Abstract
Background
The STECLA strain of Anopheles albimanus has been in continuous colony for many years and is the reference strain on which genomic studies for the species are based. Recently, the STECLA strain was demonstrated to be much less susceptible to ivermectin ingested in a blood meal (4-day LC50 of 1468 ng/ml) than all other Anopheles species tested to-date (LC50 values range from 7 to 56 ng/ml). The ability of An. albimanus to survive ingestion of ivermectin at concentrations far beyond that typically found in the blood of ivermectin-treated people or livestock (i.e., 30–70 ng/ml) could invalidate the use of ivermectin as a malaria vector control strategy in areas where An. albimanus is a primary vector.
Methods
To investigate this, host-seeking An. albimanus were captured in northern Belize and used in membrane feeding bioassays of ivermectin, employing the same methods as described earlier with the STECLA strain.
Results
Field-collected An. albimanus in Belize were 55 times more susceptible to ingested ivermectin than were the STECLA reference strain. Oral susceptibility to ivermectin in wild An. albimanus from Belize (4-day LC50 of 26 ng/ml) was equivalent to that of other Anopheles species tested.
Conclusions
Contrary to initial assessments using a highly inbred strain of mosquito, laboratory studies using a field population indicate that ivermectin treatment of livestock could reduce An. albimanus populations in areas of Central America and the Caribbean where malaria transmission may occur. Toxicity screening of ivermectin and other systemic parasiticides for malaria control should examine wild populations of the vector species being targeted.
Similar content being viewed by others
Background
Ivermectin has long been an important drug for treating livestock against parasitic nematodes and arthropods (e.g., ticks) and more recently, for treating humans against filarial nematodes that cause lymphatic filariasis and onchocerciasis. Ivermectin has potential importance in the global effort to eliminate malaria because of its ability to reduce malaria vector populations [1]. When ingested by Anopheles mosquitoes at concentrations normally found in the plasma of treated people or livestock, ivermectin has been shown to reduce the survivorship and fecundity of almost every Anopheles species in which the drug has been tested [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. The one exception has been the Central American vector, Anopheles albimanus.
In recent laboratory studies [16], it was reported that the concentration required to kill 50% (i.e., the LC50) of An. albimanus (LC50 = 1468 ng/ml) was so much higher than the maximum concentration of ivermectin typically found in the sera of treated humans or cattle (i.e., 30–70 ng/ml [1, 17,18,19]) that ivermectin would be useless as a malaria control strategy against this mosquito species. The following year a pilot trial was conducted with cattle in northern Belize [20]. One of the animals was injected with a commercial formulation of ivermectin (Labimectin®, LabiPharma, Guatemala City, GUATEMALA) following the instructions on the label. This treatment was to serve as an extra ‘negative control’. Unexpectedly, wild An. albimanus that fed on the ivermectin-injected animal experienced significantly higher mortality than did wild An. albimanus fed on untreated cattle. It appeared that wild An. albimanus mosquitoes from northern Belize were more susceptible to ingested ivermectin than were the STECLA laboratory strain of An. albimanus mosquitoes obtained from BEI Resources (Manassas, VA USA). The STECLA strain is the An. albimanus reference strain used for many studies, including a recent physical genome map [21]. This report documents the acute oral susceptibility to ivermectin of wild-caught An. albimanus in Belize (hereafter referred as An. albimanus BELIZE) and compares it with that of An. albimanus STECLA, as well as with other Anopheles species that have been similarly tested.
Methods
Mosquitoes
Host-seeking mosquitoes were collected during nighttime human landing catches in San Roman Rio Hondo, Orange Walk District, Belize. Mosquitoes were transported to the Belize Vector and Ecology Center laboratory in Orange Walk Town, Belize. Anopheles albimanus was distinguished from other anopheline species based on the characteristic banding pattern on the hind tarsi [22]. After identification, An. albimanus BELIZE were transferred into smaller (ca. 0.5 L) cylindrical plastic cages with mesh tops at a density of 15–30 mosquitoes. Mosquitoes were maintained at 26 °C with access to 8% honey solution ad libitum.
