Abstract
Background
The Community-Based Malaria Management (CBMM) strategy, introduced in 2013 and expanded to all health facilities and health posts in Afghanistan by 2016, aimed to deliver rapid diagnostic testing and more timely treatment to all communities nationwide. In this study, trends for several malaria outcome indicators were compared before and after the expansion of the CBMM strategy, using cross-sectional analysis of surveillance data.
Methods
Generalized estimating equation (GEE) models with a Poisson distribution were used to assess trends of three key outcomes before (2012–2015) and after (2016–2019) CBMM expansion. These outcomes were annual malaria incidence rate (both all and confirmed malaria incidence), malaria death rate, and malaria test positivity rate. Additional variables assessed included annual blood examination rates (ABER) and malaria confirmation rate.
Results
Average malaria incidence rates decreased from 13.1 before CBMM expansion to 10.0 per 1000 persons per year after CBMM expansion (P < 0.001). The time period after CBMM was expanded witnessed a 339% increase in confirmed malaria incidence as compared to the period before (IRR 3.39, 95% CI 2.18, 5.27; P < 0.001). In the period since the expansion of CBMM (2016–2019), overall malaria incidence rate declined by 19% each year (IRR 0.81, 95% CI 0.71,0.92; P = 0.001) and the malaria death rate declined by 85% each year (IRR 0.15, 95% CI 0.12, 0.20; P < 0.001). In comparing the before period to the after period, the ABER increased from 2.3 to 3.5 per 100 person/year, the malaria test positivity rate increased from 12.2 to 20.5%, and the confirmation rate increased from 21% before to 71% after CBMM.
Conclusions
Afghanistan’s CBMM expansion to introduce rapid diagnostic tests and provide more timely treatment for malaria through all levels of care temporally correlates with significant improvement in multiple indicators of malaria control.
Similar content being viewed by others
Background
Worldwide, malaria is a major public health problem with 241 million new infections and 627,000 deaths annually [1]. Afghanistan, a country in the World Health Organization (WHO) Eastern Mediterranean Region, has relatively low transmission of malaria [2]. The Afghanistan National Malaria and Leishmania Control Programme reported 174,893 malaria cases and zero deaths in 2019, the lowest number that has ever been reported for the country. The two main species of malaria parasites in Afghanistan are Plasmodium vivax (98% of all cases) and Plasmodium falciparum (2%) [2].
In Afghanistan, malaria incidence rates vary by location. The variation results from differences in parasites, vectors, human population density, behaviours, ecological, high temperature, humidity and agriculture (rice cultivation), socio-economic conditions, and access to health services for detection and treatment of malaria. Nationally, 27% of the Afghan population lives in areas at high risk for malaria. Areas at high risk are defined as provinces and districts with annual parasite incidence (API) rate per 1000 persons at risk of 1 or above and test positivity rate (TPR) at 9% and above. Half (50%) of the population lives in areas at medium risk (API < 1, TPR < 9%), and the remaining 23% live in areas with low and very low risk of malaria transmission or its absence in malaria free areas [3]. In 2019, more than 93% of total malaria cases were reported from six provinces that border with Pakistan (Nangarhar, Laghman, Kunar, Nooristan, Khost, and Paktika) and one district of Kabul. Nangarhar is one highest endemic province in the country and accounted for more than 45% of total malaria cases and 35% of total P. falciparum cases [2].
Malaria diagnosis either by microscopy or rapid diagnostic tests is recommended by the WHO for all suspected malaria cases before starting the treatment. Early and accurate diagnosis is essential both for effective management of the disease, and for malaria surveillance and elimination strategies. In Afghanistan, the Community-Based Management of Malaria (CBMM) strategy was designed to progressively expand access to malaria diagnosis and effective anti-malarial treatment at non-diagnostic health facilities and community including health posts [4]. Malaria diagnosis using microscopy has been available in all hospitals and Comprehensive Health Centres (CHCs) of Afghanistan. Since 2013, the focus of the CBMM in Afghanistan has changed to specifically increase access to rapid diagnostic testing (RDT) and timely treatment at the community level in all malaria endemic and non-endemic areas of Afghanistan. The programme consists of two key modules; case management, vector control; CBMM was scaled up nationwide in 2016 with the support of the Global Fund. A main pillar of this revised strategy is introducing RDT in all health facilities, not only those providing diagnosis and treatment for malaria, and expanding screening of malaria to health posts to run community-based screening programs. In addition, the CBMM expanded the community-based malaria case management program using networks of community health workers (CHW) to reach all patients with suspected malaria at a level closer to the home. Since 2016, more than 30,000 CHWs were trained on malaria case management, RDT use and distribution of long-lasting insecticidal net (LLIN) to community through mass campaign. Other malaria commodities, including medicines, were supplied to health posts and health facilities without laboratory services. As a result, in 2017 more than 90% of CHW reported screening and referral of newly identified cases of malaria, and more than 50% reported providing counselling, chloroquine treatment for vivax malaria, and artemisinin-based combination therapy for suspected and confirmed falciparum malaria cases [5].
While the magnitude of the scale-up and shift in focus of the CBMM are encouraging, the effectiveness of the programme in Afghanistan has not yet been evaluated. In this study, trends in annual malaria incidence and death rates were assessed during two time periods, 4 years before the expansion of CBMM (2012–2015), and 4 years after expansion the CBMM program (2016–2019). Additional indicators of programme impact were also tracked. The scope of analysis included both national and subnational trends in Afghanistan.
Methods
Data were extracted from the Malaria Leishmania Information System (MLIS) of the National Malaria Control Programme (NMCP) and Health Management Information System (HMIS). Data included clinical (diagnosed without a diagnostic test) and confirmed (diagnosed with a diagnostic test) malaria cases reported by approximately 2800 health facilities on a monthly basis. Patients were those with symptoms or diagnosis of malaria who visited health facilities, health posts and community member reached through outreach or mobile services. Data were initially collected on paper forms. The HMIS officers of non-governmental organizations (NGOs) and provincial malaria case managers checked the quality and completeness of the forms and entered them into the HMIS database. Hard and soft copies of collected data were shared with the provincial health directorate HMIS team on a monthly basis. The provincial HMIS and malaria officers reviewed and compiled the data and reported to the NMCP on a quarterly basis. Data were analysed and feedback provided to implementers on a quarterly basis. For this analysis, all data reported from 2012 to 2019 were used.
Analysis
To assess trends in malaria before and after the expansion of the CBMM programme in Afghanistan, seven indicators were measured (Table 1). The descriptive analysis included the following indicators: the malaria incidence rate (both all and confirmed malaria) per 1000 persons per year, malaria death rates per 100,000 persons per year, malaria test positivity rate, annual blood examination rate per 100 per year (ABER) and the malaria confirmation rate. Reporting completeness during this time period was assessed to understand the reliability of the data.
Generalized estimating equation (GEE) models with a Poisson distribution were used to assess the differences in these indicator rates before (2012–2015) and after (2016–2019) CBMM was expanded (a binary predictor variable of before and after was used). Temporal trends during the before and after years were conducted by using GEE models by stratifying on time period and using year as a predictor variable. Analyses were conducted at the provincial level with all the provinces of Afghanistan included. Stata v.15. was used for statistical analysis and ArcGIS v.10.3.1 was used to create maps of average annual malaria incidence and average annual incidence of death due to malaria during the before and after periods.
Results
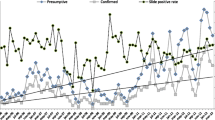
Between 2012 and 2019, the total number of malaria cases (including clinical and confirmed) fell from 391,365 to 174,893. The overall malaria incidence rate declined from 15.4 to 5.5 per 1,000 per year and the malaria confirmation rate increased from 14 to 99% (Fig. 1A). The number of malaria cases that were confirmed by testing rose from 54,840 to 173,859; clinical cases declined 336,525 to 1034 (Fig. 1B). The malaria death rate fell from 0.1416 to 0 per 100,000 per year.
Table 2 presents annual malaria data and combined data for the two time periods based on the start of CBMM expansion (2012–15 vs. 2016–19). Between 2012 and 2015, the total number of tests conducted was 2,365,753. After the expansion of CBMM (2016–2019), the total number of tests conducted was 4,097,900 (Table 2; Fig. 1B). Meanwhile, average malaria incidence rates decreased from 13.1 before CBMM expansion to 10.1 per 1000 persons per year after CBMM expansion. The malaria death rates per 100,000 decreased from 0.1345 to 0.0493 for the years after CBMM expansion. The malaria test positivity rate increased 12.2–20.5%. The ABER increased from 2.3 to 3.5 per 100 per year. The malaria confirmation rate increased from 14% to 2012 to 99% in 2019. Annual malaria testing, incidence, and deaths are presented in Appendix 1 by province from 2012 to 2019 (Fig. 2). The average annual Malaria incidence and death rates in Afghanistan before (2012–15) and after (2016–2019) the expansion of CBMM are presented in Fig. 2.
