in Gazetrap while Anopheles mediopunctatus/costai/forattini was unique to the Screen technique (Fig. 8). There were no significant patterns in the Anopheles assemblage structure when season and sampling technique levels were compared by PERMANOVA (season: pseudo-F2,15 = 1.114, P = 0.382; technique: pseudo-F2,9 = 1.173, P = 0.985) and PERMDISP (season: pseudo-F2,15 = 1.114, P = 0.382; technique: pseudo-F2,9 = 0.166, P = 0.834) tests, visually confirmed by NMDS ordination plots (Additional file 4: Table S9, Fig. S2a,b).
Relative abundance of Anopheles species by city setting, sampling technique, and season. Relative abundance was determined for each species and compared between levels of the variables Setting, Technique, and Season. Values range from 0 to 1, with relative abundances close to 0 in light blue, and close to 1, dark blue in the colour gradient
Discussion
Results of the present study demonstrated that the surroundings and urban core of a city in the Brazilian Amazon may harbour a variety of anopheline species, potential vectors of Plasmodium spp., which in turn coexist in both urban and peri-urban environments. This faunal diversity can vary according to location, season, and behavioural aspects of each species, depending on sampling procedures.
Abundance and diversity of anophelines in the urban-peri-urban setting
Anopheline abundance was the main variable affected by model predictors. The greatest number of individuals were collected in peri-urban areas, a result commonly found in other studies throughout the Amazonian region, mainly in areas with vegetation and newly anthropized areas near watercourses, like in the present study [34, 80]. Despite the low density of anophelines in the urban settings sampled in this study, a high endemicity of malaria is possible even in a low density of vectors [6]. As observed in Manaus—Amazonas state, the expansion and creation of new residential areas, such as neighbourhoods and squatters, preceded malaria outbreaks in some areas of the city [35].
On the other hand, the richness of Anopheles spp. did not vary significantly among the Porto Velho sites, though the absolute number of species found in urban areas was higher than in peri-urban areas. This can be explained by the variation in species composition, seasonality, and habits of the anopheline fauna, which can adapt to environments with recent anthropic changes [36]. Surprisingly, individual-based rarefaction/extrapolation estimates revealed a more diverse fauna in urban settings, and these findings contrast with some recent studies about the anthropic impact on Plasmodium spp. vectors [37, 38], requiring further investigation, especially those on insect dispersal between urban, peri-urban, and rural areas.
Abundance and diversity of anophelines and seasons
Malaria vectors can also easily adjust to natural environmental changes, differing in terms of regional, local and species reproductive patterns. An example is the increased density of anophelines during the rainy season due to the creation of temporary breeding sites [39]. However, in urban and peri-urban settings of Porto Velho, more individuals were collected during the dry season, although no statistical significance. This pattern differs from some findings in the region that show an increase in anophelic density during the rainy season and a decrease at the beginning of the dry season [34, 40]. One explanation for this divergence is that in anthropized environments, similar to the city of Porto Velho, the availability of artificial breeding sites may continue to exist, regardless of the season. Thus, the existence and maintenance of these larval habitats can reduce the impact of seasons on the dynamics of vector populations [41,42,43]. For instance, An. darlingi larvae have been found in dams, fishponds, clay pits, containers, and artificial ponds; An. triannulatus, in fishponds, artificial ponds, and drum/tanks; An. argyritarsis, in drum/tank, plant pots, water tanks, car parts, plastic containers, cans, dams, fishponds, clay pits, artificial ponds, and water tank. An. nuneztovari, An. peryassui, An. braziliensis, and An. deaneorum larvae were also collected in dams, fishponds, clay pits, and plastic bottle traps [41, 44,45,46,47,48,49]. Individual-based rarefaction curves indicated that the transitional season accumulated the greatest diversity of anopheline species. Such pattern was previously described for Anopheles and other mosquitoes, and at the Brazilian Amazon, can be also associated with hydrological cycles, flooding, and climatic/environmental changes [50, 51].
Abundance and diversity of anophelines and techniques
A study conducted in Rondônia in 1999 compared HLC, PHLC, and two types of Shannon trap, and found that about half of the total anophelines were captured with HLC, followed by PHLC (a quarter), and to a lesser extent in Shannon traps [27]. As expected, the presented results confirm the efficiency of the Human Landing Catch technique in assessing anopheline densities at different places and times. For instance, the HLC (and PHLC) technique has previously demonstrated a higher yield in comparison with other traps [25, 52], which can be explained by the fact that the main malaria vector species are quite anthropophilic [25]. Entomological findings also demonstrated the importance of alternative and complementary traps to caught zoophilic species in urban areas, as An. brasiliensis, An. mediopunctatus/costai/forattini, and An. minor caught only Gazetrap tent and Barrier Screen.
