Abstract
Background
Malaria remains a major cause of morbidity and death among children less than 5 years of age. In Togo, despite intensification of malaria control interventions, malaria remained highly prevalent, with significant heterogeneity from one region to another. The aim of this study is to explore further such regional differences in malaria prevalence and to determine associated risk factors.
Methods
Data from a 2017 cross-sectional nationally representative malaria indicator survey was used. Children aged 6–59 months in selected households were tested for malaria using a rapid diagnostic test (RDT), confirmed by microscopy. Univariate and multivariate logistic regression analysis were performed using Generalized Linear Models.
Results
A total of 2131 children aged 6–59 months (1983 in rural areas, 989 in urban areas) were enrolled. Overall 28% of children tested positive for malaria, ranging from 7.0% in the Lomé Commune region to 4% 7.1 in the Plateaux region. In multivariate analysis, statistically significant differences between regions persisted. Independent risk factors identified were higher children aged (aOR = 1.46, 95% CI [1.13–1.88]) for those above 24 months compared to those below; households wealth quintile (aOR = 0.22, 95% CI [0.11–0.41]) for those richest compared to those poorest quintiles; residence in rural areas (aOR = 2.02, 95% CI [1.32–3.13]).
Conclusion
Interventions that target use of combined prevention measures should prioritise on older children living in poorest households in rural areas, particularly in the regions of high malaria prevalence.
Similar content being viewed by others
Background
Malaria continues to be major public health problem. In 2020, a total of 229 million cases of malaria and 409,000 deaths were reported, with the vast majority occurring in sub-Saharan Africa (94% of malaria cases and 99% of deaths) [1]. The children under five years of age continue to be at greatest risk of severe malaria, accounting for 67% of all malaria deaths. Prevalence is influenced by environmental, vector and human-related factors [2].
In Togo, malaria is endemic with a high prevalence. In 2018, 2,002,877 cases and 905 deaths were recorded on a general population about 7 million inhabitants [3]. Children under five years of age represented 31.6% of cases. This overall prevalence hides a strong heterogeneity between the regions ranging from 2.6% in the common Lomé Commune region to 43.0% in the Savannah region [3]. The socio-economic burden on the population is significant. In one study, the average expenditure per household on malaria prevention measures was US$ 8, which represents 5–15% of monthly household income[4]. Through the Global Technical Strategy for Malaria Control (2016–2030), Togo is one of the 35 countries committed to eliminate malaria by 2030 [5]. Therefore, the Togolese government through the National Malaria Control Programme (Programme National de Lutte contre le Paludisme, PNLP) has developed five-year plans, the first of which (National Health Development Plan 2017–2022) builds on known high-impact interventions to control malaria in the country with the goal to move towards malaria elimination by 2030 [6]. The main strategies adopted in this plan are the reinforcement of mass distribution of long-lasting insecticide-treated nets (LLINs) introduced since 2008, chemoprevention of seasonal malaria (SMC) for children under 3 to 59 years of age introduced since 2013, and intermittent preventive treatment for pregnant women introduced since 2005 [6].
Despite these initiatives, malaria remains a challenge. A retrospective longitudinal study using PNLP routine data from 2008 to 2017, showed an average annual increase in malaria cases in children under five of + 13.1%, also with strong heterogeneity ranging from + 6.3% in the Lomé Commune region to + 16.7% in the Centrale region [7]. Several other studies on malaria in Togo have focused on the clinical management of cases [8, 9], the evolution of malaria incidence [7], the evaluation of the implementation of malaria control interventions [10, 11], clinical trials on malaria vector resistance to insecticides [29, 30] and socio-cultural factors [12]. So far, there are very few studies examining the risk factors associated with regional heterogeneity in malaria prevalence among children under five in Togo using national population-based survey data [7]. The aim of this study was to explore further such regional variation in malaria prevalence and to determine the associated risk factors.
