Abstract
Background
Glucose 6-phosphate dehydrogenase (G6PD) and pyruvate kinase (PKLR) deficiencies are common causes of erythrocyte haemolysis in the presence of antimalarial drugs such as primaquine and tafenoquine. The present study aimed to elucidate such an association by thoroughly investigating the haematological indices in malaria patients with G6PD and PKLRR41Q variants.
Methods
Blood samples from 255 malaria patients from Thailand, Myanmar, Laos, and Cambodia were collected to determine haematological profile, G6PD enzyme activity and G6PD deficiency variants. The multivariate analysis was performed to investigate the association between anaemia and G6PD MahidolG487A, the most common mutation in this study.
Results
The prevalence of G6PD deficiency was 11.1% (27/244) in males and 9.1% (1/11) in female. The MAFs of the G6PD MahidolG487A and PKLRR41Q variants were 7.1% and 2.6%, respectively. Compared with patients with wildtype G6PD after controlling for haemoglobinopathies, G6PD-deficient patients with hemizygous and homozygous G6PD MahidolG487A exhibited anaemia with low levels of haemoglobin (11.16 ± 2.65 g/dl, p = 0.041). These patients also exhibited high levels of reticulocytes (3.60%). The median value of G6PD activity before treatment (Day 0) was significantly lower than that of after treatment (Day 28) (5.51 ± 2.54 U/g Hb vs. 6.68 ± 2.45 U/g Hb; p < 0.001). Reticulocyte levels on Day 28 were significantly increased compared to that of on Day 0 (2.14 ± 0.92% vs 1.57 ± 1.06%; p < 0.001). PKLRR41Q had no correlation with anaemia in malaria patients. The risk of anaemia inpatients with G6PD MahidolG487A was higher than wildtype patients (OR = 3.48, CI% 1.24–9.75, p = 0.018). Univariate and multivariate analyses confirmed that G6PD MahidolG487A independently associated with anaemia (< 11 g/dl) after adjusted by age, gender, Plasmodium species, parasite density, PKLRR41Q, and haemoglobinopathies (p < 0.001).
Conclusions
This study revealed that malaria patients with G6PD MahidolG487A, but not with PKLRR41Q, had anaemia during infection. As a compensatory response to haemolytic anaemia after malaria infection, these patients generated more reticulocytes. The findings emphasize the effect of host genetic background on haemolytic anaemia and the importance of screening patients for erythrocyte enzymopathies and related mutations prior to anti-malarial therapy.
Similar content being viewed by others
Background
Glucose 6-phosphate dehydrogenase (G6PD; EC 1.1.1.49) and pyruvate kinase (PKLR; EC:2.7.1.40) deficiencies are the most common hereditary metabolic disorders affecting red blood cells [1, 2]. G6PD deficiency triggers haemolytic anaemia in states of oxidative stress because deficient erythrocytes contain low levels of NADPH, which is required for maintaining cellular redox homeostasis through glutathione recycling [2]. Millions of people worldwide, mostly in Africa, the Mediterranean, the Middle East, and Asia, are affected by this condition. G6PD deficiency is caused by mutations in the G6PD gene on chromosome X. Genetically, males are either G6PD deficient or G6PD normal, while females can be homozygous with G6PD deficiency (mutations are present on both X chromosomes) or heterozygous (one X chromosome is affected) or G6PD normal. The frequency of G6PD status follows the Hardy Weinberg equilibrium. This makes heterozygous females are more common than hemizygous males and homozygous females are the least common [2].
Approximately 186 G6PD mutations, most of which are point mutations, have been documented [3]. Each mutation has different clinical phenotypes and distinctive geographical and ethnic distributions [4]. Recently, G6PD MahidolG487A, a common Southeast Asian mutation, has been reported to reduce Plasmodium vivax density [5]. Additionally, a study in Afghanistan has demonstrates that G6PD deficiency protects against P. vivax clinical disease [6]. Even though G6PD deficiency provides clinical protection against Plasmodium spp., G6PD-deficient patients are susceptible to haemolytic anaemia when exposed to active and toxic metabolites of primaquine (PQ) and tafenoquine (TQ) [7,8,9]. PQ and TQ are anti-malarial drugs that reduce Plasmodium falciparum gametocytes for transmission and preventing the relapse of P. vivax and Plasmodium ovale malaria [8, 9]. In 2013, Howes et al. published the spatial distribution of G6PD deficiency and its mutations in malaria-endemic areas around the globe to support the safe use of PQ and TQ [10]. The diagnosis of G6PD deficiency and molecular genotyping of G6PD in malaria patients prior to PQ and TQ administration are necessary to prevent adverse outcomes [8].