Despite PHLC’s superior performance, the data also showed a similar proportion among techniques when comparing the number of individuals collected in each type of city setting and during different sampling hours. Alternative methods such as tent traps and mosquito interception traps have been proven to be feasible and effective for the surveillance of Anopheles mosquitoes, both compared to HLC/PHLC and used individually [17, 22, 23]. This variation in the efficiency of collection methods can be influenced by landscape characteristics, anopheline species, and aspects of the local human population, such as their culture and behaviour, among other factors [27]. The rarefaction analysis showed that the alternative techniques achieve higher anopheline diversity than PHLC, and together with the possibility of minimizing such challenges, make these techniques desirable in inventory and biodiversity studies. The individual protection measures can be maintained while performing alternative techniques, and the choice of a particular/single strategy should consider the research/surveillance objectives, and whether there are predefined anopheline species as the target of the study. Also, ethical issue attendance, genus specificity, non-powered components, low technical expertise, logistical feasibility, material availability, mimicry of synanthropic habitats, and high rates of engorged mosquitoes (and those looking for blood sources) were the main benefits associated with the use of these alternative traps, Barrier screen and Gazetrap.
Feeding status
A higher percentage of engorged individuals were obtained with the Screen method than with the other two capture techniques, a similar result to that recorded in recent studies, especially with anophelines [17, 18, 53, 54]. The positioning of the barrier screen between feeding sites and oviposition and/or resting sites allows for the collection of mosquitoes that were blood-fed or those that were in search of blood meals [53]. These results indicate that properly positioned interception traps can be useful in capturing blood-fed zoophilic, anthropophilic, and opportunistic Anopheles mosquitoes. Barrier screen traps have provided important insights about vector species and possible vectors, their flight and feeding behaviour, preferred hosts, and host-seeking periods and peaks [18, 54]. In addition, screen traps can complement PHLC in places with a high density of mosquitoes, reducing collection time and sampling efforts, making it a very economical collection strategy that can be used in remote locations [18], keeping in mind individual protection measures, target species, and malaria surveillance and research goals. For instance, An. darlingi was predominantly caught in PHLC.
Anopheles biting activity
Anopheline biting activity is one of the biological parameters directly related to the malaria epidemiology [55]. In northern Brazil, Anopheles mosquitoes show peaks of biting activity at dusk and dawn [56, 57]. Following the same pattern, most urban anophelines in Porto Velho showed their greatest activity between 6:00 p.m. and 8:00 p.m., corroborating other studies carried out in the Brazilian Amazon [39, 55, 58].
Furthermore, it is not uncommon to find variation in peak hours of biting activity, depending on the anopheline species and the place of collection [58,59,60]. An example of this variation is mosquitoes of the species An. darlingi, which have non-twilight habits (9:00 p.m. to 12:00 a.m.); however, they have been found biting during all hours of the night, with peaks occurring at different times [60,61,62,63]. Hence, host-seeking and biting activity of some species of anophelines may depend on several factors, since these mosquitoes are very sensitive to variations and anthropic actions, such as deforestation and urbanization, which generate changes in environmental and meteorological conditions (temperature and relative humidity), affecting the availability of suitable sites for vector reproduction. Biting behaviour can also vary according to area and/or geographic location [29].
The review by Stone & Cross [64] points out that environmental changes can exert selective pressure on the feeding behaviour of mosquitoes, which can result in differentiated patterns of malaria transmission. Other determining factors for variations in biting frequency include the season, precipitation, genetic variability, control measures adopted in the location, habits of the local population, quality and variety of hosts, and the dispersion and relative abundance of species [64, 65].
Anopheles species composition
The twelve species and one anopheline complex found in the city of Porto Velho have also been described in other studies carried out in the Brazilian Amazon, though surprisingly, the high diversity at the urban setting was previously described only for rural areas [34, 39, 56, 63, 66,67,68]. Despite this, PERMANOVA and PERMDISP tests showed no variance in the Anopheles assemblage structure between seasons and sampling techniques, probably due to the restricted number of sampled sites.