Membrane feeding
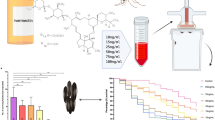
Stock solutions of ivermectin (Product No. 18898, Sigma-Aldrich, St. Louis MO, USA) at a concentration of 2 mg ivermectin per 1 ml dimethyl sulfoxide were prepared at the University of North Dakota, frozen, and transported by air to Belize City and by automobile to Belize Vector and Ecology Center, Orange Walk Town, Belize (approximately a one-hour drive). Stock solutions were diluted in water to make initial starting concentrations. Final ivermectin concentrations (i.e., 10, 25, 50, 150, 300, 1000 ng/ml) were then prepared by adding appropriate volumes of human blood to a final volume of 8 ml. The control group received blood with no additives. Blood mixtures were kept warm prior to feeding. Natural ham collagen, pre-soaked in distilled water, was used as the material through which mosquitoes probed and fed. The collagen was affixed to glass membrane feeders with rubber bands, feeders were connected to one another with rubber tubing, and warm water (37 °C) was circulated through the feeders. Membrane feeders were then placed on individual cages containing 15 to 30 wild-caught mosquitoes and the pre-warmed blood mixtures were pipetted into the feeders. Mosquitoes were allowed 90 min to feed in darkness. Afterwards, unfed mosquitoes were removed. Engorged mosquitoes were maintained at 26 °C with access to 8% honey solution ad libitum. Cages were checked every day and dead mosquitoes were counted and removed. After four days, surviving mosquitoes were counted and trial runs were terminated at that point. As much as possible, the methodologies used in this trial were consistent with that used by Dreyer et al. [16] with the notable exceptions that in this trial, the initial dilutions of ivermectin stock solution were prepared using water instead of phosphate buffered saline, and human blood (rather than cow blood) was used in this trial for the final mixtures fed to mosquitoes.
Data analysis
Mosquito mortalities observed within experimental groups were adjusted for mortality that occurred within corresponding control groups using Abbott’s formula [23]. Only experimental trials having control mortalities less than 20% were used for further data analyses. Log-probit analyses were conducted on the corrected percent moralities to estimate LC50 values (Minitab Inc., State College PA, USA). Mosquito survivorship was analysed with a Kaplan–Meier survival analysis and Log-rank Mantel-Cox test (GraphPad Software, La Jolla CA USA). A p-value of less than or equal to 0.05 was used throughout to indicate statistical difference between experimental groups.
Results
A total 352 fully engorged mosquitoes over five separate feeding trials were used to determine the acute oral toxicity of ivermectin for An. albimanus BELIZE, collected in the field from northern Belize. The estimated average membrane-feeding rate was 31.5%. Post-feeding mosquito mortality was protracted and occurred over a period of several days after ingestion of treated blood (Fig. 1), as reported for other Anopheles species ingesting ivermectin. The LC50 (lower and upper 95% confidence intervals) at day 4 post-feeding was 26.4 ng/ml (13.7–51.0); over 55-fold higher than that reported for the STECLA laboratory reference strain of An. albimanus (LC50 = 1468 ng/ml) using the same methodologies [16].