Methods
Study context
Togo is a country in West Africa, with an population of about 7 million inhabitants in 2021, with a density of 152 inhabitants/km2 [13]. It borders Burkina Faso to the north, the Gulf of Guinea to the south, Benin to the east and Ghana to the west. The country is divided into six health regions and forty-three health districts in total (Fig. 1). The health system in Togo is organized as a three-level pyramidal structure [3] (Fig. 2). The first level is composed of the central administration and the different central departments and programmes where the guidelines and national policies are developed. The regional (or intermediate) level includes six health regions which provide coordination and technical support to the peripheral health districts. The third level is represented by the health district which is the most decentralized operational entity. There are 43 health districts and 944 peripheral health units.
Study type and sample design
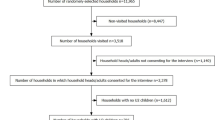
This was a secondary data analysis project using cross-sectional data from the Togo Malaria Indicator Survey (TMIS) 2017. These data provide information on malaria prevalence among children under five and pregnant women in the country [14]. The TMIS carried out a two-stage sampling method to select the sample. Using information from the last general population census in Togo in 2010 [15], each region was subdivided into Enumeration Areas (EAs). At the first step, 171 EAs were drawn with a probability proportional to the size and, 30 households were drawn randomly in each EAs selected. All women aged 15–49 reporting to usually live in the selected households or present the night before the interview with or without children under five were eligible to be interviewed. Selected households with neither a woman aged 15–49 nor a child under five were only included for the household questionnaires. In total, the sample consisted of 171 EAs, 5,130 households (1800 in urban areas and 3330 in rural areas), 4895 women from 15 to 49 years old (1684 in urban areas and 3,211 in rural areas), and 3271 children under five (2441 in rural areas and 830 in urban areas) [16].
Tools and data collection
Three questionnaires were used in the context of the 2017 TMIS: a household questionnaire, a women questionnaire, and a biomarker questionnaire. The household questionnaire recorded all household members and visitors who slept there the night before the interviewer visited the household, water source, types of toilets, habitat characteristics, possession of durable goods, and use of mosquito nets were collected. The women questionnaire was used to collect information on socio-demographic characteristics, knowledge of malaria and prevention measures, births over the last 5 years, prevalence and treatment of fever in children under five. Finger prick blood samples for malaria testing were taken from all children aged 6–59 months in the selected households, for whom the parents or responsible adults had previously given their informed consent. Screening for malaria was done with a rapid diagnostic test (RDT), namely the SD Bioline Malaria Ag Pf/Pan with a sensitivity of 94.0% and specificity of 91.4%. Children who tested positive for malaria, or who had other signs of severe malaria or other serious illnesses, were referred to the nearest health facility for "advice and action" following the national health policy in Togo. Blood collection on slides was carried out to confirm the infection status of all children using a microscope. After drying and fixing the blood smears, the prepared slides were stored in special boxes containing cold accumulators and humidity controllers. Blood samples on slides, accompanied by the transmission sheets, were regularly collected in the field and transported to the parasitology laboratory of the “Institut National d’Hygiene (INH)” to be registered, checked, and analysed. After being stained with Giemsa, the slides were examined for the presence of the parasite. Each slide was analysed independently and blindly by two different biologist technicians. In case of discrepancies between the results of the two technicians, the slide was re-examined by a senior biologist technician [16].
Study variables
The dependent variable in this study is the infection status of the child (positive/negative) on the microscopic examination of the malaria parasitaemia. The main independent variable was the region. Other independent variables included environment related factors such as altitude, household density, main water source, type of toilet facility, main material of floors, main material of walls and main material of roof. Human related factors included household wealth quintiles, age, gender, possession and use of a mosquito bed net, mother's education level, knowing mosquitoes as vectors of malaria, being exposed to malaria prevention messages, as well as ethnicity, religion and child had fever in last two weeks before the study. The household wealth quintile variable was constructed using principal component analysis on household’s assets and amenities [17]. A standardized composite measure combining household assets and possessions primarily based on selected assets, such as televisions and bicycles, materials used for housing construction, and types of water access and sanitation facilities was used [18].