Anopheles mattogrossensis, An. konderi, An. strodei, An. nuneztovari, An. benarrochi, An. minor, An. argyritarsis, An. deaneorum, An. mediopunctatus/costai/forattini, An. braziliensis, and An. peryassui presented low densities in the sampled urban sites, and are commonly found in other places in the Amazon region [37, 40, 56, 63, 66,67,68,69,70], which can be explained by host preference and the collection techniques used in this study [66], in addition to environmental and seasonal factors.
Regarding epidemiological importance, while An. darlingi is the main vector of Plasmodium spp. in the northern region of Brazil [5], the species An. triannulatus is considered a secondary vector [71], in addition to other species like An. mattogrossensis, An. strodei, An. nuneztovari, An. deaneorum, An. mediopunctatus/costai/forattini, An. braziliensis, and An. peryassui, which have previously been found to be naturally infected with human Plasmodium [4].
Anopheles darlingi was the most abundant species in urban and peri-urban settings, and this predominance over the other species was expected [34, 58, 63, 67,68,69]. Some studies indicate that An. darlingi may be the main vector responsible for the maintenance of malaria in human populations and that a reduction in the population density of this species in a given region can bring about the reduction or even the disappearance of malaria [6, 72, 73]. Several factors may contribute to the vectorial capacity of An. darlingi: the species being highly susceptible to Plasmodium species that infect humans, anthropophilic habits, its ability to transmit malaria indoors and outdoors, and biting behaviour related to anthropic changes [5, 72, 74, 75].
However, due to their dependence on the physiognomy of the landscape, other anopheline species may show dominance [55]. In some urban sites in Porto Velho, An. triannulatus presented similar density and frequency values to those of An. darlingi; this co-occurrence was previously described [40, 58, 76]. Anopheles triannulatus has an opportunistic and generalist behaviour, benefiting from host availability, being able to colonize altered or anthropized environments, and often becoming more abundant or even dominant over other species [39, 79]. This behaviour, together with the fact that it was found to be naturally infected by P. vivax, P. falciparum, and Plasmodium malariae, ranked this species as an occasional vector of malaria [4, 71].
The unexpected diversity of anopheline mosquitoes in the urban and peri-urban settings may reflect environmental changes due to disorderly urban growth and implementation of hydroelectric power plants near Porto Velho. Its recent urban growth has triggered social and environmental conditions that have enabled the persistence of urban malaria foci in some neighbourhoods of Porto Velho, mainly in peri-urban settings [77]. On the other hand, a hydroelectric artificial lake can provide larval habitats for these mosquitoes. The reproduction and survival of malaria-transmitting mosquitoes, especially An. darlingi, depends on the existence of favourable environments with watercourses, vegetation cover, and dwellings close to breeding sites [34, 68, 78], criteria utilized when selecting the sampled sites.
These findings indicate that, in addition to anophelic density and sampling techniques, studies on species taxonomy should be encouraged due to the possible participation of other species considered secondary in the dynamics of malaria transmission [42, 55]. Besides the investigation of infection rates by Plasmodium spp. And seasonal density, it is suggested that occasional zoophilic and anthropophilic anopheline species be analysed for their food sources, even in urbanized areas, and that entomological surveillance should be used continuously in these areas in order to strengthen control strategies.
Study limitations
There are still some potential limitations in the work, such as a small sample size to evaluate spatial patterns (only four sites) in urban/peri-urban areas and the trap study design. Due to their dimensions, Screen and Gazetrap remained in a fixed location throughout the study, which could compromise the outcome randomness (influence of habitat and microclimatic heterogeneity). Also, a minimal trap-distance of 20 m could result in interference/competition between techniques.
Conclusions
This study highlights the elevated number of anopheline mosquito species in urban settings of an Amazonian city, most of which are Plasmodium vectors. Furthermore, the transitional season periods can also present a high diversity of anophelines. Most of the anophelines were captured using the “gold standard” PHLC technique, and the findings suggest that the alternative techniques, Gazetrap and Screen, can complement PHLC, and depending on the purpose, they may be preferable. Non-anopheline mosquitoes, sandflies, and biting midges were also collected, and further studies should evaluate alternative trap efficiency for an integrated surveillance system of other VBD. More studies on mosquito species diversity and taxonomy should be encouraged due to the possible participation of secondary vector species in urban malaria outbreaks.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- PHLC:
-
Protected Human Landing Catch
- HLC:
-
Human Landing Catch
- GLM:
-
Generalized Linear Model
- CDC:
-
Centers for Disease Control and Prevention®
- SIVEP-Malaria:
-
Malaria Epidemiological Surveillance Information System
- FIOCRUZ-RO:
-
Fundação Oswaldo Cruz Rondônia
- INCT-EpiAmO:
-
National Institute of Epidemiology of the Western Amazon
References
WHO. World Malaria Day 2017: malaria prevention works, let’s close the gap (No. WHO/HTM/GMP/2017.6). Geneva: World Health Organization; 2017.