Discussion
With the notable exception of the An. albimanus STECLA, all Anopheles species tested thus far with ivermectin using membrane-feeding techniques, have LC50 values (i.e., 7 to 56 ng/ml, Table 1) well within the typical peak plasma concentrations of ivermectin reported for humans and livestock (e.g., 30–70 ng/ml) following standard drug administration at approved doses. Thus, all Anopheles species examined to date are theoretically susceptible to population reduction via targeted administration of ivermectin to humans and livestock. This is the first study to quantify oral susceptibility to ivermectin in a field population of Anopheles using the membrane feeding bioassay technique. Previous studies using this standardized technique have relied on laboratory strains of mosquitoes that have been in continuous colony for many years. Not surprisingly, there was more heterogeneity in the response to ingested ivermectin with the Belize field population, as indicated by wider confidence intervals around the LC50 value than observed in colonized mosquitoes (Table 1). Similarly, there was a flatter slope in the dose-response curve of wild An. albimanus BELIZE than observed for the STECLA strain of An. albimanus and for laboratory strains of Anopheles stephensi STE2 and Anopheles arabiensis DONGOLA (Table 2). Greater heterogeneity in the response to ivermectin by a wild population may have resulted from several sources, including; smaller sample sizes examined, testing mosquitoes of unknown age and physiological condition, and to the greater overall genetic diversity inherent in field populations versus inbred laboratory strains. Importantly, the findings that different populations of An. albimanus (BELIZE versus STECLA) vary widely in their susceptibilities to ivermectin and that the response to ivermectin in a wild population is more heterogenous than in laboratory populations suggest that the development of ivermectin-resistant populations of An. albimanus in nature is possible. That possibility is underscored by the fact that in our trials, two of 26 (7.7%) An. albimanus BELIZE mosquitoes that ingested 1000 ng/ml of ivermectin were able to survive the 4-day post-feeding interval.
Conclusions
This study illustrates the importance of including wild-caught indigenous populations of vectors (as opposed to sole reliance on laboratory strains) during in vitro toxicological screening of ivermectin and other systemic parasiticides. By screening wild populations of a targeted vector species, investigators may know better what to expect in field trials that involve treating entire herds of livestock.
Availability of data and materials
The data analysed during this study are available on request from the corresponding author.
Abbreviations
- LC50 :
-
Concentration of ivermectin required to kill 50% of treated mosquitoes
References
Billingsley P, Binka F, Chaccour C, Foy B, Gold, Gonzalez-Silva SM, et al. A roadmap for the development of ivermectin as a complementary malaria vector control tool. Amer J Trop Med Hyg. 2020;102:3–24.
Fritz ML, Siegert PY, Walker ED, Bayoh MN, Vulule JR, Miller JR. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.1. Ann Trop Med Parasitol. 2009;103:539–47.
Chaccour CJ, Lines J, Whitty CJM. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis. 2010;202:113–6.
Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9:365.
Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, Marques da Silva MI, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–26.
Kobylinski KC, Sylla M, Chapman PL, Sarr MD, Foy BD. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3–5.
Fritz ML, Walker ED, Miller JR. Lethal and sublethal effects of avermectin/milbemycin parasiticides on the African malaria vector, Anopheles arabiensis. J Med Entomol. 2012;49:326–31.
Naz S, Maqbool A, Ahmad M, Anjum AA. Efficacy of ivermectin for control of zoophilic malaria vectors in Pakistan. Pak J Zool. 2013;45:1585–91.
Alout H, Krajacich BJ, Meyers JI, Grubaugh ND, Brackney DE, Kobylinski KC, et al. Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malar J. 2014;13:417.
Poché RM, Burruss D, Polyakova L, Poché DM, Garlapati RB. Treatment of livestock with systemic insecticides for control of Anopheles arabiensis in western Kenya. Malar J. 2015;14:351.
Sampaio VS, Beltrán TP, Kobylinski KC, Melo GC, Lima JBP, Silva SGM, et al. Filling gaps on ivermectin knowledge: effects on the survival and reproduction of Anopheles aquasalis, a Latin American malaria vector. Malar J. 2016;15:491.
Lyimo IN, Kessy ST, Mbina KF, Daraja AA, Mnyone LL. Ivermectin-treated cattle reduces blood digestion, egg production and survival of a free-living population of Anopheles arabiensis under semi-field condition in south-eastern Tanzania. Malar J. 2017;16:239.