In the time period after CBMM expansion there was an 8% decrease in the malaria incidence rate as compared to the period before CBMM expansion (IRR 0.92, P = 0.692) (Table 3). For the time period after CBMM expansion, the confirmed malaria incidence rate increased 339% as compared to the period before CBMM was expanded (IRR 3.39, P < 0.001). There was a 65% decrease in the malaria death rate in the period after the expansion of CBMM compared to the period before (IRR 0.35, P < 0.001).
In examining only, the period since the expansion of CBMM (2016–2019), the overall malaria incidence rate declined by 19% each year (IRR 0.81, P = 0.001). The confirmed malaria incidence rate declined by 2% each year (IRR:0.98, P = 0.840). Malaria death incidence declined by 85% each year (IRR 0.15, P < 0.001) (Table 4).
Discussion
The malaria trend analysis revealed several encouraging outcomes for malaria control in Afghanistan following the scale-up of the CBMM strategy. In line with the expansion of RDT, there was an increase in the number of suspected cases that received parasitological testing in a health facility and at community levels. During the period since this expansion, the malaria incidence rate and malaria death rate declined. The magnitude of the decline in incidence is remarkable - from 15.5 to 5.5 per 1000 persons/year between 2012 and 2019. The malaria deaths rate declined from 0.1416 to 0 per 100,000 persons per year for the same periods. Additionally, number of confirmed malaria cases increased following the expansion of RDT and the number of clinical cases decreased during the period. The ABER have increased, leading to a confirmation rate of nearly 100%.
The study results are similar to positive outcomes of other community-based malaria control models. A systematic review conducted in 2019 investigated the impact of community-delivered models (namely, Integrated Community Case Management and Home Management of Malaria) on coverage and malaria outcomes compared to non-community-delivered models [6]. The result of meta-analysis indicated that the implementation of community-delivered models improved malaria-attributed mortality. Community-delivered models also reduced the risk of parasitaemia from 25 to 70% compared to non-community-delivered models [6].
There were four limitations in the study and analysis. First, surveillance and health system data were used which meant authors were not fully able to assess quality (however, there was very high reporting completeness throughout the study period). Second, data were reported as aggregated and individual characteristics such as gender, age, and other personal and behaviour data were not available. Studying the potential associations between malaria and these characteristics will help target future interventions towards malaria elimination. Third, the surveillance data did not include most of the cases, which were diagnosed or received treatment in private health sectors. It is also unclear how use of the private health sector changed over time. Lastly, treatment data were not reported to the surveillance system and, therefore, it was not possible to assess trends in this important indicator.
There are also potential confounders that may explain or partially explain the differences witnessed in malaria indicators during the before versus after CBMM scale-up. These include vector control measures, the Basic Package of Health Services (BPHS), the Essential Package of Health Services (EPHS) and strengthening of malaria surveillance, Malaria Leishmania Information System (MLIS). The diagnosis and treatment of malaria has been integrated into BPHS and EPHS services, with malaria diagnosis and treatment (including microscopy and anti-malarial therapy) provided from health post level up to regional hospitals and provided malaria reports on monthly basis. Additionally, since expansion of CBMM after 2016, approximately 6,015,826 long-lasting insecticide nets (LLIN) have been distributed to targeted provinces. The LLIN distribution programme ensured 100% operational coverage (i.e., all target provinces and districts were covered through mass distribution campaigns and through continuous distribution at antenatal clinics). The programme sought to improve coverage and accessibility for at-risk populations, including pregnant women and children. Ecological factors such as changes in temperature or rainfall, variables that could influence malaria transmission in Afghanistan were not assessed.
The trend analysis for the period after CBMM expansion shows that most of the targets of Afghanistan’s National Strategic Plan for Malaria 2018–22 are on track to being met. The plan aims to reduce malaria incidence by 73% at the national level compared with 2016. Between 2016 and 2019, the number of reported malaria cases were reduced from 385,015 to 174,893 (55%). The proportion of confirmed malaria cases increased to 99% in 2019 compared to the baseline 49% in 2016. Nonetheless, 12 provinces remain at high risk for malaria with reported annual parasite incidence rates per 1000 persons at 1 and above and test positivity rate at 9% and above.
Conclusions
In summary, the CBMM expansion which introduced rapid diagnostic tests for malaria to many primary care settings correlated with significant increase in the number of confirmed cases, while also being correlated with significant reduction in annual malaria incidence and death rates. Use of RDTs for the diagnosis of malaria could be best applied as a tool at the community level to facilitate the early treatment of malaria in settings where microscopy services are not available. The data and the study results corroborate similar studies that recommend community-based interventions as best practices for malaria control, especially in resource-limited settings.
Availability of data and materials
All details of data (case numbers) that we used for our analysis are presented in Table 2 and Appendix 1.
References
WHO. World malaria report 2021. Geneva: World Health Organization. https://www.mmv.org/sites/default/files/uploads/docs/publications/World%20Malaria%20Report_0.pdf. 2020.
National Malaria Leishmania Control Programme, Ministry of Public Health. Malaria Annual Report. https://www.ecdc.europa.eu/en/publications-data/malaria-annual-epidemiological-report-2019 2019.
National Malaria Leishmania Control Programme, Ministry of Public Health. National Strategic Plan " From Malaria Control to Elimination in Afghanistan”. https://moph.gov.af/sites/default/files/2019-07/NMSP%202018-2020%20Final.pdf. 2017.
National Malaria Leishmania Control Programme. Ministry of Public Health. National Strategy for Community-based Management of Malaria (CBMM) in Afghanistan (2016–2020). 2016.
National Malaria Leishmania Control Programme, Ministry of Public Health. Afghanistan CBHC “Community Based Health Care Evaluation Report” 2017.
Oo WH, Gold L, Moore K, Agius PA, Fowkes FJI. The impact of community-delivered models of malaria control and elimination: a systematic review. Malar J. 2019;18:269.
Acknowledgements
We would like to thank staff and directors of the Afghanistan Malaria programme for their efforts, and technical support.
Funding
This work was supported by UNDP Global Fund and the support from the UCSF’s International Traineeships in AIDS Prevention Studies (ITAPS), U.S. NIMH, R25 MH064712. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views and opinions expressed herein are those of the individual authors and not necessarily those of UNDP, UCSF, MOH, or funders.
Author information
Authors and Affiliations
Contributions
SDM (study design, implementation, data cleaning and analysis, reporting, manuscript writing), AAA, AWS, WM, TBA, MSN, HH, GQQ, ST (study design, interpretation of results, critical review of the manuscript), and SG, AM (study design, data analysis, reporting, critical review, manuscript writing, funding). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We used de-identified public health surveillance data which does not require participant consent.
Consent for publication
All co-authors have reviewed the final draft of the paper and approved it before submission to the journal.
Competing interests
Nothing to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Province wise annual malaria testing, incidence and deaths in Afghanistan from 2012 to 2019.