WHO. World Malaria Report 2021. World Health Organization; 2021.
BRASIL MS 2021. Secretaria de Vigilância em Saúde. Malária 2021. Brasília: Boletim Epidemiológico. Número especial; 2021. 100 pp. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos/especiais/2021/boletim_epidemiologico_especial_malaria_2021.pdf. Access 15 Apr 2022.
Carlos BC, Rona LD, Christophides GK, Souza-Neto JA. A comprehensive analysis of malaria transmission in Brazil. Pathog Glob Health. 2019;113:1–13.
Consoli RAGB, Lourenço-De-Oliveira R. Principais Mosquitos de Importância Sanitária no Brasil. Fundação Oswaldo Cruz; 1994.
Tadei WP, Thatcher BD, Santos JMM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59:325–35.
Campos M, Alonso DP, Conn JE, Vinetz JM, Emerson KJ, Ribolla PEM. Genetic diversity of Nyssorhynchus (Anopheles) darlingi related to biting behavior in wester Amazon. Parasit Vectors. 2019;17:12.
Recht J, Siqueira AM, Monteiro WM, Herrera SM, Herrera S, Lacerda MV. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J. 2017;16:273.
Melo JO, Padilha MAO, Barbosa RTA, Alonso WJ, Vittor AY, Laporta GZ. Evaluation of the malaria elimination policy in Brazil: a systematic review and epidemiological analysis study. Trop Biomed. 2020;37:513–35.
Wilson ML, Krogstad DJ, Arinaitwe E, Arevalo-Herrera M, Chery L, Ferreira MU, et al. Urban malaria: understanding its epidemiology, ecology, and transmission across seven diverse ICEMR network sites. Am J Trop Med Hyg. 2015;93(Suppl 3):110–23.
Baia-da-Silva DC, Brito-Sousa JD, Rodovalho SR, Peterka C, Moresco G, Lapouble OMM, et al. Current vector control challenges in the fight against malaria in Brazil. Rev Soc Bras Med Trop. 2019;52: e20180542.
Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14: e0007831.
Gomes ADC. Vigilância entomológica. Informe Epidemiológico do SUS. 2002;11:79–90.
Moss WJ, Dorsey G, Mueller I, Laufer MK, Krogstad DJ, Vinetz JM, Chery L. Malaria epidemiology and control within the international centers of excellence for malaria research. Am J Trop Med Hyg. 2015;93(Suppl 3):5.
Gama RA, Silva IMD, Geier M, Eiras AE. Development of the BG-Malaria trap as an alternative to human-landing catches for the capture of Anopheles darlingi. Mem Inst Oswaldo Cruz. 2013;108:763–71.
BIOGENTS. The BG-Sentinel: Biogents' mosquito trap for researchers. 2019. https://www.bg-sentinel.com/. Accessed 12 Dec 2021.
Moreno M, Saavedra MP, Bickersmith SA, Prussing C, Michalski A, Rios CT, et al. Intensive trapping of bloodfed Anopheles darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. PLoS Negl Trop Dis. 2017;11: e0005337.
Davidson JR, Sukowati S, Asih PBS, Syafruddin D, Baskin RN, Laurent BS, et al. Using barrier screens to characterize mosquito composition, flight activity, and abdominal status in South Lampung, Indonesia. Parasit Vectors. 2018;11:440.
Rubio-Palis Y, Moreno JE, Sánchez V, Estrada Y, Anaya W, Bevilacqua M, et al. Can Mosquito Magnet® substitute for human-landing catches to sample anopheline populations? Mem Inst Oswaldo Cruz. 2012;107:546–9.
Wong JN, Bayoh G, Olang GF, Killeen MJ, Hamel JM, Vulule JE, et al. Standardizing operational vector sampling techniques for measuring malaria transmission intensity: evaluation of six mosquito collection methods in western Kenya. Malar J. 2013;12:1–43.
Jamrozik E, Fuente-Núñez V, Reis A, Ringwald P, Selgelid MJ. Ethical aspects of malaria control and research. Malar J. 2015;14:518.
Russell TL, Beebe NW, Bugoro H, Apairamo A, Cooper RD, Collins FH, et al. Determinants of host feeding success by Anopheles farauti. Malar J. 2016;15:152.