Data analysis
For each of the factors recorded, we assessed associations with malaria infection in univariate logistic regression using Generalized Linear Models (GLM), calculating odds ratios. This choice is explained by the fact that the GML is a very reasonable and general approach which consists in using variables (x's) to estimate the probability p that y = 1 [19]. Any association that was found to be statistically significant at a level of p ≤ 0.10 in univariate analysis was assessed as a potential confounder which could explain the observed heterogeneity in the multivariate analysis. In multivariable model, region of residence and all the potential confounders as well as any other factors that were significantly associated in univariate analysis (p ≤ 0.10) were included. Each of the secondary exposures were then removed one at a time, starting with the one with the highest p-value. If this resulted in a change of more than 10% in the odds ratio of region or if the likelihood ratio test comparing the complex model with the simple model was significant, the secondary exposure was retained. This process was continued until all remaining secondary exposures were either important as confounders or their removal would result in a significantly less precise model [20]. The Interactions test between our main variable of interest (region of residence) and each of the secondary exposures was checked. For this purpose, categorical variables with more than two levels were recoded to binary. Dose response curves were generated using the predictor effect plots [21] to show the trend between the malaria prevalence and certain associated risk factors. Data were analysed using the software R version 4.0.4.
Results
Characteristics of the study population
A total of 3,271 children aged between 0 and 59 months from 5,130 households were enrolled in the survey, of whom 2131 aged 6–59 months were tested for the presence of malaria parasites. There were almost equal numbers of female and male children, with a median age of 27 months (IQR 13–43). Most children (66.7%) were from rural areas, except for the Lomé Commune region, which is an urban agglomeration that includes the country's capital. The largest group of children in our sample (24.1%) were from the poorest quintile of households, while only 16.6% were from the richest quintile (Table 1). In addition, most of the children were living in houses where the main materials of the floor, wall and roof were not upgraded (Table 1).
Children living in the Plateaux region had the highest prevalence of malaria (47.1%) followed by children living in the Savanes (35.0%) and Maritime (31.3%) regions. Intermediate prevalence levels of malaria were recorded in Kara (18.3%) and Centrale (19.7%), and the lowest prevalence level (7.0%) in Lomé region (Fig. 3; Table 2).
Regional heterogeneity of malaria in Togo
The region of residence was significantly associated with the child's malaria status (Table 2). Persistence of regional heterogeneity was explored following correction for the study variables described in the methods. Then, the Plateaux and Maritime regions remained at increased risk compared to Lomé, while the other regions no longer were at increased risk. The probability of a positive malaria test for children living in the Plateaux decreased from 13.7 times higher risk to 4.2 times higher risk compared to those living in the Lomé Commune region. (see Table 2). The same observation was made for the Maritime region, where the probability of a positive malaria test decreased from 6.9 times higher risk to 2 times higher risk compared to those living in the Lomé Commune region.
Other significant risk factors related to increased malaria prevalence among children
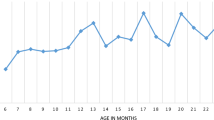
Table 2 summarized variables that had an independent statistically significant impact on the malaria prevalence. Children living in rural areas were at twice as high risk (adj. OR = 2.02, 95% CI [1.32–3.13]) to test positive for malaria compared to those living in urban areas. Malaria prevalence was highest among children whose mother had no education (38.5%), followed by those whose mother had primary education (26.3%), and finally those whose mother had secondary education and above (13.0%), showing a clear dose–response association (Fig. 4). Child age, household wealth quintile, altitude also showed an dose–response trend (Fig. 4). The probability of malaria in children decreased with increasing altitude, maternal education, and household wealth, while malaria prevalence increased with child’s age in months (Fig. 4). The interaction tests were not significant.