Province | Population | Total microscopy and rapid tests for Malaria diagnosis | Plasmodium vivax (PV) Malaria cases | PV incidence rate per 1000 persons per year | Plasmodium falciparum (PF) Malaria cases | PF incidence rate per 1000 persons per year | Total Confirmed Malaria cases | Total clinical malaria cases | Reported malaria cases (clinical and confirmed) | Malaria test positivity rate (per 100 malaria tests per year) | Malaria confirmation rate (per 100 reported cases per year) | Malaria incidence rate (per 1000 persons per year) | Annual blood examination rate (per 100 population per year) | Total malaria deaths |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Badakhshan | ||||||||||||||
2012 | 889,700 | 17,646 | 1154 | 1.30 | 4 | 0.004 | 1158 | 13,573 | 14,731 | 6.6% | 7.9% | 16.56 | 1.98% | 0 |
2013 | 919,900 | 14,408 | 1140 | 1.24 | 4 | 0.004 | 1144 | 10,592 | 11,736 | 7.9% | 9.7% | 12.76 | 1.57% | 0 |
2014 | 935,327 | 23,665 | 2261 | 2.42 | 239 | 0.256 | 2500 | 5769 | 8269 | 10.6% | 30.2% | 8.84 | 2.53% | 0 |
2015 | 950,953 | 20,646 | 1810 | 1.90 | 77 | 0.081 | 1887 | 5636 | 7523 | 9.1% | 25.1% | 7.91 | 2.17% | 0 |
2016 | 966,789 | 12,104 | 1201 | 1.24 | 22 | 0.023 | 1223 | 4733 | 5956 | 10.1% | 20.5% | 6.16 | 1.25% | 0 |
2017 | 982,835 | 17,078 | 1110 | 1.13 | 10 | 0.010 | 1120 | 526 | 1646 | 6.6% | 68.0% | 1.67 | 1.74% | 0 |
2018 | 1,017,499 | 15,114 | 731 | 0.72 | 6 | 0.006 | 737 | 95 | 832 | 4.9% | 88.6% | 0.82 | 1.49% | 0 |
2019 | 1,035,658 | 13,435 | 407 | 0.39 | 23 | 0.022 | 430 | 0 | 430 | 3.2% | 100.0% | 0.42 | 1.30% | 0 |
Badghis | ||||||||||||||
2012 | 464,100 | 1682 | 27 | 0.06 | 0 | 0.000 | 27 | 6666 | 6693 | 1.6% | 0.4% | 14.42 | 0.36% | 0 |
2013 | 479,800 | 1997 | 28 | 0.06 | 4 | 0.008 | 32 | 3095 | 3127 | 1.6% | 1.0% | 6.52 | 0.42% | 0 |
2014 | 487,838 | 955 | 13 | 0.03 | 2 | 0.004 | 15 | 3150 | 3165 | 1.6% | 0.5% | 6.49 | 0.20% | 0 |
2015 | 495,958 | 1042 | 18 | 0.04 | 2 | 0.004 | 20 | 3739 | 3759 | 1.9% | 0.5% | 7.58 | 0.21% | 0 |
2016 | 504,185 | 10,356 | 3228 | 6.40 | 197 | 0.391 | 3425 | 2225 | 5650 | 33.1% | 60.6% | 11.21 | 2.05% | 0 |
2017 | 512,518 | 5476 | 418 | 0.82 | 12 | 0.023 | 430 | 1590 | 2020 | 7.9% | 21.3% | 3.94 | 1.07% | 0 |
2018 | 530,574 | 6545 | 23 | 0.04 | 2 | 0.004 | 25 | 195 | 220 | 0.4% | 11.4% | 0.41 | 1.23% | 0 |
2019 | 540,009 | 4518 | 12 | 0.02 | 1 | 0.002 | 13 | 0 | 13 | 0.3% | 100.0% | 0.02 | 0.84% | 0 |
Baghlan | ||||||||||||||
2012 | 848,500 | 5567 | 18 | 0.02 | 0 | 0.000 | 18 | 646 | 664 | 0.3% | 2.7% | 0.78 | 0.66% | 0 |
2013 | 855,400 | 3246 | 21 | 0.02 | 3 | 0.004 | 24 | 347 | 371 | 0.7% | 6.5% | 0.43 | 0.38% | 0 |
2014 | 894,838 | 7906 | 8 | 0.01 | 1 | 0.001 | 9 | 543 | 552 | 0.1% | 1.6% | 0.62 | 0.88% | 0 |
2015 | 910,784 | 5451 | 31 | 0.03 | 5 | 0.005 | 36 | 95 | 131 | 0.7% | 27.5% | 0.14 | 0.60% | 0 |
2016 | 926,969 | 5297 | 69 | 0.07 | 10 | 0.011 | 79 | 25 | 104 | 1.5% | 76.0% | 0.11 | 0.57% | 0 |
2017 | 946,394 | 10,799 | 122 | 0.13 | 8 | 0.008 | 130 | 19 | 149 | 1.2% | 87.2% | 0.16 | 1.14% | 0 |
2018 | 977,297 | 10,147 | 70 | 0.07 | 1 | 0.001 | 71 | 17 | 88 | 0.7% | 80.7% | 0.09 | 1.04% | 0 |
2019 | 995,814 | 9218 | 52 | 0.05 | 3 | 0.003 | 55 | 0 | 55 | 0.6% | 100.0% | 0.06 | 0.93% | 0 |
Balkh | ||||||||||||||
2012 | 1,219,200 | 3431 | 154 | 0.13 | 0 | 0.000 | 154 | 3583 | 3737 | 4.5% | 4.1% | 3.07 | 0.28% | 0 |
2013 | 1,318,000 | 3680 | 138 | 0.10 | 4 | 0.003 | 142 | 3734 | 3876 | 3.9% | 3.7% | 2.94 | 0.28% | 0 |
2014 | 1,298,247 | 11,520 | 153 | 0.12 | 65 | 0.050 | 218 | 1187 | 1405 | 1.9% | 15.5% | 1.08 | 0.89% | 0 |
2015 | 1,325,659 | 11,261 | 155 | 0.12 | 25 | 0.019 | 180 | 1457 | 1637 | 1.6% | 11.0% | 1.23 | 0.85% | 0 |
2016 | 1,353,626 | 3867 | 12 | 0.01 | 82 | 0.061 | 94 | 1137 | 1231 | 2.4% | 7.6% | 0.91 | 0.29% | 0 |
2017 | 1,382,155 | 5942 | 67 | 0.05 | 5 | 0.004 | 72 | 559 | 631 | 1.2% | 11.4% | 0.46 | 0.43% | 0 |
2018 | 1,442,847 | 6412 | 56 | 0.04 | 10 | 0.007 | 66 | 843 | 909 | 1.0% | 7.3% | 0.63 | 0.44% | 0 |
2019 | 1,475,649 | 8627 | 59 | 0.04 | 1 | 0.001 | 60 | 0 | 60 | 0.7% | 100.0% | 0.04 | 0.58% | 0 |
Bamyan | ||||||||||||||
2012 | 418,500 | 680 | 27 | 0.06 | 0 | 0.000 | 27 | 833 | 860 | 4.0% | 3.1% | 2.05 | 0.16% | 0 |
2013 | 432,700 | 804 | 34 | 0.08 | 7 | 0.016 | 41 | 691 | 732 | 5.1% | 5.6% | 1.69 | 0.19% | 0 |
2014 | 439,899 | 952 | 58 | 0.13 | 9 | 0.020 | 67 | 578 | 645 | 7.0% | 10.4% | 1.47 | 0.22% | 0 |
2015 | 447,218 | 2281 | 27 | 0.06 | 16 | 0.036 | 43 | 673 | 716 | 1.9% | 6.0% | 1.60 | 0.51% | 0 |
2016 | 427,067 | 2299 | 167 | 0.39 | 357 | 0.836 | 524 | 312 | 836 | 22.8% | 62.7% | 1.96 | 0.54% | 0 |
2017 | 462,144 | 1940 | 9 | 0.02 | 0 | 0.000 | 9 | 121 | 130 | 0.5% | 6.9% | 0.28 | 0.42% | 0 |
2018 | 478,424 | 1660 | 49 | 0.10 | 4 | 0.008 | 53 | 5 | 58 | 3.2% | 91.4% | 0.12 | 0.35% | 0 |
2019 | 486,928 | 707 | 19 | 0.04 | 1 | 0.002 | 20 | 0 | 20 | 2.8% | 100.0% | 0.04 | 0.15% | 0 |
Daykundi | ||||||||||||||
2012 | 431,300 | 2097 | 82 | 0.19 | 14 | 0.032 | 96 | 2913 | 3009 | 4.6% | 3.2% | 6.98 | 0.49% | 1 |