PK deficiency (PKD), the second most common enzyme deficiency, causes haemolytic anaemia worldwide with an estimated prevalence of 0.005% (1/20,000) in the Caucasian population. The prevalence of PKD in the European population was estimated to be less than 0.05% (5/10,000) [1], 3.4% in the Hong Kong population and 2.2% in Chinese infants [11, 12]. The prevalence of PKD in Southeast Asian countries has yet to be determined. PKD is caused by loss-of-function mutations in PK predominantly expressed in the liver and red blood cells (PKLR). More than 150 mutations of PKLR have been reported [13]. Evidence in murine models has suggested that PKD confers a protective effect against malaria [14]. Recently, a novel point mutation (161A > G) resulting in an amino acid change at residue 41 from arginine (R), which is highly conserved in the PK family, to glutamine (Q) (R41Q) in the N-terminus of PK has been reported [15]. However, the haematological parameters in malaria patients with G6PD and PKLRR41Q mutations have not been thoroughly investigated. The main aim of the present study was to examine the haematological profiles in malaria patients with G6PD or PKLR mutations.
Methods
Study subjects and sample collection
The study protocol was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (Bangkok, Thailand) (COA No. 040/2013, IRB No. 459/55). All patients were screened by passive case detection (PCD) protocol and provided written informed consent prior to enrollment in this study. A total of 255 uncomplicated malaria patients who were admitted to the Hospital for Tropical Diseases in Thailand during 2011–2012 with blood slide positivity for Plasmodium spp. and had no history of anti-malarial drug treatment 2 weeks prior were recruited for this study. Blood samples were collected at the Hospital for Tropical Diseases in Bangkok, Thailand and transferred on ice to a research laboratory at the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand within an hour for immediate measurement of G6PD activity and haematological parameters. Complete blood count (CBC) was measured using an BC-6800 Auto Hematology Analyzer (Mindray Medical International, China).
Identify Plasmodium spp.
Giemsa staining of thick and thin blood smears prepared from finger pricks was examined every 12 h from initiation of treatment until they were negative. Blood smears were examined daily until patients were discharged. Plasmodium spp. were identified under a microscope by an independent parasitologist at the Hospital for Tropical Diseases and further confirmed by polymerase chain reaction (PCR)-based analysis [16].
Measurement of G6PD activity
G6PD activity in the blood of malaria patients was measured in triplicate along with the normal and G6PD-deficient controls (G6888, G5888; Trinity Biotech, Ireland) using a quantitative assay kit for G6PD (Trinity Biotech, Ireland) prior to treatment and repeated weekly until patients were discharged. This assay measured NADPH production by G6PD in the blood of patients in parallel with positive and negative controls. Detection was carried out at a wavelength of 340 nm. The haemoglobin level was measured using Hb201 (HemoCue, Sweden) and used to calculate G6PD activity. Leftover blood samples were kept at − 20 °C for molecular typing.
Identification of G6PD, PKLR R41Q, and thalassaemia mutations
Genomic DNA from frozen blood samples was extracted using the phenol–chloroform method. PCR–restriction fragment length polymorphism (PCR–RFLP) was used to identify G6PD MahidolG487A, G6PD ViangchanG871A, and G6PD KaipingG1388A, as described previously [17], and PKLRR41Q according to a previous report [15]. PKLRR41Q was amplified by PKLR-R41Q-F: 5'–GCC AAC GGG GTA TCT ACG GC–3' and PKLR-R41Q-R: 5'–GCA GAG GTG TTC CAG GAA GG–3'. After digestion with AciI, the PCR product size of PKLRR41Q was 121 base pairs (bp). The normal allele produced two fragments of 102 bp and 19 bp. For G6PD-deficient patients with unknown mutations by the PCR–RFLP method, the coding exons of G6PD (exons 3–12) were amplified using primers described previously [16]. The PCR products were sequenced (Macrogen, Korea). The sequencing data were analysed using BioEdit software version 2.1 with the G6PD reference sequence (GenBank accession number X55448.1).
For thalassaemia mutations, multiplex gap-polymerase chain reaction was performed as previously described with minor modifications [18] to detect -α3.7, -α4.2, –SEA and –FIL variants of the α-globin gene. PCR–RFLP was used to identify HbCS and HbE, as described previously [19, 20].
Statistical analysis
All statistical analyses were performed using SPSS version 22 (IBM SPSS software, IL, USA). Data are expressed as percentages, median ± interquartile range (IQR), and mean ± standard deviation (SD). Following a computation approach reported previously, the adjusted male median (AMM) of G6PD activity was used to determine the cut-off values for G6PD deficiency [7]. The AMM values were defined as 100% activity of all male subjects after removing subjects with severe G6PD activity (≤ 10% of the overall median G6PD activity). The cut-off points for G6PD deficiency, G6PD intermediate (mild deficiency), and normal were median values less than 30%, 30 to 70%, and over 70% of the AMM, respectively. The G6PD activity of patients before (Day 0) and after treatment (Day 28) were compared using the Wilcoxon signed-rank test. The two-tailed Student’s t-test was used to analyse the differences in quantitative variables. Haemoglobin less than 11 g/dl is considered anaemia [21]. The risk of anaemia in G6PD status and mutations was analysed by odd ratio (OR). In a study of the association between haematological parameters, G6PD deficiency/G6PD normal and G6PD MahidolG487A/G6PD wildtype, a multiple linear regression was performed adjusted for age, gender, parasite species, parasite density, and haemoglobinopathies. A statistically significant difference was defined as two-sided with a p-value less than 0.05.