Lima JBP, Galardo AKR, Bastos LS, Lima AWS, Rosa-Freitas MG. MosqTent: an individual portable protective double-chamber mosquito trap for anthropophilic mosquitoes. PLoS Negl Trop Dis. 2017;11: e0005245.
Hawkes FM, Dabiré RK, Sawadogo SP, Torr SJ, Gibson G. Exploiting Anopheles responses to thermal, odour and visual stimuli to improve surveillance and control of malaria. Sci Rep. 2017;7:17283.
Laurent BST, Sukowati S, Burton TA, Bretz D, Zio M, Syah F, et al. Comparative evaluation of anopheline sampling methods in three localities in Indonesia. Malar J. 2018;17:13.
Sanou A, Guelbéogo WM, Nelli L, Toé KH, Zongo S, Ouédraogo P, et al. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar J. 2019;18:386.
Lima JBP, Rosa-Freitas MG, Rodovalho CM, Santos F, Lourenço-de-Oliveira R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? A review. Mem Inst Oswaldo Cruz. 2014;109:685–705.
BRAZIL MS, 2019. Guia para o planejamento das ações de captura de Anofelinos pela Técnica de Atração por Humano Protegido (TAHP) e acompanhamento dos riscos à saúde do profissional capturador. Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Ministério da Saúde, Brasília. 2019; 27. https://bvsms.saude.gov.br/bvs/publicacoes/guia_planejamento_acoes_captura_anofelinos_tecnica_atracao_humano_protegido.pdf. Access 10 May 2022.
Forattini OP. Culicidologia Médica: Identificação, Biologia, Epidemiologia. 2ª edn. Editora da Universidade de São Paulo; 2002.
Harbach R. Mosquito Taxonomic Inventory: valid species list. 2022. https://mosquito-taxonomic-inventory.myspecies.info/sites/mosquito-taxonomic-inventory.info/files/Valid%20Species%20List_117.pdf. Accessed Aug 2022.
O’Hara R, Kotze J. Do not log-transform count data. Methods Ecol Evol. 2010;1:118–22.
Hsieh TC, Ma KH, Chao A. iNEXT: an R package for rarefaction and extrapolation of species diversity (H ill numbers). Methods Ecol Evol. 2016;7:1451–6.
Gotelli N, Ellison M. A primer of ecological statistics. Sunderland: Sinauer Associates Inc; 2013. p. 109–16.
Gil LHS, Rodrigues MDS, Katsuragawa TH. Seasonal distribution of malaria vectors (Diptera: Culicidae) in rural localities of Porto Velho, Rondônia. Brazilian Amazon Rev Inst Med Trop. 2015;57:263–7.
Saraiva MDGG, Amorim RDS, Moura MAS, Martinez-Espinosa FE, Barbosa MDGV. Expansão urbana e distribuição espacial da malária no município de Manaus, Estado do Amazonas. Rev Soc Bras Med Trop. 2009;42:515–22.
Gil LHS, Alves FP, Zieler H, Salcedo JM, Durlacher RR, Cunha RP, et al. Seasonal malaria transmission and variation of anopheline density in two distinct endemic areas in Brazilian Amazonia. J Med Entomol. 2003;40:636–41.
Chaves LSM, Bergo ES, Conn JE, Laporta GZ, Prist PR, Sallum MAM. Anthropogenic landscape decreases mosquito biodiversity and drives malaria vector proliferation in the Amazon rainforest. PLoS ONE. 2021;16: e0245087.
Doumbe-Belisse P, Kopya E, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, et al. Urban malaria in sub-Saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J. 2021;20:364.
Tadei WP, Thatcher DB. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Rev Soc Bras Med Trop. 2000;42:87–94.
Gil LHS, Tada MS, Katsuragawa TH, Ribolla PEM, Silva LHPD. Urban and suburban malaria in Rondônia (Brazilian Western Amazon) II: perennial transmissions with high anopheline densities are associated with human environmental changes. Mem Inst Oswaldo Cruz. 2007;102:271–6.
Collucci E, Sallum MAM. Records of Anopheles (Nyssorhynchus) (Diptera, Culicidae) in artificial containers in Ribeirão Preto City, State of São Paulo, Brazil. Rev Bras Entomol. 2006;50:431–2.
Reis IC, Codeço CT, Camara DCP, Carvajal JJ, Pereira GR, Keppeler EC, et al. Diversity of Anopheles spp. (Diptera: Culicidae) in an Amazonian urban area. Neotrop Entomol. 2018;47:412–7.