2013 | 378,900 | 2594 | 47 | 0.12 | 4 | 0.011 | 51 | 1956 | 2007 | 2.0% | 2.5% | 5.30 | 0.68% | 0 |
2014 | 417,476 | 1798 | 40 | 0.10 | 10 | 0.024 | 50 | 2029 | 2079 | 2.8% | 2.4% | 4.98 | 0.43% | 0 |
2015 | 424,339 | 1633 | 34 | 0.08 | 10 | 0.024 | 44 | 1756 | 1800 | 2.7% | 2.4% | 4.24 | 0.38% | 0 |
2016 | 468,178 | 2818 | 132 | 0.28 | 66 | 0.141 | 198 | 2493 | 2691 | 7.0% | 7.4% | 5.75 | 0.60% | 0 |
2017 | 493,634 | 4376 | 52 | 0.11 | 19 | 0.038 | 71 | 1374 | 1445 | 1.6% | 4.9% | 2.93 | 0.89% | 0 |
2018 | 544,788 | 3434 | 124 | 0.23 | 5 | 0.009 | 129 | 853 | 982 | 3.8% | 13.1% | 1.80 | 0.63% | 0 |
2019 | 507,610 | 4845 | 15 | 0.03 | 1 | 0.002 | 16 | 0 | 16 | 0.3% | 100.0% | 0.03 | 0.95% | 0 |
Farah | ||||||||||||||
2012 | 480,500 | 1221 | 40 | 0.08 | 2 | 0.004 | 42 | 438 | 480 | 3.4% | 8.8% | 1.00 | 0.25% | 0 |
2013 | 490,600 | 1186 | 15 | 0.03 | 5 | 0.010 | 20 | 337 | 357 | 1.7% | 5.6% | 0.73 | 0.24% | 0 |
2014 | 498,951 | 658 | 17 | 0.03 | 1 | 0.002 | 18 | 310 | 328 | 2.7% | 5.5% | 0.66 | 0.13% | 0 |
2015 | 507,405 | 815 | 13 | 0.03 | 7 | 0.014 | 20 | 381 | 401 | 2.5% | 5.0% | 0.79 | 0.16% | 0 |
2016 | 515,973 | 3143 | 33 | 0.06 | 5 | 0.010 | 38 | 258 | 296 | 1.2% | 12.8% | 0.57 | 0.61% | 0 |
2017 | 524,657 | 4942 | 110 | 0.21 | 2 | 0.004 | 112 | 311 | 423 | 2.3% | 26.5% | 0.81 | 0.94% | 0 |
2018 | 543,237 | 5096 | 84 | 0.15 | 0 | 0.000 | 84 | 23 | 107 | 1.6% | 78.5% | 0.20 | 0.94% | 0 |
2019 | 553,058 | 1899 | 25 | 0.05 | 1 | 0.002 | 26 | 0 | 26 | 1.4% | 100.0% | 0.05 | 0.34% | 0 |
Faryab | ||||||||||||||
2012 | 931,800 | 2989 | 31 | 0.03 | 1 | 0.001 | 32 | 4471 | 4503 | 1.1% | 0.7% | 4.83 | 0.32% | 0 |
2013 | 964,600 | 3527 | 26 | 0.03 | 3 | 0.003 | 29 | 3901 | 3930 | 0.8% | 0.7% | 4.07 | 0.37% | 0 |
2014 | 981,197 | 5136 | 914 | 0.93 | 98 | 0.100 | 1012 | 2898 | 3910 | 19.7% | 25.9% | 3.98 | 0.52% | 0 |
2015 | 998,147 | 3808 | 0 | 0.00 | 1 | 0.001 | 1 | 2461 | 2462 | 0.0% | 0.0% | 2.47 | 0.38% | 0 |
2016 | 1,015,335 | 3966 | 14 | 0.01 | 0 | 0.000 | 14 | 2559 | 2573 | 0.4% | 0.5% | 2.53 | 0.39% | 0 |
2017 | 1,032,765 | 7098 | 3 | 0.00 | 7 | 0.007 | 10 | 1099 | 1109 | 0.1% | 0.9% | 1.07 | 0.69% | 0 |
2018 | 1,069,540 | 3747 | 30 | 0.03 | 0 | 0.000 | 30 | 472 | 502 | 0.8% | 6.0% | 0.47 | 0.35% | 0 |
2019 | 1,089,228 | 3799 | 12 | 0.01 | 1 | 0.001 | 13 | 1 | 14 | 0.3% | 92.9% | 0.01 | 0.35% | 0 |
Ghazni | ||||||||||||||
2012 | 1,149,400 | 10,086 | 1179 | 1.03 | 61 | 0.053 | 1240 | 4109 | 5349 | 12.3% | 23.2% | 4.65 | 0.88% | 0 |
2013 | 1,188,600 | 14,258 | 1307 | 1.10 | 41 | 0.034 | 1348 | 4001 | 5349 | 9.5% | 25.2% | 4.50 | 1.20% | 1 |
2014 | 1,240,437 | 16,606 | 1329 | 1.07 | 83 | 0.067 | 1412 | 3241 | 4653 | 8.5% | 30.3% | 3.75 | 1.34% | 0 |
2015 | 1,228,831 | 17,392 | 832 | 0.68 | 80 | 0.065 | 912 | 2723 | 3635 | 5.2% | 25.1% | 2.96 | 1.42% | 0 |
2016 | 1,249,376 | 22,488 | 841 | 0.67 | 67 | 0.054 | 908 | 2297 | 3205 | 4.0% | 28.3% | 2.57 | 1.80% | 0 |
2017 | 1,270,192 | 17,949 | 959 | 0.76 | 85 | 0.067 | 1044 | 961 | 2005 | 5.8% | 52.1% | 1.58 | 1.41% | 0 |
2018 | 1,315,041 | 8154 | 610 | 0.46 | 23 | 0.017 | 633 | 359 | 992 | 7.8% | 63.8% | 0.75 | 0.62% | 0 |
2019 | 1,338,597 | 9905 | 750 | 0.56 | 129 | 0.096 | 879 | 1 | 880 | 8.9% | 99.9% | 0.66 | 0.74% | 0 |
Ghor | ||||||||||||||
2012 | 646,300 | 361 | 19 | 0.03 | 34 | 0.053 | 53 | 1817 | 1870 | 14.7% | 2.8% | 2.89 | 0.06% | 1 |
2013 | 668,000 | 585 | 15 | 0.02 | 29 | 0.043 | 44 | 1586 | 1630 | 7.5% | 2.7% | 2.44 | 0.09% | 0 |
2014 | 679,085 | 415 | 5 | 0.01 | 22 | 0.032 | 27 | 1407 | 1434 | 6.5% | 1.9% | 2.11 | 0.06% | 0 |
2015 | 690,296 | 339 | 7 | 0.01 | 29 | 0.042 | 36 | 1004 | 1040 | 10.6% | 3.5% | 1.51 | 0.05% | 0 |
2016 | 701,653 | 1332 | 23 | 0.03 | 5 | 0.007 | 28 | 540 | 568 | 2.1% | 4.9% | 0.81 | 0.19% | 0 |
2017 | 713,158 | 4755 | 39 | 0.05 | 11 | 0.015 | 50 | 485 | 535 | 1.1% | 9.3% | 0.75 | 0.67% | 0 |
2018 | 738,224 | 3360 | 43 | 0.06 | 4 | 0.005 | 47 | 291 | 338 | 1.4% | 13.9% | 0.46 | 0.46% | 0 |
2019 | 751,254 | 4637 | 13 | 0.02 | 0 | 0.000 | 13 | 0 | 13 | 0.3% | 100.0% | 0.02 | 0.62% | 0 |
Hilmand | ||||||||||||||
2012 | 864,600 | 8643 | 314 | 0.36 | 32 | 0.037 | 346 | 11,296 | 11,642 | 4.0% | 3.0% | 13.47 | 1.00% | 0 |
2013 | 867,600 | 10,776 | 375 | 0.43 | 63 | 0.073 | 438 | 12,939 | 13,377 | 4.1% | 3.3% | 15.42 | 1.24% | 0 |
2014 | 909,395 | 14,840 | 204 | 0.22 | 104 | 0.114 | 308 | 12,845 | 13,153 | 2.1% | 2.3% | 14.46 | 1.63% | 0 |
2015 | 924,711 | 13,146 | 106 | 0.11 | 11 | 0.012 | 117 | 12,631 | 12,748 | 0.9% | 0.9% | 13.79 | 1.42% | 0 |
2016 | 894,805 | 11,746 | 106 | 0.12 | 13 | 0.015 | 119 | 7611 | 7730 | 1.0% | 1.5% | 8.64 | 1.31% | 1 |
2017 | 938,184 | 21,474 | 192 | 0.20 | 32 | 0.034 | 224 | 8922 | 9146 | 1.0% | 2.4% | 9.75 | 2.29% | 0 |
2018 | 1,395,514 | 24,637 | 101 | 0.07 | 10 | 0.007 | 111 | 978 | 1089 | 0.5% | 10.2% | 0.78 | 1.77% | 0 |
2019 | 1,420,682 | 15,466 | 51 | 0.04 | 3 | 0.002 | 54 | 0 | 54 | 0.3% | 100.0% | 0.04 | 1.09% | 0 |
Hirat | ||||||||||||||
2012 | 1,744,700 | 1927 | 9 | 0.01 | 0 | 0.000 | 9 | 5373 | 5382 | 0.5% | 0.2% | 3.08 | 0.11% | 0 |
2013 | 1,816,100 | 2285 | 3 | 0.00 | 0 | 0.000 | 3 | 2791 | 2794 | 0.1% | 0.1% | 1.54 | 0.13% | 0 |