Results
Demographic data and prevalence of G6PD and PKLR R41Q mutations in malaria patients
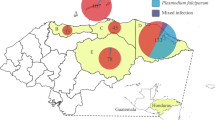
A total of malaria patients consisting of 189 individuals from Myanmar (74.1%), 35 Thais (13.7%), 16 Karen (6.3%), 8 Cambodians (3.1%), 4 Mons (1.6%), 2 Laotians (0.8%), and 1 unknown ethnicity (0.4%) are summarized in Table 1. These patients were from malaria-endemic areas, including the Thailand-Myanmar border (N = 25), Thailand-Cambodia border (N = 5) and several provinces of Thailand (N = 225), as described in a previous report [16] (Fig. 1). Two hundred forty-four patients (95.7%) were male, and eleven patients (4.3%) were female. The skewness of the gender ratio was influenced by male labor migration. The mean age of all patients was 27.94 ± 9.93 years (range 14–60 years). The numbers of patients infected with P. falciparum and P. vivax were 106 (41.6%) and 145 (56.9%), respectively. Three patients (1.2%) had coinfection of P. falciparum and P. vivax. One patient (0.4%) was infected with Plasmodium malariae.
The overall median value of G6PD activity in this cohort (n = 255) was 5.66 ± 2.55 U/g Hb (median ± IQR), ranging from 0.00 to 14.59 U/g Hb. The median values of G6PD activity in males (n = 244) and in females (n = 11) were 5.68 ± 2.49 and 4.80 ± 3.10 U/g Hb, ranging from 0.00 to 14.59 U/g Hb and 1.04 to 7.95 U/g Hb, respectively. The adjusted male median (AMM) G6PD activity in G6PD normal was 5.77 U/g Hb. The cut-off values for G6PD deficiency and G6PD intermediate were < 1.73 U/g Hb (< 30% of the AMM) and 4.04 U/g Hb (30–70% of the AMM), respectively (Fig. 2). G6PD activity exhibited bimodal distribution in males and normal distribution in females. The median values of G6PD activity in G6PD deficiency and G6PD intermediate were 0.49 ± 0.39 U/g Hb (range from 0.00 to 1.04 U/g Hb) and 3.59 ± 0.82 U/g Hb (range from 1.86 to 4.04 U/g Hb) (Fig. 2). According to these cut-off values, 27 male (11.1%; of a total of 244) and 1 female (9.1%; of a total of 11) patients were identified as G6PD deficient. The prevalence of G6PD intermediate in this study was 5.7% (14/ 244) in males and 27.3% (3/ 11) in females. Of 45 patients with G6PD deficiency and intermediate G6PD, 19 patients (42.2%) were infected with P. falciparum, 25 patients (55.6%) were infected with P. vivax, and 1 patient (2.2%) was coinfected with P. falciparum and P. vivax. Of all 28 patients with G6PD deficiency, 18 patients carried G6PD MahidolG487A (17 hemizygous deficient males, 1 homozygous deficient female), 3 patients carried G6PD ViangchanG871A (3 hemizygous deficient males), 1 patient carried G6PD KaipingG1388A (1 hemizygous deficient male) and 1 patient carried G6PD AuresT143C (1 hemizygous deficient male) (Table 1). In the remaining 5 patients, both PCR–RFLP and DNA sequencing methods could not detect mutations in the G6PD gene. The minor allele frequency (MAF) of G6PD MahidolG487A was 7.1% in these populations (Table 1). Individually, 17 hemizygous G6PD MahidolG487A and 3 hemizygous G6PD ViangchanG871A were 0.40 ± 0.46 U/g Hb (range from 0.00 to 0.89 U/g Hb) and 0.63 U/g Hb (range from 0.31 to 0.66 U/g Hb), respectively. G6PD activity levels in 1 hemizygous male with G6PD KaipingG1388A, 1 hemizygous male with G6PD AuresT143C, and 1 homozygous female with G6PD MahidolG487A were 1.02 U/g Hb, 0.40 U/g Hb, and 1.04 U/g Hb, respectively.