Rohr JR, Barrett CB, Civitello DJ, Craft ME, Delius B, DeLeo GA, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2:445–56.
Carreira-Alves JR. Finding of anophelines, subgenus Nyssorhynchus, in artificial containers. Brazil Rev Saúde Públ. 2001;35:407–8.
Silva AMD. Imaturos de mosquito (Diptera, Culicidae) de áreas urbana e rural no norte do Estado do Paraná, Brasil. Iheringia Série Zoologia. 2002;92:31–6.
Silva CMD, Sérvio HS, Ramos RAN, Faustino MADG, Alves LC, Carvalho GAD. Ocorrência de formas imaturas de culicídeos (Insecta: Diptera) na região nordeste do Brasil. Rev Bras Parasitol Vet. 2014;23:200–5.
dos Reis IC, Codeço CT, Degener CM, Keppeler EC, Muniz MM, de Oliveira FGS, et al. Contribution of fish farming ponds to the production of immature Anopheles spp. in a malaria-endemic Amazonian town. Malar J. 2015;14:452.
Rufalco-Moutinho P, Moura Kadri S, Peres Alonso D, Moreno M, Carrasco-Escobar G, Prussing C, et al. Ecology and larval population dynamics of the primary malaria vector Nyssorhynchus darlingi in a high transmission setting dominated by fish farming in western Amazonian Brazil. PLoS ONE. 2021;16: e0246215.
Arcos A, Ferreira FADS, Cunha HBD, Tadei WP. Characterization of artificial larval habitats of Anopheles darlingi (Diptera: Culicidae) in the Brazilian Central Amazon. Rev Bras Entomol. 2018;62:267–74.
Tadei WP, Rodrigues IB, Rafael MS, Sampaio RTDM, Mesquita HG, Pinheiro VCS, et al. Adaptative processes, control measures, genetic background, and resilience of malaria vectors and environmental changes in the Amazon region. Hydrobiologia. 2017;789:179–96.
Julião GR, Abad-Franch F, Lourenço-de-Oliveira R, Luz SLB. Measuring mosquito diversity patterns in an Amazonian terra firme rain forest. J Med Entomol. 2014;47:121–8.
Mathenge EM, Killeen GF, Oulo DO, Irungu LW, Ndegwa PN, Knols BGJ. Development of an exposure-free bednet trap for sampling Afrotropical malaria vectors. Med Vet Entomol. 2002;16:77–84.
Burkot TR, Russell TL, Reimer LJ, Bugoro H, Beebe NW, Cooper RD, et al. Barrier screens: a method to sample blood-fed and host-seeking exophilic mosquitoes. Malar J. 2013;12:4.
Pollard EJ, Russell TL, Burkot TR. Maximizing mosquito collections from barrier screens: the impacts of physical design and operation parameters. Parasit Vectors. 2019;12:31.
Martins LMO, David MR, Maciel-De-Freitas R, Silva-Donascimento TF. Diversity of Anopheles mosquitoes from four landscapes in the highest endemic region of malaria transmission in Brazil. J Vector Ecol. 2018;43:235–44.
Lourenço-De-Oliveira R, Guimarães AEDG, Arlé M, Silva TFD, Castro MG, Motta MA, et al. Anopheline species, some of their 108 habits and relation to malaria in endemic areas of Rondônia State, Amazon region of Brazil. Mem Inst Oswaldo Cruz. 1989;84:501–14.
Charlwood JD. Biological variation in Anopheles darlingi Root. Mem Inst Oswaldo Cruz. 1996;91:391–8.
Gama RA, Santos RL, Santos FD, Silva IM, Resende MC, Eiras ÁE. Periodicity of capture of the Anopheles darlingi Root (Diptera: Culicidae) in Porto Velho, Rondônia. Brazil Neotrop Entomol. 2009;38:677–82.
Barbosa LMC, Souto RNP. Aspectos ecológicos de Anopheles (Nyssorhyncus) darlingi Root 1926 e Anopheles (Nyssorhyncus) marajoara Galvão e Damasceno 1942 (Diptera: Culicidae) nos bairros Marabaixo I e Zerão, Macapá, Amapá. Brasil Biota Amaz. 2011;1:19–25.
Ferreira RAM, Da Cunha AC, Souto RNP. Distribuição mensal e atividade horária de Anopheles (Diptera: Culicidae) em uma área rural da Amazônia Oriental. Biota Amaz. 2013;3:64–75.