Discussion
The prevalence of malaria in Togo in children under 5 years of age was high and varied by region, with the highest prevalence in the Plateaux region. After adjusting for several known risk factors, the effect of region of residence decreased but remained significant. This suggests that the repeatedly observed heterogeneity by region of residence reflects residual confounding by other variables not considered in the study. Such variables could include temperature, humidity, the presence of standing water in the household, the proximity of the household to a body of water, availability and quality of health care. The persistence of a higher prevalence in two of the six regions, Plateaux and Maritime, could potentially be explained by climatic conditions favourable for high mosquito densities in this region. The high rainfall provides an excellent breeding ground for the Anopheles mosquito [22]. Entomological studies in Nigeria [23] and Eritrea [24], also showed higher number of mosquitoes in the irrigated community compared to those in the non-irrigated community.
The observed increased risk of malaria for children living in rural areas may also be associated with more favourable conditions for mosquito development. Studies in Ghana [25] and Burkina Faso [26] found that proximity of dwellings to agricultural areas and rural water reservoirs increased the risk of malaria morbidity due to greater exposure to malaria vectors. The difference in risk between rural and urban children in our study was greater in the Lomé Commune region, the most urbanized region in Togo. Urbanization reduces the risk of malaria because it creates an environment which is suboptimal for mosquito persistence as it reduces the number of breeding sites [25, 26]. Urbanization also changes health practices, which can result in more effective prevention (use of insecticides, mosquito nets, preventive treatments), information dissemination (concentration of public and private media), and better access to health facilities [27, 28]. The difference in malaria risk by household wealth quintile suggests that malaria risk depends on wealth quintile [29, 30]. In contrast to our study, similar studies in Uganda [31] and Kenya [32], did not observe an association between household wealth index and malaria prevalence. This can be explained by the fact that these two studies were conducted in single rural communities, rather than nationwide including both urban and rural populations and a mix of different communities.
The higher malaria prevalence in older age groups observed in our study is consistent with observations in Kenya [33] and Tanzania [34]. An explanation could be that younger children have anti-malaria antibodies that have been transmitted to them by their mothers [34, 35]. Or that they spent less time outdoors in the evenings compared to older children, resulting in less exposure and are likely to be put under bed nets regularly [36]. Other explanations may include the fact that they have received Seasonal Malaria Chemo-prevention (SMC). This could guide considerations of more targeted malaria control strategies for older children, such as extending the age group covered by SMC.
Children living in areas with high malaria transmission intensity develop immunity with age because of continuous exposure to infected mosquito bites [37]. This immunity develops first against severe forms of malaria and then against non-severe malaria [35]. This could explain that older children are more likely to have malaria parasites without developing clinical disease, in contrast to younger children who still have less mature immunity and therefore still struggle more with malaria infections [35]. A mother's educational level showed a protective effect against malaria infection for her child. The same results were found using cross-sectional survey data from three African countries (Angola, Tanzania, Uganda) [38] and also in Democratic Republic of Congo [39]. An educated mother in general has a higher capacity for self-reliance and to break with certain traditions and beliefs that are not favourable to health. She is also more likely to be able to keep the household healthy and improve hygiene [40]. This positive effect of a mother's education on child survival is true even in poor households [41, 42]. Indeed, educated mothers are known to be more receptive to awareness raising campaigns to prevent children from getting sick, and are quicker to consult and use health services [42].
The dataset used for these analyses was derived from a cross-sectional survey and therefore has some limitations. For example, a causal relationship between the explanatory variables and the prevalence of malaria in children under five cannot be established. Also, data were collected on adherence to the first dose of seasonal malaria chemoprevention (SMC) drug in 2017. SMC was only available in three regions and 1061 children were exposed to the treatment as reported by caregivers with 99.9% adherence during the survey. Because of this low number of children exposed to SMC in the study sample, this were unable to control for this variable in the analyses. The large and nationally representative study population is an important strength of the study. Another strength of the study is that the malaria infection was confirmed by microscopy, which is the gold standard. In addition, the use of Generalized Linear Models allowed explaining part of the regional heterogeneity of malaria prevalence in under-five in Togo and the associated risk factors. This information can thus be used in the design of appropriate interventions.