2014 | 1,852,790 | 5567 | 24 | 0.01 | 1 | 0.001 | 25 | 1713 | 1738 | 0.4% | 1.4% | 0.94 | 0.30% | 0 |
2015 | 1,890,202 | 6593 | 11 | 0.01 | 1 | 0.001 | 12 | 1325 | 1337 | 0.2% | 0.9% | 0.71 | 0.35% | 0 |
2016 | 1,928,327 | 3295 | 12 | 0.01 | 3 | 0.002 | 15 | 947 | 962 | 0.5% | 1.6% | 0.50 | 0.17% | 0 |
2017 | 1,967,180 | 9607 | 4 | 0.00 | 17 | 0.009 | 21 | 196 | 217 | 0.2% | 9.7% | 0.11 | 0.49% | 0 |
2018 | 2,050,514 | 7736 | 12 | 0.01 | 0 | 0.000 | 12 | 117 | 129 | 0.2% | 9.3% | 0.06 | 0.38% | 0 |
2019 | 2,095,117 | 4681 | 19 | 0.01 | 2 | 0.001 | 21 | 0 | 21 | 0.4% | 100.0% | 0.01 | 0.22% | 0 |
Jawzjan | ||||||||||||||
2012 | 503,100 | 2263 | 20 | 0.04 | 0 | 0.000 | 20 | 3264 | 3284 | 0.9% | 0.6% | 6.53 | 0.45% | 0 |
2013 | 521,400 | 2462 | 6 | 0.01 | 15 | 0.029 | 21 | 3967 | 3988 | 0.9% | 0.5% | 7.65 | 0.47% | 0 |
2014 | 530,751 | 1957 | 11 | 0.02 | 4 | 0.008 | 15 | 1675 | 1690 | 0.8% | 0.9% | 3.18 | 0.37% | 0 |
2015 | 540,255 | 2790 | 24 | 0.04 | 8 | 0.015 | 32 | 3001 | 3033 | 1.1% | 1.1% | 5.61 | 0.52% | 0 |
2016 | 549,900 | 1738 | 3 | 0.01 | 5 | 0.009 | 8 | 1169 | 1177 | 0.5% | 0.7% | 2.14 | 0.32% | 0 |
2017 | 559,691 | 2243 | 3 | 0.01 | 17 | 0.030 | 20 | 447 | 467 | 0.9% | 4.3% | 0.83 | 0.40% | 0 |
2018 | 579,833 | 3196 | 17 | 0.03 | 0 | 0.000 | 17 | 21 | 38 | 0.5% | 44.7% | 0.07 | 0.55% | 0 |
2019 | 590,866 | 6700 | 13 | 0.02 | 0 | 0.000 | 13 | 0 | 13 | 0.2% | 100.0% | 0.02 | 1.13% | 0 |
Kabul | ||||||||||||||
2012 | 3,818,700 | 23,300 | 2013 | 0.53 | 20 | 0.005 | 2033 | 10,352 | 12,385 | 8.7% | 16.4% | 3.24 | 0.61% | 1 |
2013 | 4,086,500 | 23,568 | 1133 | 0.28 | 74 | 0.018 | 1207 | 7441 | 8648 | 5.1% | 14.0% | 2.12 | 0.58% | 1 |
2014 | 4,227,261 | 28,007 | 2013 | 0.48 | 37 | 0.009 | 2050 | 7971 | 10,021 | 7.3% | 20.5% | 2.37 | 0.66% | 0 |
2015 | 4,372,977 | 30,343 | 3394 | 0.78 | 81 | 0.019 | 3475 | 9769 | 13,244 | 11.5% | 26.2% | 3.03 | 0.69% | 1 |
2016 | 4,523,718 | 53,469 | 10,844 | 2.40 | 257 | 0.057 | 11,101 | 11,628 | 22,729 | 20.8% | 48.8% | 5.02 | 1.18% | 5 |
2017 | 4,679,648 | 58,254 | 13,468 | 2.88 | 269 | 0.057 | 13,737 | 4678 | 18,415 | 23.6% | 74.6% | 3.94 | 1.24% | 2 |
2018 | 4,860,880 | 63,631 | 11,208 | 2.31 | 180 | 0.037 | 11,388 | 3799 | 15,187 | 17.9% | 75.0% | 3.12 | 1.31% | 1 |
2019 | 5,029,850 | 43,034 | 5906 | 1.17 | 126 | 0.025 | 6032 | 832 | 6864 | 14.0% | 87.9% | 1.36 | 0.86% | 0 |
Kandahar | ||||||||||||||
2012 | 1,127,000 | 6510 | 134 | 0.12 | 10 | 0.009 | 144 | 12,154 | 12,298 | 2.2% | 1.2% | 10.91 | 0.58% | 0 |
2013 | 1,119,000 | 6215 | 99 | 0.09 | 5 | 0.004 | 104 | 7935 | 8039 | 1.7% | 1.3% | 7.18 | 0.56% | 0 |
2014 | 1,200,929 | 5613 | 70 | 0.06 | 1 | 0.001 | 71 | 6548 | 6619 | 1.3% | 1.1% | 5.51 | 0.47% | 0 |
2015 | 1,226,593 | 7088 | 150 | 0.12 | 1 | 0.001 | 151 | 4859 | 5010 | 2.1% | 3.0% | 4.08 | 0.58% | 0 |
2016 | 1,193,020 | 12,086 | 88 | 0.07 | 2 | 0.002 | 90 | 2908 | 2998 | 0.7% | 3.0% | 2.51 | 1.01% | 0 |
2017 | 1,279,520 | 11,921 | 101 | 0.08 | 63 | 0.049 | 164 | 242 | 406 | 1.4% | 40.4% | 0.32 | 0.93% | 0 |
2018 | 1,351,169 | 12,122 | 125 | 0.09 | 18 | 0.013 | 143 | 379 | 522 | 1.2% | 27.4% | 0.39 | 0.90% | 0 |
2019 | 1,368,036 | 17,490 | 338 | 0.25 | 19 | 0.014 | 357 | 1 | 358 | 2.0% | 99.7% | 0.26 | 1.28% | 0 |
Kapisa | ||||||||||||||
2012 | 413,000 | 5369 | 388 | 0.94 | 1 | 0.002 | 389 | 1594 | 1983 | 7.2% | 19.6% | 4.80 | 1.30% | 0 |
2013 | 426,800 | 7527 | 275 | 0.64 | 1 | 0.002 | 276 | 1120 | 1396 | 3.7% | 19.8% | 3.27 | 1.76% | 0 |
2014 | 433,867 | 5148 | 109 | 0.25 | 0 | 0.000 | 109 | 720 | 829 | 2.1% | 13.1% | 1.91 | 1.19% | 0 |
2015 | 441,010 | 5855 | 140 | 0.32 | 0 | 0.000 | 140 | 653 | 793 | 2.4% | 17.7% | 1.80 | 1.33% | 0 |
2016 | 448,245 | 8768 | 763 | 1.70 | 39 | 0.087 | 802 | 2403 | 3205 | 9.1% | 25.0% | 7.15 | 1.96% | 0 |
2017 | 455,574 | 12,061 | 2091 | 4.59 | 49 | 0.108 | 2140 | 1152 | 3292 | 17.7% | 65.0% | 7.23 | 2.65% | 0 |
2018 | 471,574 | 14,229 | 1608 | 3.41 | 12 | 0.025 | 1620 | 75 | 1695 | 11.4% | 95.6% | 3.59 | 3.02% | 0 |
2019 | 479,875 | 11,687 | 1098 | 2.29 | 8 | 0.017 | 1106 | 0 | 1106 | 9.5% | 100.0% | 2.30 | 2.44% | 0 |
Khost | ||||||||||||||
2012 | 537,800 | 10,861 | 1610 | 2.99 | 61 | 0.113 | 1671 | 10,461 | 12,132 | 15.4% | 13.8% | 22.56 | 2.02% | 0 |
2013 | 556,000 | 14,161 | 1298 | 2.33 | 121 | 0.218 | 1419 | 9018 | 10,437 | 10.0% | 13.6% | 18.77 | 2.55% | 1 |
2014 | 565,211 | 26,224 | 1560 | 2.76 | 286 | 0.506 | 1846 | 5207 | 7053 | 7.0% | 26.2% | 12.48 | 4.64% | 1 |
2015 | 574,582 | 19,670 | 1435 | 2.50 | 492 | 0.856 | 1927 | 6445 | 8372 | 9.8% | 23.0% | 14.57 | 3.42% | 0 |
2016 | 584,075 | 23,215 | 2473 | 4.23 | 349 | 0.598 | 2822 | 4292 | 7114 | 12.2% | 39.7% | 12.18 | 3.97% | 1 |
2017 | 593,691 | 24,731 | 3600 | 6.06 | 407 | 0.686 | 4007 | 1675 | 5682 | 16.2% | 70.5% | 9.57 | 4.17% | 0 |
2018 | 614,584 | 23,897 | 2864 | 4.66 | 106 | 0.172 | 2970 | 406 | 3376 | 12.4% | 88.0% | 5.49 | 3.89% | 0 |
2019 | 625,473 | 20,294 | 1778 | 2.84 | 21 | 0.034 | 1799 | 1 | 1800 | 8.9% | 99.9% | 2.88 | 3.24% | 0 |
Kunar | ||||||||||||||