The histograms show the distribution of G6PD activity (U/g Hb); a G6PD activity (U/g Hb) in the study population (male and female) and b G6PD activity (U/g Hb) in individual G6PD genotypes in males. G6PD activity less than 1.73 U/g Hb (< 30% AMM), between 1.73 and 4.04 U/g Hb (30–70% AMM), and more than 4.04 U/g Hb (> 70% AMM) were indicated G6PD deficiency, G6PD intermediate, and G6PD normal, respectively
The PKLRR41Q mutation was detected in 12 patients (4.7%). These patients were from Thailand and Thailand borders (Fig. 1). The number of patients with PKLRR41Q who were infected with P. falciparum and P. vivax was equal. As shown in Table 1, PKLRR41Q was detected in malaria patients with an MAF of 2.6% in the study population (13 of 510), 2.6% in individuals from Myanmar (10 of 378), 2.9% in Thais (2 of 70) and 6.3% in Cambodians (1 of 16).
Haematological profiles of malaria patients with G6PD and PKLR mutations
At the first visit prior malaria treatment, malaria patients with G6PD deficiency (n = 28), compared to malaria patients with normal G6PD activity levels (n = 209), exhibited a significant decrease in the haemoglobin levels (11.03 ± 2.51 g/dl vs. 12.65 ± 1.97 g/dl; p = 0.003). These patients also had a significant increase in the reticulocyte count (2.80 ± 2.05% vs. 1.49 ± 1.07%; p = 0.005). Malaria patients with G6PD MahidolG487A mutation (n = 17) compared to wildtype patients without common Southeast Asian (SEA) mutations including the G6PD MahidolG487A (n = 215) exhibited a significant decrease in haemoglobin levels (11.16 ± 2.65 g/dl vs. 12.66 ± 1.92 g/dl; p = 0.041). These patients also had an increase of reticulocyte levels (3.61 ± 2.44% vs. 1.47 ± 1.05%; p = 0.008) (Table 2). There were not statistically differences in malaria patients with PKLRR41Q compared to those with wildtype. The coexistence of G6PD deficiency and thalassaemia/haemoglobinopathies is very common in this region. One hundred twenty-nine malaria patients with thalassaemia and haemoglobinopathies were found in this study population. After excluding these patients, an association between G6PD deficiency and anaemia in malaria patients was found. The data were shown in Additional file 1: Table S1.
In the longitudinal monitoring of G6PD activity before (Day 0) and after (Day 28) treatment, there was a total of 174 malaria patients with complete haematological data were analysed. These patients included G6PD deficiency, thalassaemia, and haemoglobinopathies. The median value of G6PD activity on Day 28 was significantly higher than that of on Day 0 (6.68 ± 2.45 U/g Hb vs. 5.51 ± 2.54 U/g Hb; p < 0.001) (Fig. 3a). In G6PD normal and intermediate group, the median values of G6PD activity on Day 28 were significantly increased, compared with that of on Day 0 (G6PD normal: 7.34 ± 2.39 U/g Hb vs. 6.32 ± 2.15 U/g Hb; p < 0.001; G6PD intermediate: 6.62 ± 1.59 U/g Hb vs. 3.49 ± 0.90 U/g Hb; p < 0.001). The median value of G6PD activity in G6PD deficiency group on Day 28 was significantly different from that of on Day 0 (0.67 ± 0.71 U/g Hb vs. 0.55 ± 0.31 U/g Hb; p = 0.043). Additionally, reticulocyte levels on Day 28 were significantly increased compared to that of on Day 0 (2.14 ± 0.92% vs 1.57 ± 1.06%; p < 0.001) (mean ± SD) (Fig. 3b). In groups of G6PD normal and G6PD intermediate, the mean values were significantly increased on Day 28 compared with that of on Day 0 (G6PD normal: 2.15 ± 0.95% vs. 1.51 ± 1.10%; p < 0.001; G6PD intermediate: 2.06 ± 0.55% vs. 1.24 ± 0.41%; p = 0.001). However, there were not statistically significant in reticulocyte count between Day 0 and Day 28 in G6PD deficiency group.
Anaemia according to the most commonly used in malaria studies can be grouped into three categories base on haemoglobin concentrations including mild anaemia (Hb < 11 g/dl), moderate anaemia (Hb < 8 g/dl), and severe anaemia (Hb < 5 g/dl) [21]. Mild to moderate anaemia was found in 50.0% (14 of 28 cases) of G6PD-deficient individuals and 17.7% (37 of 209 cases) of normal patients (OR = 4.65; CI%: 2.04–10.57, p < 0.001). Besides, mild to moderate anaemia was observed in 41.2% (7 of 17 cases) of patients with G6PD MahidolG487A and 16.7% (36 of 215 cases) of patient with wildtype G6PD (non-common SEA mutation) (OR = 3.48; CI%: 1.24 – 9.75, p = 0.018). The univariate analysis revealed that haemoglobin levels were significantly associated with both G6PD deficiency/G6PD normal (p < 0.001) and G6PD MahidolG487A/ G6PD Wildtype (p < 0.001) (Table 3). After adjusting by various variables including age, gender, Plasmodium species, parasite density, PKLRR41Q, thalassaemia and haemoglobinopathies, the multivariate analysis revealed that haemoglobin levels were significantly associated with G6PD deficiency/G6PD normal (p < 0.001) and G6PD MahidolG487A/ G6PD Wildtype (p < 0.001) (Table 3).