Conclusion
This study showed a strong regional heterogeneity of malaria prevalence in children under five years of age in Togo. Part of this heterogeneity could be explained by factors such as place of residence, child’s age, mother's education level, household wealth quintile, exposure to malaria prevention messages and altitude, but after adjusting for these, significant heterogeneity persisted. These findings support designing targeted interventions and strategies tailored to older children aged more than 24 months living in rural areas and from households with a poor wealth index. Particular emphasis could be placed on designing key malaria prevention messages for the high malaria prevalence regions. Prioritizing high prevalence regions for SMC and the combined use of preventive measures is recommended for better control of malaria in Togo.
Availability of data and materials
DHS datasets are publicly available on www.dhsprogram.org.
Abbreviations
- CHW:
-
Community Health Workers
- CBRS:
-
“Comité Bioéthique de Recherche en Santé
- DALY:
-
Disability-Adjusted Life Years
- EA:
-
Enumeration Areas
- GLM:
-
Generalized Linear Models
- INSEED:
-
National Institute of Statistics
- IRS:
-
Indoor Residual Spraying
- LLIN:
-
Long-lasting Insecticidal Nets
- PNLP:
-
National Malaria Control Program
- RDT:
-
Rapid Diagnostic Test
- SMC:
-
Seasonal Malaria Chemo-prevention
- TMIS:
-
Togo Malaria Indicator Survey
- WHO:
-
World Health Organization
References
WHO. Fact sheet about Malaria [Internet]. Geneva, World Health Organization, 2019 [cited 2021 Jan 20]. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria
Protopopoff N, Bortel WV, Speybroeck N, Geertruyden J-PV, Baza D, D’Alessandro U, et al. Ranking malaria risk factors to guide malaria control efforts in African Highlands. PLoS One. 2009;4:e8022.
Ministry of Health/Togo. Annual performance report 2018 [Internet]. [cited 2021 Jan 9]. Available from: https://www.afro.who.int/sites/default/files/2019-09/rapport%20annuel%20de%20performance%202018%20du%20mshp.pdf
Sanoussi Y, Ametoglo M. Ampleur Et déterminants des dépenses catastrophiques de santé: cas des ménages togolais (Magnitude and Determinants of Catastrophic Health Expenditure: Case of Togolese Households) [Internet]. Rochester, NY: Social Science Research Network; 2019 Aug [cited 2021 Jan 23]. Report No.: ID 3440106. Available from: https://papers.ssrn.com/abstract=3440106
WHO. Global technical strategy for malaria, 2016–2030 [Internet]. Geneva, World Health Organization, 2015 [cited 2020 Dec 28]. Available from: http://apps.who.int/iris/bitstream/10665/176712/1/9789241564991_eng.pdf?ua=1
Ministry of Health/Togo. National Health Development Plan (PNDS) 2017–2022 [Internet]. [cited 2021 Jan 9]. Available from: https://sante.gouv.tg/node/359
Bakai TA, Thomas A, Iwaz J, Atcha-Oubou T, Tchadjobo T, Khanafer N, et al. Changes in registered malaria cases and deaths in Togo from 2008 to 2017. Int J Infect Dis. 2020;101:298–305.
Gbadoé AD, Kini-Caussi M, Koffi S, Traoré H, Atakouma DY, Tatagan-Agbi K, et al. Évolution du paludisme grave de l’enfant au Togo de 2000 à 2002. Med Mal Infect. 2006;36:52–4.
Déti EK, Flénon J, Zohoun T, Maurice-Tison S, Salamon R, Atakouma YD. Prise en charge à domicile du paludisme chez l’enfant : propositions d’actions à partir des résultats d’une enquête CAP menée auprès des mères d’enfants de moins de 5 ans à Notsé (Togo). Sante. 2008;18:155–61.
Thomas A, Bakai TA, Atcha-Oubou T, Tchadjobo T, Voirin N. Implementation of a malaria sentinel surveillance system in Togo: a pilot study. Malar J. 2020;19:330.
Wang Q, Zhang Z, Yu W, Lu C, Li G, Pan Z, et al. Surveillance of the efficacy of artemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria among children under 5 years of age in Est-Mono District, Togo, in 2017. Front Pharmacol. 2020;11:784.