2012 | 421,700 | 40,847 | 7464 | 17.70 | 200 | 0.474 | 7664 | 33,376 | 41,040 | 18.8% | 18.7% | 97.32 | 9.69% | 0 |
2013 | 436,000 | 39,298 | 5354 | 12.28 | 173 | 0.397 | 5527 | 39,103 | 44,630 | 14.1% | 12.4% | 102.36 | 9.01% | 0 |
2014 | 443,272 | 65,356 | 12,534 | 28.28 | 1277 | 2.881 | 13,811 | 23,198 | 37,009 | 21.1% | 37.3% | 83.49 | 14.74% | 3 |
2015 | 450,652 | 55,648 | 12,150 | 26.96 | 308 | 0.683 | 12,458 | 32,670 | 45,128 | 22.4% | 27.6% | 100.14 | 12.35% | 4 |
2016 | 440,231 | 67,782 | 19,235 | 43.69 | 914 | 2.076 | 20,149 | 28,522 | 48,671 | 29.7% | 41.4% | 110.56 | 15.40% | 3 |
2017 | 465,706 | 115,289 | 37,373 | 80.25 | 1235 | 2.652 | 38,608 | 13,023 | 51,631 | 33.5% | 74.8% | 110.87 | 24.76% | 1 |
2018 | 482,115 | 132,366 | 40,427 | 83.85 | 1119 | 2.321 | 41,546 | 6348 | 47,894 | 31.4% | 86.7% | 99.34 | 27.46% | 0 |
2019 | 490,690 | 97,434 | 30,015 | 61.17 | 141 | 0.287 | 30,156 | 0 | 30,156 | 31.0% | 100.0% | 61.46 | 19.86% | 0 |
Kunduz | ||||||||||||||
2012 | 935,600 | 11,155 | 123 | 0.13 | 0 | 0.000 | 123 | 5028 | 5151 | 1.1% | 2.4% | 5.51 | 1.19% | 0 |
2013 | 972,200 | 9260 | 53 | 0.05 | 0 | 0.000 | 53 | 3480 | 3533 | 0.6% | 1.5% | 3.63 | 0.95% | 0 |
2014 | 990,937 | 9437 | 46 | 0.05 | 6 | 0.006 | 52 | 1001 | 1053 | 0.6% | 4.9% | 1.06 | 0.95% | 1 |
2015 | 1,010,037 | 6895 | 21 | 0.02 | 0 | 0.000 | 21 | 494 | 515 | 0.3% | 4.1% | 0.51 | 0.68% | 0 |
2016 | 961,309 | 7123 | 54 | 0.06 | 4 | 0.004 | 58 | 344 | 402 | 0.8% | 14.4% | 0.42 | 0.74% | 0 |
2017 | 1,049,249 | 8284 | 123 | 0.12 | 6 | 0.006 | 129 | 377 | 506 | 1.6% | 25.5% | 0.48 | 0.79% | 0 |
2018 | 1,091,116 | 11,793 | 120 | 0.11 | 0 | 0.000 | 120 | 29 | 149 | 1.0% | 80.5% | 0.14 | 1.08% | 0 |
2019 | 1,113,676 | 8881 | 93 | 0.08 | 0 | 0.000 | 93 | 0 | 93 | 1.0% | 100.0% | 0.08 | 0.80% | 0 |
Laghman | ||||||||||||||
2012 | 417,200 | 37,619 | 4129 | 9.90 | 70 | 0.168 | 4199 | 30,394 | 34,593 | 11.2% | 12.1% | 82.92 | 9.02% | 0 |
2013 | 431,200 | 37,810 | 2142 | 4.97 | 49 | 0.114 | 2191 | 24,334 | 26,525 | 5.8% | 8.3% | 61.51 | 8.77% | 0 |
2014 | 438,346 | 50,427 | 8171 | 18.64 | 800 | 1.825 | 8971 | 23,292 | 32,263 | 17.8% | 27.8% | 73.60 | 11.50% | 2 |
2015 | 445,588 | 78,359 | 17,784 | 39.91 | 902 | 2.024 | 18,686 | 37,723 | 56,409 | 23.8% | 33.1% | 126.59 | 17.59% | 0 |
2016 | 445,238 | 169,476 | 64,194 | 144.18 | 3240 | 7.277 | 67,434 | 34,473 | 101,907 | 39.8% | 66.2% | 228.88 | 38.06% | 1 |
2017 | 460,352 | 130,626 | 45,363 | 98.54 | 2789 | 6.058 | 48,152 | 21,184 | 69,336 | 36.9% | 69.4% | 150.62 | 28.38% | 0 |
2018 | 476,537 | 167,947 | 53,724 | 112.74 | 3923 | 8.232 | 57,647 | 11,940 | 69,587 | 34.3% | 82.8% | 146.03 | 35.24% | 0 |
2019 | 484,952 | 159,817 | 38,792 | 79.99 | 1173 | 2.419 | 39,965 | 177 | 40,142 | 25.0% | 99.6% | 82.78 | 32.96% | 0 |
Logar | ||||||||||||||
2012 | 367,000 | 3008 | 285 | 0.78 | 6 | 0.016 | 291 | 1638 | 1929 | 9.7% | 15.1% | 5.26 | 0.82% | 0 |
2013 | 379,400 | 2701 | 157 | 0.41 | 18 | 0.047 | 175 | 1213 | 1388 | 6.5% | 12.6% | 3.66 | 0.71% | 0 |
2014 | 385,638 | 5128 | 851 | 2.21 | 27 | 0.070 | 878 | 1056 | 1934 | 17.1% | 45.4% | 5.02 | 1.33% | 0 |
2015 | 392,045 | 4528 | 542 | 1.38 | 35 | 0.089 | 577 | 1896 | 2473 | 12.7% | 23.3% | 6.31 | 1.15% | 0 |
2016 | 398,535 | 8510 | 1242 | 3.12 | 62 | 0.156 | 1304 | 1435 | 2739 | 15.3% | 47.6% | 6.87 | 2.14% | 0 |
2017 | 405,109 | 11,846 | 1822 | 4.50 | 19 | 0.047 | 1841 | 580 | 2421 | 15.5% | 76.0% | 5.98 | 2.92% | 0 |
2018 | 419,377 | 13,952 | 1627 | 3.88 | 26 | 0.062 | 1653 | 108 | 1761 | 11.8% | 93.9% | 4.20 | 3.33% | 0 |
2019 | 426,821 | 12,110 | 694 | 1.63 | 6 | 0.014 | 700 | 0 | 700 | 5.8% | 100.0% | 1.64 | 2.84% | 0 |
Nangarhar | ||||||||||||||
2012 | 1,409,600 | 244,604 | 29,108 | 20.65 | 517 | 0.367 | 29,625 | 116,035 | 145,660 | 12.1% | 20.3% | 103.33 | 17.35% | 30 |
2013 | 1,462,600 | 236,080 | 25,217 | 17.24 | 1441 | 0.985 | 26,658 | 80,157 | 106,815 | 11.3% | 25.0% | 73.03 | 16.14% | 18 |
2014 | 1,489,787 | 279,057 | 41,554 | 27.89 | 2542 | 1.706 | 44,096 | 63,032 | 107,128 | 15.8% | 41.2% | 71.91 | 18.73% | 24 |
2015 | 1,517,388 | 274,610 | 53,087 | 34.99 | 2582 | 1.702 | 55,669 | 97,207 | 152,876 | 20.3% | 36.4% | 100.75 | 18.10% | 45 |
2016 | 1,545,448 | 315,613 | 67,114 | 43.43 | 3187 | 2.062 | 70,301 | 64,644 | 134,945 | 22.3% | 52.1% | 87.32 | 20.42% | 35 |
2017 | 1,573,973 | 416,333 | 92,948 | 59.05 | 3951 | 2.510 | 96,899 | 25,208 | 122,107 | 23.3% | 79.4% | 77.58 | 26.45% | 7 |
2018 | 1,635,872 | 495,795 | 105,650 | 64.58 | 2405 | 1.470 | 108,055 | 12,731 | 120,786 | 21.8% | 89.5% | 73.84 | 30.31% | 0 |
2019 | 1,668,481 | 423,073 | 74,825 | 44.85 | 1093 | 0.655 | 75,918 | 8 | 75,926 | 17.9% | 100.0% | 45.51 | 25.36% | 0 |
Nimroz | ||||||||||||||
2012 | 147,700 | 83 | 1 | 0.01 | 0 | 0.000 | 1 | 382 | 383 | 1.2% | 0.3% | 2.59 | 0.06% | 0 |
2013 | 152,800 | 82 | 2 | 0.01 | 0 | 0.000 | 2 | 307 | 309 | 2.4% | 0.6% | 2.02 | 0.05% | 0 |
2014 | 162,135 | 81 | 1 | 0.01 | 0 | 0.000 | 1 | 241 | 242 | 1.2% | 0.4% | 1.49 | 0.05% | 0 |
2015 | 164,978 | 197 | 1 | 0.01 | 5 | 0.030 | 6 | 246 | 252 | 3.0% | 2.4% | 1.53 | 0.12% | 0 |