Discussion
Malaria infection causes haemolysis of infected erythrocytes. PQ and TQ, 8-aminoquinoline, are essential anti-malarial drugs commonly used for radical cure of P. vivax infections. It also reduces transmission of P. falciparum. However, PQ and TQ cause acute haemolytic complications in patients with G6PD deficiency. Although there have been many reports of G6PD deficiency status and its mutations in malaria patients living in the Southeast Asia, data on the haematological parameters of G6PD and other erythrocytic mutations including PK in malaria patients are limited. These data may be beneficial for the administration of anti-malarial treatment especially PQ and TQ prescription.
The overall frequency of patients with P. vivax infection was slightly higher than that of with P. falciparum infection, confirming that the frequencies of P. vivax and P. falciparum-infected cases are approximately equal, with high chances of coinfection in the international border between the territory of Myanmar and the western region of Thailand [22, 23]. Overall, 28 (11.0%) participants were G6PD deficient, which was presented as 11.1% (27/244) of males and 9.1% (1/11) of females. This finding is consistent with previous studies reporting that G6PD deficiency was found in approximately 10.0–13.7% of the male population of these ethnic groups [24, 25]. Although, the prevalence of G6PD deficiency in females (9.1%) in this population was higher than that reported previously (5.3%) [26], as a result of the small number of the female patients, the frequency of G6PD mutation in females follows Hardy–Weinberg equilibrium. Based on the population wide AMM, a total of 17 individuals (6.7%) exhibited intermediate G6PD activity, which was present in 5.7% (14/244) of males and 27.3% (3/11) of females. According to the results of this study and previous report, G6PD deficiency was more common in males than in females whereas intermediate was more common in females than in males [27].
These results showed that the G6PD MahidolG487A mutation was more common among individuals from Myanmar, in Thai, and in Karen malaria patients, whereas G6PD ViangchanG871A mutation was more common among Thai and Cambodian malaria patients. In general, G6PD ViangchanG871A is more common in Thai people than G6PD MahidolG487A [26]. Genetic admixture could explain the equal prevalence of G6PD ViangchanG871A and G6PD MahidolG487A in this Thai population. The G6PD MahidolG487A mutation in malaria patients had an MAF of 7.1% in the study population (18 of 255), 7.4% in individuals from Myanmar (14 of 189), 5.7% in Thais (2 of 35) and 12.5% in Karen (2 of 16). These findings agreed with the spatial distribution of G6PD deficient mutations in the Southeast Asia, where G6PD MahidolG487A and G6PD ViangchanG871A mutations are commonly observed on the western and eastern Indochina Peninsula, respectively [5, 17, 26, 28]. Although the G6PD genotype is a key factor of enzyme activity [29], some G6PD-deficient patients were unable to detect any mutations in G6PD coding regions. This could be explained by the methylation of CpG or CpNpG islands on G6PD promotor, resulting in gene silencing [30, 31]. Another possibility is that the presence of mutation in the 5’untranslated region (UTR) that has been reported to reduce enzyme activity [32].
The frequency of PK in this Southeast Asian population was comparable to what was reported by van Bruggen et al., who found this mutation in 13 out of 340 healthy unrelated Southeast Asian subjects with an MAF of 3.2% in individuals from Myanmar, 1% in Thais, 1.5% in Cambodians, 1.8% in Laotians, and 2.9% in Mons [15]. Possible contributing factors for the discrepancy between these findings include the differences in population size, homogeneity within each ethnic group and the place of origin of each subject (malaria vs non-malaria endemic areas).
Correlations between altered G6PD activity due to mutations in malaria patients and haematological phenotypes prior to treatment with anti-malarial drugs have not been well studied. Based on the International Classification of Diseases, 11th Revision (ICD-11) considering classification of G6PD deficiency under haemolytic anaemias (code: 3A10.00) [33], the data demonstrated that malaria patients with G6PD deficiency prior to treatment, particularly the G6PD MahidolG487A mutation, displayed signs of haemolytic anaemia, including low haemoglobin, RBC count, haematocrit, and high reticulocyte count. However, this study showed no signs of haemolytic anaemia in other G6PD and PKLRR41Q mutations. This is possibly due to the small number of patients enrolled, which limit the chance to detect haemolytic anaemia in malaria patients carrying G6PD AuresT143C, G6PD ViangchanG871A and G6PD KaipingG1388A. According to the World Health Organization (WHO), G6PD variants are categorized based on the degree of enzyme deficiency and severity of haemolysis. G6PD MahidolG487A and G6PD AuresT143C are in a class III mutation (moderately deficient) and G6PD ViangchanG871A and G6PD KaipingG1388A are in a class II mutation (severely deficient) [3]. Increased G6PD activity levels after treatment in G6PD intermediate and normal groups was associated with reticulocytosis. The underlying mechanism for this phenomenon includes a post-treatment response of the bone marrow, which is suppressed during malaria infection [34,35,36,37,38,39].