Kpanake L, Dassa KS, Mullet E. Why most Togolese people do not seek care for malaria in health care facilities: a theory-driven inventory of reasons. Psychol Health Med. 2009;14:502–10.
National Institute of Statistics (INSEED). Socio-Demographic Indicators [Internet]. 2021 [cited 2021 Oct 24]. Available from: https://inseed.tg/
The DHS Program - Togo: Malaria Indicator Survey (MIS), 2017 [Internet]. [cited 2020 Dec 29]. Available from: https://dhsprogram.com/methodology/survey/survey-display-497.cfm
National Institute of Statistics (INSEED). General Census of Population and Housing_Togo 2010 [Internet]. Togo: INSEED; 2011 Dec [cited 2020 Dec 29] p. 65. Available from: https://inseed.tg/statistiques-demographiques/
Ministry of Health, ICF. Togo Malaria Indicator Survey 2017 [Internet]. 2018 Feb [cited 2021 Jun 13] p. 165. Available from: https://dhsprogram.com/publications/publication-MIS29-MIS-Final-Reports.cfm
Krefis AC, Schwarz NG, Nkrumah B, Acquah S, Loag W, Sarpong N, et al. Principal component analysis of socioeconomic factors and their association with malaria in children from the Ashanti Region. Ghana Malar J. 2010;9:201.
Shaukat B, Javed SA, Imran W. Wealth index as substitute to income and consumption: assessment of household poverty determinants using demographic and health survey data. JPoverty. 2020;24:24–44.
Myers RH, Montgomery DC. A Tutorial on Generalized Linear Models. J Qual Technol. 1997;29:274–91.
Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. UCLA Statistics/American Statistical Association [Internet]. 2014 Aug [cited 2021 Nov 14]; Available from: https://dspace.mit.edu/handle/1721.1/91154
Fox J, Weisberg S. Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. J Stat Softw. 2018;87:1–27.
Carnevale P, Robert V. Les anophèles: biologie, transmission du Plasmodium et lutte antivectorielle. IRD Éditions; 2017. 404 p.
Amaechi EC, Ukpai OM, Ohaeri CC, Ejike UB, Irole-Eze OP, Egwu O, et al. Distribution and seasonal abundance of Anopheline mosquitoes and their association with rainfall around irrigation and non-irrigation areas in Nigeria. Cuadernos de Investigación UNED. 2018;10:267–72.
Kifle MM, Teklemariam TT, Teweldeberhan AM, Tesfamariam EH, Andegiorgish AK, Azaria KE. Malaria risk stratification and modeling the effect of rainfall on malaria incidence in Eritrea. J Environ Public Health. 2019;2019:7314129.
Afoakwah C, Deng X, Onur I. Malaria infection among children under-five: the use of large-scale interventions in Ghana. BMC Public Health. 2018;18:536.
Ouédraogo M, Samadoulougou S, Rouamba T, Hien H, Sawadogo JEM, Tinto H, et al. Spatial distribution and determinants of asymptomatic malaria risk among children under 5 years in 24 districts in Burkina Faso. Malar J. 2018;17:460.
Le GP. paludisme en Afrique au sud du Sahara : comparaison entre les milieux urbains et ruraux. Sante. 1991;1:33–8.
Bouba Djourdebbé F. Facteurs environnementaux immédiats et santé des enfants dans les zones de l’Observatoire de population de Ouagadougou (Burkina Faso). 2016 [cited 2021 Jan 22]; Available from: https://papyrus.bib.umontreal.ca/xmlui/handle/1866/13592
Kanmiki EW, Awoonor-Williams JK, Phillips JF, Kachur SP, Achana SF, Akazili J, et al. Socio-economic and demographic disparities in ownership and use of insecticide-treated bed nets for preventing malaria among rural reproductive-aged women in northern Ghana. PLoS ONE. 2019;14: e0211365.
Uzochukwu BS, Onwujekwe OE. Socio-economic differences and health seeking behaviour for the diagnosis and treatment of malaria: a case study of four local government areas operating the Bamako initiative programme in south-east Nigeria. Int J Equity Health. 2004;3:6.