Tadei WP, Mascarenhas BM, Podestá MG. Biologia de anofelinos amazônicos. VIII. Conhecimentos sobre a distribuição de espécies de Anopheles na região de Tucuruí-Marabá (Pará). Acta Amaz. 1983;13:103–40.
Silva-Vasconcelos AD, Kató MYN, Mourão EN, Souza RTLD, Lacerda RNDL, Sibajev A, et al. Biting indices, hostseeking activity and natural infection rates of anopheline species in Boa Vista, Roraima, Brazil from 1996 to 1998. Mem Inst Oswaldo Cruz. 2002;9:151–61.
Cruz RMB, Gil LHS, De Almeida Silva EA, Da Silva Araújo M, Katsuragawa TH. Mosquito abundance and behavior in the influence area of the hydroelectric complex on the Madeira River, Western Amazon, Brazil. Trans R Soc Trop Med Hyg. 2009;103:1174–6.
Stone C, Gross K. Evolution of host preference in anthropophilic mosquitoes. Malar J. 2018;17:257.
Rozendaal JA. Observations on the distribution of anophelines in Suriname with particular reference to the malaria vector Anopheles darlingi. Mem Inst Oswaldo Cruz. 1990;85:221–34.
Klein TA, Lima JB, Tang AT. Biting behavior of Anopheles mosquitoes in Costa Marques, Rondônia. Brazil Rev Soc Bras Med Trop. 1991;24:13–20.
Gama RA, Silva IMD, Monteiro HADO, Eiras ÁE. Fauna of Culicidae in rural areas of Porto Velho and the first record of Mansonia (Mansonia) flaveola (Coquillet, 1906), for the state of Rondônia. Brazil Rev Soc Bras Med Trop. 2012;45:125–7.
Rodrigures MS, Batista EP, Silva AA, Costa FM, Neto VA, Gil LHS. Change in Anopheles richness and composition in response to artificial flooding during the creation of the Jirau hydroelectric dam in Porto Velho, Brazil. Malar J. 2017;16:87.
Morais AS, Urbinatti PR, Sallum MAM, Kuniy AA, Moresco GG, Fernandes A, et al. Brazilian mosquito (Diptera: Culicidae) fauna: I. Anopheles species from Porto Velho, Rondonia state, western Amazon, Brazil. Rev Inst Med Trop Sao Paulo. 2012;54:331–5.
Barbosa LMC, Souto RNP, dos Anjos Ferreira RM, Scarpassa VM. Behavioral patterns, parity rate and natural infection analysis in anopheline species involved in the transmission of malaria in the northeastern Brazilian Amazon region. Acta Trop. 2016;164:216–25.
Pimenta PF, Orfano AS, Bahia AC, Duarte AP, Ríos-Velásquez CM, Melo FF, et al. An overview of malaria transmission from the perspective of Amazon Anopheles vectors. Mem Inst Oswaldo Cruz. 2015;110:23–47.
Deane LM. Malaria vectors in Brazil. Mem Inst Oswaldo Cruz. 1986;81:5–14.
Tadei WP, Santos JMM, Scarpassa VM, Rodrigues IB. Incidência, distribuição e aspectos ecológicos de espécies de Anopheles (Diptera: Culicidae), em regiões naturais e sob impacto ambiental da Amazônia brasileira. In: Ferreira EJG, Santos GM, Leão ELM, Oliveira LA, editors. Editora Bases Científicas para Estratégias de Preservação e Desenvolvimento da Amazônia, vol. 2. Manaus: Instituto Nacional de Pesquisas da Amazônia; 1993. p. 167–96.
Klein TA, Lima JB, Tada MS, Miller R. Comparative susceptibility of anopheline mosquitoes in Rondônia, Brazil to infection by Plasmodium vivax. Am J Trop Med Hyg. 1991;45:463–70.
Klein TA, Lima JB, Tada MS. Comparative susceptibility of anopheline mosquitoes to Plasmodium falciparum in Rondônia. Brazil Am J Trop Med Hyg. 1991;44:598–603.
Souza-Santos R. Distribuição sazonal de vetores da malária em Machadinho d’Oeste, Rondônia, Região Amazônica, Brasil. Cad Saude Publica. 2002;18:1013–8.
Marques RD, Angelo JR, Lima AAD, Fuller T, Barcellos C. Production of Urban Space and the occurrence of malaria in the Brazilian Amazon: the Porto Velho case. Cien Saude Colet. 2021;26:4263–74.
Ferreira IM, Yokoo EM, Souza-Santos R, Galvão ND, Atanakasantos M. Factors associated with the incidence of malaria in settlement areas in the district of Juruena, Mato Grosso state, Brazil. Cien Saúde Colet. 2012;17:2415–24.