G6PD MahidolG487A was an independent risk factor for anaemia based on age, gender, parasite species, parasite density, PKLRR41Q, thalassaemia, and haemoglobinopathies. G6PD-deficient RBCs are exposed to oxidative stress caused by active neutrophil-produced ROS [40], leading to a decline in haemoglobin levels and generates reticulocytes [41]. According to the in-silico study by Bharti et al., G6PD enzymes with the Mahidol487A mutation lose their crucial catalytic interaction with substrate [42]. In addition, Boonyuen et al. have reported that G6PD MahidolG487A causes a local conformational change and affects backbone folding. This results in a reduction in thermostability in the absence or presence of NADP+ and a reduction in Kcat, thereby reducing catalytic efficiency [42, 43]. The ability of erythrocytes to produce NADPH is diminished. NADPH is a reducing cofactor of glutathione reductase (GR), which reduces oxidized glutathione (GSSG) to reduced glutathione (GSH). GSH maintains the reduced state of the sulfhydryl group of haemoglobin and membrane proteins. In erythrocytes with the G6PD Mahidol487A mutation, oxidation of membrane proteins causes the cells to rigid, nondeformable, and finally haemolysis. A recent report has indicated that patients with G6PD MahidolG487A presented symptoms of acute haemolytic anaemia after taking an incorrect dose of PQ [44]. Although this study had no haematological data to support the clinical impact of anti-malarial drugs on patients with G6PD MahidolG487A and other mutations, these findings provided evidence for malaria infection-induced haemolysis in patients with G6PD deficiency. This may be useful for G6PD deficiency testing requirements and administration of anti-malarial drugs including PQ and TQ to prevent relapse of P. vivax and sterilize mature P. falciparum gametocytes with a low risk of adverse events. For radical cure of P. vivax in the Southeast Asia, a high daily dose of PQ (0.5 mg/kg/day) for 14 days has been recommended by the WHO [45]. In Thailand and Cambodia, the lower 0.25 mg/kg daily for 14 days given as directly observed treatment (DOT) with G6PD deficiency testing has been recommended, whereas 0.5 mg/kg daily for 14 days at health centres and 0.75 mg/kg once a week for 8 weeks given as DOT since 2014 by malaria volunteers in the community has been recommended in Myanmar [46]. However, testing G6PD deficiency before PQ treatment was not routinely implemented in Myanmar and Cambodia but with poor implementation in Thailand [45]. For a gametocytocide of P. falciparum, a single dose of PQ (0.25 mg/kg) along with artemisinin-based combination treatment (ACT) without a requirement for G6PD deficiency testing has been recommended by the WHO [46,47,48].
Conclusions
In summary, the presence of G6PD MahidolG487A and PKLRR41Q was found in malaria patients in the Southeast Asia with MAFs of 7.1% and 2.5%, respectively. A deficiency in the enzyme G6PD by the G6PD MahidolG487A mutation exhibits a statistically significant correlation with haemolytic anaemia during malaria infection. Together, this study underlines the impact of host genetic background on haemolytic reactions and the benefit of screening for red cell enzymopathies and related mutations in patients before anti-malarial drug administration.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- G6PD:
-
Glucose 6-phosphate dehydrogenase
- PKLR:
-
Pyruvate kinase
- PQ:
-
Primaquine
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- PCR–RFLP:
-
Polymerase chain reaction–restriction fragment length polymorphism
- MAF:
-
Minor allele frequency
- Hb:
-
Haemoglobin
- RBCs:
-
Red blood cells
- Hct:
-
Haematocrit
- MCV:
-
Mean corpuscular volume
- MCH:
-
Mean corpuscular haemoglobin
- MCHC:
-
Mean corpuscular Hb concentration
- RDW:
-
Red cell distribution width
- PKD:
-
PK deficiency
- IQR:
-
Interquartile range
- AMM:
-
Adjusted male median
- DOT:
-
Directly observed treatment
- ACT:
-
Artemisinin-based combination treatment
References
Beutler E, Gelbart T. Estimating the prevalence of pyruvate kinase deficiency from the gene frequency in the general white population. Blood. 2000;95:3585–8.
Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74.
Minucci A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of the “old” and update of the new mutations. Blood Cells Mol Dis. 2012;48:154–65.
Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am. 2016;30:373–93.
Louicharoen C, Patin E, Paul R, Nuchprayoon I, Witoonpanich B, et al. Positively selected G6PD-Mahidol mutation reduces Plasmodium vivax density in Southeast Asians. Science. 2009;326:1546–9.
Awab GR, Aaram F, Jamornthanyawat N, Suwannasin K, Pagornrat W, et al. Protective effect of Mediterranean-type glucose-6-phosphate dehydrogenase deficiency against Plasmodium vivax malaria. Elife. 2021;10: e62448.