Pullan RL, Bukirwa H, Staedke SG, Snow RW, Brooker S. Plasmodium infection and its risk factors in eastern Uganda. Malar J. 2010;9:2.
Snow RW, Peshu N, Forster D, Bomu G, Mitsanze E, Ngumbao E, et al. Environmental and entomological risk factors for the development of clinical malaria among children on the Kenyan coast. Trans R Soc Trop Med Hyg. 1998;92:381–5.
Bashir IM, Nyakoe N, van der Sande M. Targeting remaining pockets of malaria transmission in Kenya to hasten progress towards national elimination goals: an assessment of prevalence and risk factors in children from the Lake endemic region. Malar J. 2019;18:233.
Roberts D, Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar J. 2016;15:246.
Carnevale P, Vaugelade J, Programme WHOMA. Paludismes, morbidité palustre et mortalité infantile et juvénile en Afrique sub-saharienne. 1987 [cited 2020 Dec 29]; Available from: https://apps.who.int/iris/handle/10665/59447
Oguoma VM, Anyasodor AE, Adeleye AO, Eneanya OA, Mbanefo EC. Multilevel modelling of the risk of malaria among children aged under five years in Nigeria. Trans R Soc Trop Med Hyg. 2021;115:482–94.
Musa MI, Shohaimi S, Hashim NR, Krishnarajah I. A climate distribution model of malaria transmission in Sudan. Geospat Health. 2012;7:27–36.
Njau JD, Stephenson R, Menon MP, Kachur SP, McFarland DA. Investigating the Important correlates of maternal education and childhood malaria infections. Am J Trop Med Hyg. 2014;91:509–19.
Ma C, Claude KM, Kibendelwa ZT, Brooks H, Zheng X, Hawkes M. Is maternal education a social vaccine for childhood malaria infection? A cross-sectional study from war-torn Democratic Republic of Congo. Pathog Glob Health. 2017;111:98–106.
Mattos T, Mackinnon MA, Boorse D. The Intersection of Gender , Education , and Health : A Community-level Survey of Education and Health Outcomes for Women in Southeastern Togo [Internet]. 2012 [cited 2020 Dec 29]. Available from: /paper/The-Intersection-of-Gender-%2C-Education-%2C-and-Health-Mattos-Mackinnon/a776c16b09a17705b70fd4749afecdea109892d0
Peña R, Wall S, Persson LA. The effect of poverty, social inequity, and maternal education on infant mortality in Nicaragua, 1988–1993. Am J Public Health. 2000;90:64–9.
Caldwell J, McDonald P. Influence of maternal education on infant and child mortality: levels and causes. Health Policy Educ. 1982;2:251–67.
Acknowledgements
Thanks to the ICF International and DHS (Demographic and Health Surveys) Program for providing and granting permission for the use of the data in this study and to the Ministry of health, public hygiene and universal access to care, Togo for their support and advise. Our special thanks are owed to DGD finding, Institute of Tropical Medicine, Antwerp MPH staff and jury. Thanks to Prof. Epco Hasker for his advice on data analysis.
Funding
No funding was available for this study.
Author information
Authors and Affiliations
Contributions
KG and MS designed the study. SS, GK and MS performed the data analysis. WG, AAK and DKE drafted the manuscript. All co-authors contributed significantly to the revision of the manuscript and provided scientific guidance. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval for the study protocol was obtained from the ICF International’s Institutional Review Board and the Togo Bioethics Committee for Health Research (CBRS). Moreover, informed consent was obtained from study participants prior data collection. For this study, Dataset and permission to conduct secondary data analysis were granted by the DHS program. The data that were used in this analysis were anonymous and no personal identifier or link was received from the DHS program.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kombate, G., Gmakouba, W., Scott, S. et al. Regional heterogeneity of malaria prevalence and associated risk factors among children under five in Togo: evidence from a national malaria indicators survey. Malar J 21, 168 (2022). https://doi.org/10.1186/s12936-022-04195-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04195-6