2016 | 161,033 | 770 | 5 | 0.03 | 8 | 0.050 | 13 | 105 | 118 | 1.7% | 11.0% | 0.73 | 0.48% | 0 |
2017 | 170,790 | 1048 | 6 | 0.04 | 0 | 0.000 | 6 | 94 | 100 | 0.6% | 6.0% | 0.59 | 0.61% | 0 |
2018 | 176,898 | 1054 | 4 | 0.02 | 0 | 0.000 | 4 | 68 | 72 | 0.4% | 5.6% | 0.41 | 0.60% | 0 |
2019 | 180,200 | 1664 | 9 | 0.05 | 0 | 0.000 | 9 | 0 | 9 | 0.5% | 100.0% | 0.05 | 0.92% | 0 |
Nuristan | ||||||||||||||
2012 | 138,600 | 5109 | 423 | 3.05 | 23 | 0.166 | 446 | 3220 | 3666 | 8.7% | 12.2% | 26.45 | 3.69% | 0 |
2013 | 143,200 | 4797 | 355 | 2.48 | 26 | 0.182 | 381 | 3412 | 3793 | 7.9% | 10.0% | 26.49 | 3.35% | 0 |
2014 | 145,574 | 6290 | 647 | 4.44 | 19 | 0.131 | 666 | 3040 | 3706 | 10.6% | 18.0% | 25.46 | 4.32% | 1 |
2015 | 147,967 | 10,134 | 1725 | 11.66 | 43 | 0.291 | 1768 | 4332 | 6100 | 17.4% | 29.0% | 41.23 | 6.85% | 0 |
2016 | 150,391 | 14,529 | 3732 | 24.82 | 134 | 0.891 | 3866 | 2714 | 6580 | 26.6% | 58.8% | 43.75 | 9.66% | 0 |
2017 | 152,845 | 22,795 | 7033 | 46.01 | 247 | 1.616 | 7280 | 4891 | 12,171 | 31.9% | 59.8% | 79.63 | 14.91% | 0 |
2018 | 158,211 | 31,952 | 11,687 | 73.87 | 432 | 2.731 | 12,119 | 5100 | 17,219 | 37.9% | 70.4% | 108.84 | 20.20% | 0 |
2019 | 160,993 | 30,266 | 10,343 | 64.25 | 108 | 0.671 | 10,451 | 0 | 10,451 | 34.5% | 100.0% | 64.92 | 18.80% | 0 |
Paktika | ||||||||||||||
2012 | 407,100 | 10,362 | 1025 | 2.52 | 69 | 0.169 | 1094 | 12,980 | 14,074 | 10.6% | 7.8% | 34.57 | 2.55% | 2 |
2013 | 420,700 | 16,120 | 1351 | 3.21 | 59 | 0.140 | 1410 | 14,105 | 15,515 | 8.7% | 9.1% | 36.88 | 3.83% | 2 |
2014 | 427,692 | 22,779 | 2251 | 5.26 | 131 | 0.306 | 2382 | 18,568 | 20,950 | 10.5% | 11.4% | 48.98 | 5.33% | 0 |
2015 | 434,742 | 24,769 | 2040 | 4.69 | 177 | 0.407 | 2217 | 15,052 | 17,269 | 9.0% | 12.8% | 39.72 | 5.70% | 0 |
2016 | 441,883 | 27,688 | 2046 | 4.63 | 261 | 0.591 | 2307 | 5690 | 7997 | 8.3% | 28.8% | 18.10 | 6.27% | 1 |
2017 | 449,116 | 37,823 | 5471 | 12.18 | 651 | 1.450 | 6122 | 6379 | 12,501 | 16.2% | 49.0% | 27.83 | 8.42% | 0 |
2018 | 748,910 | 39,002 | 5621 | 7.51 | 550 | 0.734 | 6171 | 3469 | 9640 | 15.8% | 64.0% | 12.87 | 5.21% | 0 |
2019 | 762,108 | 33,888 | 3063 | 4.02 | 164 | 0.215 | 3227 | 3 | 3230 | 9.5% | 99.9% | 4.24 | 4.45% | 0 |
Paktya | ||||||||||||||
2012 | 516,300 | 13,839 | 1060 | 2.05 | 23 | 0.045 | 1083 | 7823 | 8906 | 7.8% | 12.2% | 17.25 | 2.68% | 0 |
2013 | 525,500 | 10,437 | 916 | 1.74 | 53 | 0.101 | 969 | 5551 | 6520 | 9.3% | 14.9% | 12.41 | 1.99% | 0 |
2014 | 542,896 | 14,974 | 1405 | 2.59 | 110 | 0.203 | 1515 | 3751 | 5266 | 10.1% | 28.8% | 9.70 | 2.76% | 0 |
2015 | 551,987 | 13,276 | 1380 | 2.50 | 38 | 0.069 | 1418 | 3022 | 4440 | 10.7% | 31.9% | 8.04 | 2.41% | 0 |
2016 | 532,780 | 13,108 | 1288 | 2.42 | 93 | 0.175 | 1381 | 1131 | 2512 | 10.5% | 55.0% | 4.71 | 2.46% | 0 |
2017 | 570,534 | 20,717 | 1678 | 2.94 | 117 | 0.205 | 1795 | 1112 | 2907 | 8.7% | 61.7% | 5.10 | 3.63% | 0 |
2018 | 590,668 | 17,274 | 897 | 1.52 | 25 | 0.042 | 922 | 506 | 1428 | 5.3% | 64.6% | 2.42 | 2.92% | 0 |
2019 | 601,230 | 12,690 | 476 | 0.79 | 14 | 0.023 | 490 | 0 | 490 | 3.9% | 100.0% | 0.81 | 2.11% | 0 |
Panjsher | ||||||||||||||
2012 | 143,700 | 4242 | 65 | 0.45 | 0 | 0.000 | 65 | 349 | 414 | 1.5% | 15.7% | 2.88 | 2.95% | 0 |
2013 | 137,700 | 3895 | 20 | 0.15 | 0 | 0.000 | 20 | 239 | 259 | 0.5% | 7.7% | 1.88 | 2.83% | 0 |
2014 | 151,004 | 2565 | 21 | 0.14 | 0 | 0.000 | 21 | 110 | 131 | 0.8% | 16.0% | 0.87 | 1.70% | 0 |
2015 | 153,487 | 2106 | 18 | 0.12 | 0 | 0.000 | 18 | 149 | 167 | 0.9% | 10.8% | 1.09 | 1.37% | 0 |
2016 | 144,535 | 1227 | 10 | 0.07 | 3 | 0.021 | 13 | 68 | 81 | 1.1% | 16.0% | 0.56 | 0.85% | 0 |
2017 | 158,548 | 1290 | 47 | 0.30 | 1 | 0.006 | 48 | 46 | 94 | 3.7% | 51.1% | 0.59 | 0.81% | 0 |
2018 | 164,115 | 1273 | 72 | 0.44 | 18 | 0.110 | 90 | 15 | 105 | 7.1% | 85.7% | 0.64 | 0.78% | 0 |
2019 | 167,000 | 928 | 72 | 0.43 | 2 | 0.012 | 74 | 0 | 74 | 8.0% | 100.0% | 0.44 | 0.56% | 0 |
Parwan | ||||||||||||||
2012 | 620,900 | 3276 | 12 | 0.02 | 8 | 0.013 | 20 | 1316 | 1336 | 0.6% | 1.5% | 2.15 | 0.53% | 1 |
2013 | 642,300 | 2803 | 36 | 0.06 | 0 | 0.000 | 36 | 497 | 533 | 1.3% | 6.8% | 0.83 | 0.44% | 0 |
2014 | 653,362 | 2518 | 33 | 0.05 | 0 | 0.000 | 33 | 519 | 552 | 1.3% | 6.0% | 0.84 | 0.39% | 0 |
2015 | 664,502 | 2986 | 54 | 0.08 | 0 | 0.000 | 54 | 510 | 564 | 1.8% | 9.6% | 0.85 | 0.45% | 0 |
2016 | 675,795 | 2516 | 132 | 0.20 | 1 | 0.001 | 133 | 588 | 721 | 5.3% | 18.4% | 1.07 | 0.37% | 0 |
2017 | 687,243 | 2883 | 440 | 0.64 | 20 | 0.029 | 460 | 304 | 764 | 16.0% | 60.2% | 1.11 | 0.42% | 0 |
2018 | 711,621 | 3705 | 597 | 0.84 | 1 | 0.001 | 598 | 35 | 633 | 16.1% | 94.5% | 0.89 | 0.52% | 0 |
2019 | 724,561 | 2407 | 191 | 0.26 | 2 | 0.003 | 193 | 0 | 193 | 8.0% | 100.0% | 0.27 | 0.33% | 0 |
Samangan | ||||||||||||||
2012 | 362,500 | 299 | 22 | 0.06 | 2 | 0.006 | 24 | 540 | 564 | 8.0% | 4.3% | 1.56 | 0.08% | 0 |
2013 | 335,700 | 242 | 10 | 0.03 | 0 | 0.000 | 10 | 424 | 434 | 4.1% | 2.3% | 1.29 | 0.07% | 0 |
2014 | 381,459 | 1697 | 27 | 0.07 | 0 | 0.000 | 27 | 835 | 862 | 1.6% | 3.1% | 2.26 | 0.44% | 0 |