Domingo GJ, Satyagraha AW, Anvikar A, Baird K, Bancone G, et al. G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 2013;12:391.
WHO. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale: Policy brief. Geneva: World Health Organization; 2016. Contract No.: WHO/HTM/GMP/2016.9.
Stone W, Mahamar A, Smit MJ, Sanogo K, Sinaba Y, et al. Single low-dose tafenoquine combined with dihydroartemisinin-piperaquine to reduce Plasmodium falciparum transmission in Ouelessebougou, Mali: a phase 2, single-blind, randomised clinical trial. Lancet Microbe. 2022;3:e336–47.
Howes RE, Dewi M, Piel FB, Monteiro WM, Battle KE, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J. 2013;12:418.
Fung RH, Keung YK, Chung GS. Screening of pyruvate kinase deficiency and G6PD deficiency in Chinese newborn in Hong Kong. Arch Dis Child. 1969;44:373–6.
Wu ZL, Yu WD, Chen SC. Frequency of erythrocyte pyruvate kinase deficiency in Chinese infants. Am J Hematol. 1985;20:139–44.
Zanella A, Fermo E, Bianchi P, Valentini G. Red cell pyruvate kinase deficiency: molecular and clinical aspects. Br J Haematol. 2005;130:11–25.
Min-Oo G, Fortin A, Tam MF, Nantel A, Stevenson MM, Gros P. Pyruvate kinase deficiency in mice protects against malaria. Nat Genet. 2003;35:357–62.
van Bruggen R, Gualtieri C, Iliescu A, Louicharoen Cheepsunthorn C, Mungkalasut P, et al. Modulation of malaria phenotypes by pyruvate kinase (PKLR) variants in a Thai population. PLoS ONE. 2015;10: e0144555.
Para S, Mungkalasut P, Chanda M, Nuchprayoon I, Krudsood S, Cheepsunthorn CL. An observational study of the effect of hemoglobinopathy, alpha thalassemia and hemoglobin E on P. vivax parasitemia. Mediterr J Hematol Infect Dis. 2018;10: e2018015.
Nuchprayoon I, Louicharoen C, Charoenvej W. Glucose-6-phosphate dehydrogenase mutations in Mon and Burmese of southern Myanmar. J Hum Genet. 2008;53:48–54.
Chong SS, Boehm CD, Higgs DR, Cutting GR. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood. 2000;95:360–2.
Tachavanich K, Viprakasit V, Chinchang W, Glomglao W, Pung-Amritt P, Tanphaichitr VS. Clinical and hematological phenotype of homozygous hemoglobin E: revisit of a benign condition with hidden reproductive risk. Southeast Asian J Trop Med Public Health. 2009;40:306–16.
Makonkawkeyoon L, Sanguansermsri T, Asato T, Nakashima Y, Takei H. Rapid detection of chain termination mutations in the alpha 2 globin gene. Blood. 1993;82:3503–4.
White NJ. Anaemia and malaria. Malar J. 2018;17:371.
Recht J, Ashley EA, White NJ. Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: divergent policies and practices in malaria endemic countries. PLoS Negl Trop Dis. 2018;12: e0006230.
WHO. World malaria report 2015. Geneva: World Health Organization; 2015.
Bancone G, Chu CS, Somsakchaicharoen R, Chowwiwat N, Parker DM, et al. Characterization of G6PD genotypes and phenotypes on the northwestern Thailand-Myanmar border. PLoS ONE. 2014;9: e116063.
Kotepui M, Uthaisar K, PhunPhuech B, Phiwklam N. Prevalence and hematological indicators of G6PD deficiency in malaria-infected patients. Infect Dis Poverty. 2016;5:36.
Nuchprayoon I, Sanpavat S, Nuchprayoon S. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Thailand: G6PD Viangchan (871GA) is the most common deficiency variant in the Thai population. Hum Mutat. 2002;19:185.
Ley B, Kibria MG, Khan WA, Auburn S, Phru CS, et al. Wide range of G6PD activities found among ethnic groups of the Chittagong Hill Tracts. Bangladesh PLoS Negl Trop Dis. 2020;14: e0008697.
Bancone G, Menard D, Khim N, Kim S, Canier L, et al. Molecular characterization and mapping of glucose-6-phosphate dehydrogenase (G6PD) mutations in the Greater Mekong Subregion. Malar J. 2019;18:20.
Gómez-Manzo S, Marcial-Quino J, Vanoye-Carlo A, Serrano-Posada H, Ortega-Cuellar D, et al. Glucose-6-phosphate dehydrogenase: update and analysis of new mutations around the world. Int J Mol Sci. 2016;17:2069.
Toniolo D, Martini G, Migeon BR, Dono R. Expression of the G6PD locus on the human X chromosome is associated with demethylation of three CpG islands within 100 kb of DNA. EMBO J. 1988;7:401–6.