Silva-Do-Nascimento TF, Lourenço-De-Oliveira R. Diverse population dynamics of three Anopheles species belonging to the Triannulatus Complex (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 2007;102:975–82.
Luiz HS, Gil MS, Tada TH, Katsuragawa PEM, Ribolla LHP da, Silva (2007) Urban and suburban malaria in Rondônia (Brazilian Western Amazon) II: perennial transmissions with high anopheline densities are associated with human environmental changes. Memórias do Instituto Oswaldo Cruz 102(3) 271-276 https://doi.org/10.1590/S0074-02762007005000013
Acknowledgements
We are very grateful for the financial support from the partnership between FAPERO (Fundação de Amparo ao Desenvolvimento das Ações Científicas e Tecnológicas e à Pesquisa do Estado de Rondônia) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES). LHS Gil, for his support in taxonomic delimitation of some Anopheles species. TFS Nascimento and the LATHEMA/IOC team for confirming species’ taxonomies.
Funding
The Brazilian Ministry of Science and Technology (MCTI), CNPq, CAPES, and FAPERO partially funded this work, by means of the National Institute of Epidemiology of the Western Amazon (INCT EpiAmO-Grant#465657/2014-1). ACA Meireles received fellowship support from FAPERO/CAPES (Ch. FAPERO/CAPES No. 012/2016, TO#071/2016).
Author information
Authors and Affiliations
Contributions
ACAM: designed the mosquito sampling protocol, performed field collections, taxonomic identification, data analysis, and drafted the manuscript, GRJ: designed the study and mosquito sampling protocol, performed data analysis, acquired grants, and wrote the manuscript, AAL: contributed to the initial study design and to the site selection, LRS: collaborated in the taxonomic identification and field collections, MFS: helped in building the alternative traps and performing field collections, FGFR, CAM, LHMF: participated in the field collections. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
According to Normative Instruction no. 141 (December 19, 2006), the capture and transport of harmful synanthropic fauna are exempt from authorization by the SISBIO System (Biodiversity Authorization and Information System—ICMBio/MMA). Mosquito outdoor collection was formally authorized by each dwelling’s owner, using a Free and Informed Consent Form (CEP Fiocruz/IOC #2,647,939/2018).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Site Selection Criteria. Table S1. Number of malaria cases in the urban and peri-urban localities from the city of Porto Velho, between 2015-2017. Figure S1. Location of 25 sites sampled for anopheline presence in Porto Velho, Rondônia, Brazil. Table S2. Details on 25 sites where anopheline presence was recorded, and selected sites in bold.
Additional file 2.
Residual Diagnostics, Model Validation, and Temporal Autocorrelation Analyses - Anopheline Abundance Data.
Additional file 3.
Non-anopheline mosquitoes and other Dipterans. Table S3. Number of non-anopheline mosquitoes, biting midges, and sand flies caught with Gazetrap, PHLC, and Screen, at urban and peri-urban settings. Table S4. Number of non-anopheline mosquitoes, biting midges, and sand flies caught during seasonal periods, at urban and peri-urban settings.
Additional file 4.
Supplementary results for the number of individuals/species of Anopheles. Model Output – Best-fitted models. PERMANOVA / PERMDISP estimates and NMDS plots. Table S5. Anopheles species and individuals (N) captured at urban and peri-urban settings of Porto Velho, state of Rondônia, Brazilian Amazon. Table S6. Anopheles species and individuals (N) captured through Gazetrap, PHLC, and Barrier Screen in Porto Velho, state of Rondônia, Brazilian Amazon. Table S7. Anopheles species and individuals (N) captured in the Dry, Rainy, and Transitional seasons, at Porto Velho, state of Rondônia, Brazilian Amazon. Table S8. Summary of estimates from the best approximation models for anopheline abundance and richness. Table S9. PERMANOVA and PERMDISP analyses for Anopheles assemblage structure between seasons and sampling techniques. Fig. S2. Non-metric multidimensional scaling (NMDS) plots for graphical visualization of the PERMANOVA and PERMDISP analyses of Anopheles assemblage structure.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Meireles, A.A., da Silva, L.R., Simplício, M.F. et al. Anopheline diversity in urban and peri-urban malaria foci: comparison between alternative traps and seasonal effects in a city in the Western Brazilian Amazon. Malar J 21, 258 (2022). https://doi.org/10.1186/s12936-022-04274-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04274-8