2015 | 387,928 | 2049 | 4 | 0.01 | 0 | 0.000 | 4 | 681 | 685 | 0.2% | 0.6% | 1.77 | 0.53% | 0 |
2016 | 394,487 | 1195 | 14 | 0.04 | 26 | 0.066 | 40 | 885 | 925 | 3.3% | 4.3% | 2.34 | 0.30% | 0 |
2017 | 401,134 | 2066 | 9 | 0.02 | 0 | 0.000 | 9 | 108 | 117 | 0.4% | 7.7% | 0.29 | 0.52% | 0 |
2018 | 415,343 | 1058 | 2 | 0.00 | 1 | 0.002 | 3 | 184 | 187 | 0.3% | 1.6% | 0.45 | 0.25% | 0 |
2019 | 422,859 | 883 | 4 | 0.01 | 0 | 0.000 | 4 | 0 | 4 | 0.5% | 100.0% | 0.01 | 0.21% | 0 |
Sar-e-Pul | ||||||||||||||
2012 | 522,900 | 1341 | 58 | 0.11 | 1 | 0.002 | 59 | 4056 | 4115 | 4.4% | 1.4% | 7.87 | 0.26% | 0 |
2013 | 451,000 | 1591 | 92 | 0.20 | 3 | 0.007 | 95 | 2875 | 2970 | 6.0% | 3.2% | 6.59 | 0.35% | 0 |
2014 | 550,238 | 1442 | 49 | 0.09 | 6 | 0.011 | 55 | 1922 | 1977 | 3.8% | 2.8% | 3.59 | 0.26% | 0 |
2015 | 559,577 | 6391 | 105 | 0.19 | 13 | 0.023 | 118 | 1367 | 1485 | 1.8% | 7.9% | 2.65 | 1.14% | 0 |
2016 | 569,043 | 1708 | 8 | 0.01 | 5 | 0.009 | 13 | 919 | 932 | 0.8% | 1.4% | 1.64 | 0.30% | 0 |
2017 | 578,639 | 3392 | 8 | 0.01 | 1 | 0.002 | 9 | 288 | 297 | 0.3% | 3.0% | 0.51 | 0.59% | 0 |
2018 | 599,137 | 2027 | 4 | 0.01 | 2 | 0.003 | 6 | 143 | 149 | 0.3% | 4.0% | 0.25 | 0.34% | 0 |
2019 | 609,986 | 4546 | 6 | 0.01 | 0 | 0.000 | 6 | 0 | 6 | 0.1% | 100.0% | 0.01 | 0.75% | 0 |
Takhar | ||||||||||||||
2012 | 917,700 | 9009 | 140 | 0.15 | 1 | 0.001 | 141 | 16,297 | 16,438 | 1.6% | 0.9% | 17.91 | 0.98% | 0 |
2013 | 950,100 | 8109 | 178 | 0.19 | 4 | 0.004 | 182 | 11,950 | 12,132 | 2.2% | 1.5% | 12.77 | 0.85% | 0 |
2014 | 966,576 | 22,810 | 592 | 0.61 | 37 | 0.038 | 629 | 4789 | 5418 | 2.8% | 11.6% | 5.61 | 2.36% | 0 |
2015 | 983,336 | 19,671 | 394 | 0.40 | 36 | 0.037 | 430 | 3011 | 3441 | 2.2% | 12.5% | 3.50 | 2.00% | 0 |
2016 | 1,000,336 | 12,812 | 482 | 0.48 | 25 | 0.025 | 507 | 3400 | 3907 | 4.0% | 13.0% | 3.91 | 1.28% | 0 |
2017 | 1,017,575 | 21,014 | 562 | 0.55 | 11 | 0.011 | 573 | 316 | 889 | 2.7% | 64.5% | 0.87 | 2.07% | 0 |
2018 | 1,053,852 | 24,713 | 410 | 0.39 | 2 | 0.002 | 412 | 17 | 429 | 1.7% | 96.0% | 0.41 | 2.35% | 0 |
2019 | 1,073,319 | 21,326 | 471 | 0.44 | 1 | 0.001 | 472 | 0 | 472 | 2.2% | 100.0% | 0.44 | 1.99% | 0 |
Uruzgan | ||||||||||||||
2012 | 328,000 | 4564 | 110 | 0.34 | 10 | 0.030 | 120 | 2109 | 2229 | 2.6% | 5.4% | 6.80 | 1.39% | 0 |
2013 | 339,200 | 4534 | 162 | 0.48 | 34 | 0.100 | 196 | 3746 | 3942 | 4.3% | 5.0% | 11.62 | 1.34% | 0 |
2014 | 380,469 | 3170 | 83 | 0.22 | 6 | 0.016 | 89 | 3115 | 3204 | 2.8% | 2.8% | 8.42 | 0.83% | 0 |
2015 | 386,818 | 4394 | 95 | 0.25 | 15 | 0.039 | 110 | 2414 | 2524 | 2.5% | 4.4% | 6.53 | 1.14% | 0 |
2016 | 343,069 | 4440 | 98 | 0.29 | 5 | 0.015 | 103 | 832 | 935 | 2.3% | 11.0% | 2.73 | 1.29% | 0 |
2017 | 362,253 | 5275 | 53 | 0.15 | 5 | 0.014 | 58 | 889 | 947 | 1.1% | 6.1% | 2.61 | 1.46% | 0 |
2018 | 361,030 | 7134 | 111 | 0.31 | 22 | 0.061 | 133 | 966 | 1099 | 1.9% | 12.1% | 3.04 | 1.98% | 0 |
2019 | 428,466 | 7264 | 69 | 0.16 | 9 | 0.021 | 78 | 0 | 78 | 1.1% | 100.0% | 0.18 | 1.70% | 0 |
Wardak | ||||||||||||||
2012 | 558,400 | 3907 | 232 | 0.42 | 4 | 0.007 | 236 | 979 | 1215 | 6.0% | 19.4% | 2.18 | 0.70% | 0 |
2013 | 577,100 | 3660 | 160 | 0.28 | 4 | 0.007 | 164 | 543 | 707 | 4.5% | 23.2% | 1.23 | 0.63% | 0 |
2014 | 586,623 | 3788 | 329 | 0.56 | 13 | 0.022 | 342 | 753 | 1095 | 9.0% | 31.2% | 1.87 | 0.65% | 0 |
2015 | 596,287 | 5311 | 449 | 0.75 | 17 | 0.029 | 466 | 873 | 1339 | 8.8% | 34.8% | 2.25 | 0.89% | 0 |
2016 | 606,077 | 5052 | 536 | 0.88 | 12 | 0.020 | 548 | 364 | 912 | 10.8% | 60.1% | 1.50 | 0.83% | 0 |
2017 | 615,992 | 6294 | 589 | 0.96 | 31 | 0.050 | 620 | 252 | 872 | 9.9% | 71.1% | 1.42 | 1.02% | 0 |
2018 | 637,634 | 4622 | 776 | 1.22 | 14 | 0.022 | 790 | 31 | 821 | 17.1% | 96.2% | 1.29 | 0.72% | 0 |
2019 | 648,866 | 4035 | 359 | 0.55 | 20 | 0.031 | 379 | 8 | 387 | 9.4% | 97.9% | 0.60 | 0.62% | 0 |
Zabul | ||||||||||||||
2012 | 284,600 | 13,511 | 2133 | 7.49 | 57 | 0.200 | 2190 | 6460 | 8650 | 16.2% | 25.3% | 30.39 | 4.75% | 0 |
2013 | 294,100 | 12,447 | 1677 | 5.70 | 25 | 0.085 | 1702 | 6241 | 7943 | 13.7% | 21.4% | 27.01 | 4.23% | 0 |
2014 | 299,125 | 21,899 | 554 | 1.85 | 46 | 0.154 | 600 | 4117 | 4717 | 2.7% | 12.7% | 15.77 | 7.32% | 0 |
2015 | 304,126 | 15,338 | 321 | 1.06 | 3 | 0.010 | 324 | 2894 | 3218 | 2.1% | 10.1% | 10.58 | 5.04% | 0 |
2016 | 309,192 | 25,039 | 530 | 1.71 | 43 | 0.139 | 573 | 1133 | 1706 | 2.3% | 33.6% | 5.52 | 8.10% | 0 |
2017 | 314,325 | 15,440 | 191 | 0.61 | 14 | 0.045 | 205 | 1042 | 1247 | 1.3% | 16.4% | 3.97 | 4.91% | 0 |
2018 | 371,043 | 15,776 | 278 | 0.75 | 6 | 0.016 | 284 | 556 | 840 | 1.8% | 33.8% | 2.26 | 4.25% | 0 |
2019 | 377,648 | 10,405 | 697 | 1.85 | 40 | 0.106 | 737 | 2 | 739 | 7.1% | 99.7% | 1.96 | 2.76% | 0 |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahmoodi, S.D., Atarud, A.A., Sediqi, A.W. et al. Trends in malaria indicators after scale-up of community-based malaria management in Afghanistan. Malar J 21, 165 (2022). https://doi.org/10.1186/s12936-022-04174-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04174-x