Garinis GA, Patrinos GP, Spanakis NE, Menounos PG. DNA hypermethylation: when tumour suppressor genes go silent. Hum Genet. 2002;111:115–27.
Sanders S, Smith DP, Thomas GA, Williams ED. A glucose-6-phosphate dehydrogenase (G6PD) splice site consensus sequence mutation associated with G6PD enzyme deficiency. Mutat Res. 1997;374:79–87.
Updating the WHO G6PD classification of variants and the International Classification of Diseases, 11th Revision (ICD-11) [Internet]. Malaria Policy Committee Meeting. 2019. Accessed Feb 2020.
Leowattana W, Krudsood S, Tangpukdee N, Brittenham G, Looareesuwan S. Defective erythropoietin production and reticulocyte response in acute Plasmodium falciparum malaria-associated anaemia. Southeast Asian J Trop Med Public Health. 2008;39:581–8.
Kurtzhals JA, Rodrigues O, Addae M, Commey JO, Nkrumah FK, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol. 1997;97:169–74.
el Hassan AM, Saeed AM, Fandrey J, Jelkmann W. Decreased erythropoietin response in Plasmodium falciparum malaria-associated anaemia. Eur J Haematol. 1997;59:299–304.
Chang KH, Stevenson MM. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int J Parasitol. 2004;34:1501–16.
Burgmann H, Looareesuwan S, Kapiotis S, Viravan C, Vanijanonta S, et al. Serum levels of erythropoietin in acute Plasmodium falciparum malaria. Am J Trop Med Hyg. 1996;54:280–3.
Burchard GD, Radloff P, Philipps J, Nkeyi M, Knobloch J, Kremsner PG. Increased erythropoietin production in children with severe malarial anaemia. Am J Trop Med Hyg. 1995;53:547–51.
Postma NS, Mommers EC, Eling WM, Zuidema J. Oxidative stress in malaria; implications for prevention and therapy. Pharm World Sci. 1996;18:121–9.
Bancone G, Chu CS. G6PD variants and haemolytic sensitivity to primaquine and other drugs. Front Pharmacol. 2021;12: 638885.
Bharti RS, Vashisht K, Ahmed N, Nayak A, Pande V, Mishra N. First report of glucose-6-phosphate dehydrogenase (G6PD) variants (Mahidol and Acores) from malaria-endemic regions of northeast India and their functional evaluations in-silico. Acta Trop. 2020;202: 105252.
Boonyuen U, Chamchoy K, Swangsri T, Saralamba N, Day NP, Imwong M. Detailed functional analysis of two clinical glucose-6-phosphate dehydrogenase (G6PD) variants, G6PDViangchan and G6PDViangchan+Mahidol: decreased stability and catalytic efficiency contribute to the clinical phenotype. Mol Genet Metab. 2016;118:84–91.
Chu CS, Bancone G, Soe NL, Carrara VI, Gornsawun G, Nosten F. The impact of using primaquine without prior G6PD testing: a case series describing the obstacles to the medical management of haemolysis. Wellcome Open Res. 2019;4:25.
WHO. World Malaria Report 2017. Geneva: World Health Organization; 2017.
WHO. Updated WHO Policy Recommendation: single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. Geneva: World Health Organization; 2012.
Bancone G, Chowwiwat N, Somsakchaicharoen R, Poodpanya L, Moo PK, et al. Single low dose primaquine (0.25 mg/kg) does not cause clinically significant haemolysis in G6PD deficient subjects. PLoS ONE. 2016;11: e0151898.
Dysoley L, Kim S, Lopes S, Khim N, Bjorges S, et al. The tolerability of single low dose primaquine in glucose-6-phosphate deficient and normal falciparum-infected Cambodians. BMC Infect Dis. 2019;19:250.
Acknowledgements
The authors are grateful to and would like to specifically thank staff at the Critical Care Research Unit, Department of Clinical Tropical Medicine, Mahidol University for providing data, which was important for this study. In additional, the Second Century Fund (C2F), Chulalongkorn University, for providing scholarship.
Funding
This study was supported by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund).
Author information
Authors and Affiliations
Contributions
CLC and SK designed the research study. PM, PK and WJ collected samples and performed the research. PM and CLC analysed, interpreted the patients’ data and drafted the manuscript. CLC and PC revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (COA No. 040/2013 IRB No. 459/55). The protocol of this study was performed according to the Declaration of Helsinki for the participation of human individuals. Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Mean and standard deviation (SD) for clinical parameters of malaria patients without thalassaemia and haemoglobinopathies (p-values were determined using the Student’s t-test.)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mungkalasut, P., Kiatamornrak, P., Jugnam-Ang, W. et al. Haematological profile of malaria patients with G6PD and PKLR variants (erythrocytic enzymopathies): a cross-sectional study in Thailand. Malar J 21, 250 (2022). https://doi.org/10.1186/s12936-022-04267-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04267-7