Kobylinski KC, Ubalee R, Ponlawat A, Nitatsukprasert C, Phasomkulsolsil S, Wattanakul T, et al. Ivermectin susceptibility and sporontocidal effect in Greater Mekong Subregion Anopheles. Malar J. 2017;16:280.
Kobylinski KC, Escobedo-Vargas KS, López-Sifuentes VM, Durand S, Smith ES, Baldeviano GC, et al. Ivermectin susceptibility, sporontocidal effect, and inhibition of time to re-feed in the Amazonian malaria vector Anopheles darlingi. Malar J. 2017;16:474.
Chaccour CJ, Ngha’Bi K, Abizanda G, Irigoyen Barrio A, Aldaz A, Okumu F, et al. Targeting cattle for malaria elimination: marked reduction of Anopheles arabiensis survival for over six months using a slow-release ivermectin implant formulation. Parasites Vectors. 2018;11:287.
Dreyer SM, Morin KJ, Vaughan JA. Differential susceptibilities of Anopheles albimanus and Anopheles stephensi mosquitoes to ivermectin. Malar J. 2018;17:148.
Lanusse C, Lifschitz A, Virkel G, Alvarez L, Sánchez S, Sutra JF, et al. Comparative plasma disposition kinetics of ivermectin, moxidectin and doramectin in cattle. J Vet Pharmacol Ther. 1997;20:91–9.
Lifschitz A, Sallovitz J, Imperiale F, Pis A, Jauregui Lorda J, Lanusse C. Pharmacokinetic evaluation of four ivermectin generic formulations in calves. Vet Parasitol. 2004;119:247–57.
Ndong TB, Kane Y, Ba MA, Sane I, Sutra JF, Alvinerie M. Pharmacokinetics of ivermectin in zebu Gobra (Bos indicus). Vet Parasitol. 2005;128:169–73.
Dreyer SM, Leiva D, Magaña M, Pott M, Kay J, Cruz A, et al. Fipronil and ivermectin treatment of cattle reduced the survival and ovarian development of field-collected Anopheles albimanus in a pilot trial conducted in northern Belize. Malar J. 2019;18:296.
Artemov GN, Peery AN, Jiang X, Tu Z, Stegniy VN, Sharakhova MV, et al. The physical genome mapping of Anopheles albimanus corrected scaffold misassemblies and identified interarm rearrangements in genus Anopheles. G3. 2017;7:155–64.
Wilkerson RC, Strickman D, Litwak TR. Illustrated key to the female anopheline mosquitoes of Central America and Mexico. J Am Mosq Control Assoc. 1990;6:7–34.
Abbott WS. Abbott’s formula a method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–7.
Acknowledgements
The authors wish to thank the mosquito collectors in San Roman who assisting in collecting the mosquitoes used in this trial. The Anopheles albimanus STECLA strain was obtained as eggs (i.e., MRA-126, contributed by Mark Q. Benedict) from the BEI Resources Manassas, VA, funded in part by the National Institute of Allergy & Infectious Diseases, National Institute of Health, USA.
Funding
This study was funded by Grant R21AI119771 from the National Institute of Allergy and Infectious Diseases, NIH, USA. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: SMD and JAV. Conducted the laboratory work: SMD, KJM, MM, and MP. Conducted data analysis and wrote the manuscript: SMD and JAV. Provided logistical and infrastructure support from the Belize Vector Ecology Center: NLA and JPG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. The source of human blood used in this study came from local blood bank. No identifiers were associated with the blood and it use in this research was authorized under an Exemption 4 as described in the United States 45 Code of Federal Regulations part 46.104.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dreyer, S.M., Morin, K.J., Magaña, M. et al. Oral susceptibility to ivermectin is over fifty times greater in a wild population of Anopheles albimanus mosquitoes from Belize than the STECLA laboratory reference strain of this mosquito. Malar J 21, 72 (2022). https://doi.org/10.1186/s12936-022-04092-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04092-y